Abstract
A severe outbreak of diarrhea associated with poor growth was reported in ten newly weaned goat kids that originated from a research farm (Group A). Two of these kids underwent necropsy examination. Five goat kids of the same age maintained in the same pen showed no clinical signs (Group B). The clinical, gross pathological and histopathological features of the clinically sick animals were consistent with severe coccidiosis. Group A animals had significantly lower levels of serum vitamin B12 (<200 pg/ml) compared with group B animals (2000 pg/ml). In addition, kids belonging to group A had significantly higher Eimeria arloingi oocysts per gram (OPG) of faeces (101,400/g) compared with kids of group B (9,154/g). Microscopy and molecular tools (18S rRNA and COI genes) confirmed that the goat kids were infected with the caprine protozoan parasite E. arloingi. This study provides a definitive association between low levels of serum vitamin B12 and clinical E. arloingi infection, and also provides support to our previous studies that demonstrated how low levels of serum vitamin B12 leads to an impairment of neutrophil function and thereby potential lowered immunity to pathogens.
Keywords:
Cobalt; vitamin B12; Eimeria arloingi; 18S rRNA; COI; goats
Resumo
Um surto grave de diarreia, associado à baixo crescimento, foi relatado em dez cabritos recém-desmamados, originários de uma fazenda de pesquisa (Grupo A). Dois animais foram submetidos a exame necroscópico. Cinco cabritos da mesma idade e mantidos na mesma instalação não apresentaram sinais clínicos (Grupo B). As características clínicas e as lesões macroscópicas e microscópicas dos animais clinicamente doentes eram consistentes com coccidiose grave. Os animais do grupo A apresentaram níveis significativamente mais baixos de vitamina B12 sérica (<200 pg / ml) em comparação com os animais do grupo B (2000 pg/ml). Além disso, os animais pertencentes ao grupo A apresentaram um número de oocistos de Eimeria arloingi por grama (OPG) de fezes (101,400/g) significativamente mais alto do que os animais do grupo B (9,154/g). As análises microscópica e molecular (genes 18S rRNA e COI) confirmaram que os cabritos estavam infectados com o protozoário E. arloingi. Este estudo fornece uma associação definitiva entre baixos níveis de vitamina B12 no soro e infecção clínica por E. arloingi. Também fornece suporte aos estudos anteriores, que demonstraram como baixos níveis de vitamina B12 no soro comprometem a função dos neutrófilos e, consequentemente, a imunidade a patógenos.
Palavras-chave:
Cobalto; vitamina B12; Eimeria arloingi; 18S rRNA; COI; cabras
Introduction
In previous studies, we demonstrated that newly weaned goat kids having low levels of serum vitamin B12 (< 200 pg/ml) exhibited poor growth rates and bone lengths (Kadim et al., 2006Kadim IT, Mahgoub O, Al-Ajmi D, Al-Habsi KR, Johnson EH. Comparative effects of low levels of dietary cobalt and parenteral injections of Vitamin B12 on body dimensions in different breeds of Omani goats. Small Rumin Res 2006; 66(1-3): 244-252. http://dx.doi.org/10.1016/j.smallrumres.2005.09.018.
http://dx.doi.org/10.1016/j.smallrumres....
), decreased nutrient digestibility (Kadim et al., 2003Kadim IT, Johnson EH, Mahgoub O, Srikandakumar A, Al-Ajmi D, Ritchie A, et al. Effect of low levels of dietary cobalt on apparent nutrient digestibility in Omani goats. Anim Feed Sci Technol 2003; 109(1-4): 209-216. http://dx.doi.org/10.1016/S0377-8401(03)00174-3.
http://dx.doi.org/10.1016/S0377-8401(03)...
) and poor meat quality (Kadim et al., 2004Kadim IT, Mahgoub O, Srikandakumar A, Al-Ajmi DS, Al-Maqbaly RS, Al-Saqri NM, et al. Comparative effect of low levels of dietary cobalt and parenteral injection of vitamin B12 on carcass and meat quality characteristics in Omani goats. Meat Sci 2004; 66(4): 837-844. http://dx.doi.org/10.1016/j.meatsci.2003.08.003. PMid:22061016.
http://dx.doi.org/10.1016/j.meatsci.2003...
). In addition, we also observed hepatic lipidosis in goat kids fed diets with low levels of cobalt resulting in deficiency of serum vitamin B12 (Johnson et al., 1999Johnson EH, Muirhead DE, Annamalai K, King GJ, Al-Busaidy R, Shahul Hameed M. Hepatic lipidosis associated with cobalt deficiency in Omani goats. Vet Res Commun 1999; 23(4): 215-221. http://dx.doi.org/10.1023/A:1006244925482. PMid:10461798.
http://dx.doi.org/10.1023/A:100624492548...
, 2004Johnson EH, Al-Habsi K, Kaplan E, Srikandakumar A, Kadim IT, Annamalai K, et al. Caprine hepatic lipidosis induced through the intake of low levels of dietary cobalt. Vet J 2004; 168(2): 174-179. http://dx.doi.org/10.1016/j.tvjl.2003.10.012. PMid:15301766.
http://dx.doi.org/10.1016/j.tvjl.2003.10...
). Deficiency of vitamin B12 in serum significantly impairs antibody response, and also leads to lower oxidative respiratory burst of neutrophils during phagocytosis (Johnson et al., 2010Johnson EH, Al-Habsi K, Al-Busaidy R, Khalaf SK. The effect of low levels of dietary cobalt on the chemiluminescence response of polymorphonuclear leukocytes of goats. Res Vet Sci 2010; 88(1): 61-63. http://dx.doi.org/10.1016/j.rvsc.2009.06.008. PMid:19679325.
http://dx.doi.org/10.1016/j.rvsc.2009.06...
, 2016Johnson EH, Al-Habsi K, Al-Busaidi R, Al-Abri M. Impaired antibody response and phagocytosis in goats fed a diet low in cobalt. Small Rumin Res 2016; 140: 27-31. http://dx.doi.org/10.1016/j.smallrumres.2016.05.013.
http://dx.doi.org/10.1016/j.smallrumres....
). Other authors have observed that sheep deficient in vitamin B12 are more susceptible to parasites such as Ostertagia ostertagi (Paterson & MacPherson, 1990Paterson JE, MacPherson A. The influence of a low cobalt intake on the neutrophil function and severity of Ostertagia infection in cattle. Br Vet J 1990; 146(6): 519-530. http://dx.doi.org/10.1016/0007-1935(90)90055-8. PMid:2271909.
http://dx.doi.org/10.1016/0007-1935(90)9...
).
Eimeria infections in goats are commonly asymptomatic. However, some species such as E. ninakohlyakimovae and E. arloingi are considered pathogenic and have been associated with poor body growth (Chartier & Paraud, 2012Chartier C, Paraud C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res 2012; 103(1): 84-92. http://dx.doi.org/10.1016/j.smallrumres.2011.10.022.
http://dx.doi.org/10.1016/j.smallrumres....
; Ruiz et al., 2012Ruiz A, Guedes AC, Muñoz MC, Molina JM, Hermosilla C, Martín S, et al. Control strategies using diclazuril against coccidiosis in goat kids. Parasitol Res 2012; 110(6): 2131-2136. http://dx.doi.org/10.1007/s00436-011-2746-0. PMid:22193521.
http://dx.doi.org/10.1007/s00436-011-274...
). A positive correlation has been seen between undetermined Eimeria species oocyst counts and low levels of serum vitamin B12 levels in six month old goats (Al-Zadjali et al., 2004Al-Zadjali A, Johnson EH, Srikandakumar A. Serum vitamin B12 levels in Omani goats. Trop Anim Health Prod 2004; 36(5): 473-482. http://dx.doi.org/10.1023/B:TROP.0000035009.66980.2b. PMid:15449837.
http://dx.doi.org/10.1023/B:TROP.0000035...
).
In the present study, we describe an outbreak of diarrhea and poor growth in a group of newly weaned goat kids that were found infected with E. arloingi. Based on previous experience with this farm it was also suspected that some of the animals might also have low levels of serum vitamin B12 and decreased red blood cell counts despite all of the animals having access to mineral blocks containing cobalt. We measured serum vitamin B12 levels in these clinically affected animals, as well as in asymptomatic kids from the same pen, and also evaluated their stool samples not only quantitatively for the presence of Eimeria oocysts but also employed morphological and molecular tools to confirm the species of Eimeria causing pathogenicity.
Material and Methods
Study animals
Faecal and blood samples were collected at the Agricultural Experiment Station (AES), Sultan Qaboos University from two groups (A and B) of goat kids. These kids were separated from their dams at weaning, yet kept in the same pen at densities of 20-30 heads per pen. Group A was comprised of kids (n=10) that presented severe diarrhea and a history of rapid weight loss. Group B was comprised of apparently healthy kids (n=5), with no obvious clinical signs of diarrhea or weight loss. Animals at the AES were free of internal parasites, as they were routinely subjected to anthelmintics. Both groups were fed 150 g/day per head of pelleted concentrate (containing 0.2 ppm Co/kg DM), Rhodes grass hay (Chioris gayanna) ad libitum and provided with access to mineral blocks (containing 40 mg/kg of Co).
Microscopic procedure
Sporulation of Eimeria oocysts was done at room temperature (~32 °C) by adding 2% K2Cr2O7 to 2g of faeces. Sporulated oocysts of Eimeria were isolated 72-97 hr later by flotation technique using saturated salt or 50% sucrose solution, as previously performed by Al-Habsi et al. (2017a)Al-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
. Oocysts per gram (OPG) of faeces were determined by a modified McMaster test, as described by Zajac & Conboy (2012)Zajac AZ, Conboy GA. Veterinary clinical parasitology. 8th ed. Ames: John Wiley & Sons; 2012.. A 3-axis hydraulic micromanipulator (MO-102, Nirashige, Japan) was used as previously performed by Al-Habsi et al. (2017a)Al-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
to isolate selected morphotypes and to transfer Eimeria oocysts to separate slides for morphometric measurements. Length and width measurements (n=50 from total samples) were done and species were confirmed from works of Levine et al. (1962)Levine ND, Ivens V, Fritz TE. Eimeria christenseni sp. n. and other coccidia (Protozoa: Eimeriidae) of the goat. J Parasitol 1962; 48(2): 255-269. http://dx.doi.org/10.2307/3275578. PMid:14464632.
http://dx.doi.org/10.2307/3275578...
and Al-Habsi et al. (2017a)Al-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
.
A week after the collection of faecal and blood samples, two kids from group A died and were necropsied. Tissue samples from the liver, mesenteric lymph nodes, and small and large intestine were fixed in 10% buffered formalin. Later, tissues were embedded in paraffin, sectioned at 5μm, stained with hematoxylin and eosin, and examined microscopically. The remaining eight kids were treated for three consecutive days with oral coccidiostat (55 mg/kg body weight sulfadimethoxine, Albon®, Zoetis Inc USA), and a vitamin B12 supplement (Lovit Amino Plus Liquid, Kaesler Nutrition®, Germany).
Chemical procedure for vitamin B12
Serum vitamin B12 levels were determined for animals from both group A and group B goat kids, as previously performed by Johnson et al. (2004)Johnson EH, Al-Habsi K, Kaplan E, Srikandakumar A, Kadim IT, Annamalai K, et al. Caprine hepatic lipidosis induced through the intake of low levels of dietary cobalt. Vet J 2004; 168(2): 174-179. http://dx.doi.org/10.1016/j.tvjl.2003.10.012. PMid:15301766.
http://dx.doi.org/10.1016/j.tvjl.2003.10...
using a Microparticle Enzyme Immunoassay (MEIA) (IMX B12; Abbott Laboratories) according to the manufacturer’s recommendations. The assay was based on the competitive binding of serum vitamin B12 and a standard B12 where the alkaline phosphatase conjugate attaches to an intrinsic factor bound on a matrix.
Molecular procedure
DNA was extracted from 0.25 g of faecal material using repeated bead beating plus column (RBB+C) method as performed by Yu & Morrison (2004)Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004; 36(5): 808-812. http://dx.doi.org/10.2144/04365ST04. PMid:15152600.
http://dx.doi.org/10.2144/04365ST04...
. Briefly, cell lysis was achieved by bead beating in the presence of 4% (w/v) sodium dodecyl sulfate (SDS), 500 mM NaCl, and 50 mM EDTA. The impurities and the SDS were removed by precipitation with ammonium acetate, and then the nucleic acids were recovered by precipitation with isopropanol. Genomic DNA was purified via sequential digestions with RNase and proteinase K, followed by the use of QIAamp columns. Subsequently, DNA was also extracted from single Eimeria oocysts using micromanipulator (as delineated under the microscopic procedure) and transferred into a PCR tube for extraction. At annealing temperature of 58 °C, DNA was amplified at 18S rRNA and COI loci, using two-step PCR, with primers as shown in Table 1. PCR products of ∼1510 bp and 723 bp were expected from 18S rRNA and COI amplifications respectively. Horizontal electrophoresis was performed using 1.0% agarose gels (Promega, Madison, USA).
Target genes and primers used for amplification and sequencing of Eimeria isolates in Omani goats.
Sequence searches of positive isolates for Eimeria were conducted using BLAST (NCBI, 2020National Center for Biotechnology Information – NCBI [online]. 2020 [cited 2020 Mar 17]. Available from: http://blast.ncbi.nlm.nih.gov/Blast.cgi
http://blast.ncbi.nlm.nih.gov/Blast.cgi...
) and nucleotide sequences were analyzed using Chromas Lite version 2.0 (Technelysium, 2020Technelysium [online]. 2020 [cited 2020 Mar 17]. Available from: http://www.technelysium.com
http://www.technelysium.com...
) and aligned with reference genotypes from GenBank using ClustalW (2020)ClustalW [online]. 2020 [cited 2020 Mar 17]. Available from: https://www.genome.jp/tools-bin/clustalw
https://www.genome.jp/tools-bin/clustalw...
. Maximum likelihood (ML) and neighbor-joining (NJ) analyses were conducted using Tamura-Nei model and pairwise deletion for gaps based on the most appropriate model selection using Model Test in MEGA 7: Molecular Evolutionary Genetics Analysis version 7.0 (Tamura et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. http://dx.doi.org/10.1093/molbev/mst197. PMid:24132122.
http://dx.doi.org/10.1093/molbev/mst197...
). Bootstrap analyses were conducted using 1000 replicates to assess the reliability of inferred tree topologies.
Results
Eimeria morphometry and density
Eimeria arloingi was identified by microscopy in faecal samples of all animals in both groups. All examined oocysts (n=50) were elliptical with bi-layered walls with an average length and width of 26.8 μm (range; 24.4-30.4 μm) and 19.6 μm (range; 17.1-21.6 μm). Sporocysts were ovoid with an average length size of 12.1 μm (10.4-14.1). No polar granules were noted, while sporocyst residuum was observed in most of the examined oocysts (Figure 1A, B).
Nomarski interference-contrast photomicrographs of the Eimeria oocysts from weaned Omani goats; (A) unsporulated E. arloingi, and (B) sporulated E. arloingi showing oocyst wall (OW), micropolar cap (MC), sporont (SPO), sporozoites (SP), and sporocyst residuum (SR), Scale bar = 20 μm; (C) Density of Eimeria spp. using modified McMaster in Omani goat kids before and after treatment with a coccidiostat.
An average of 101,400 (range; 86,190-116,610) OPG of faeces was found in kids from group A. In group B kids, this value was considerably lower, i.e., 2,150 (range; 1,825-2,472) OPG. After treatment of group A kids with a coccidiostat, the OPG got reduced to 9,154 (range; 7,781-10,527) (Figure 1C).
Histopathology and vitamin B12 levels
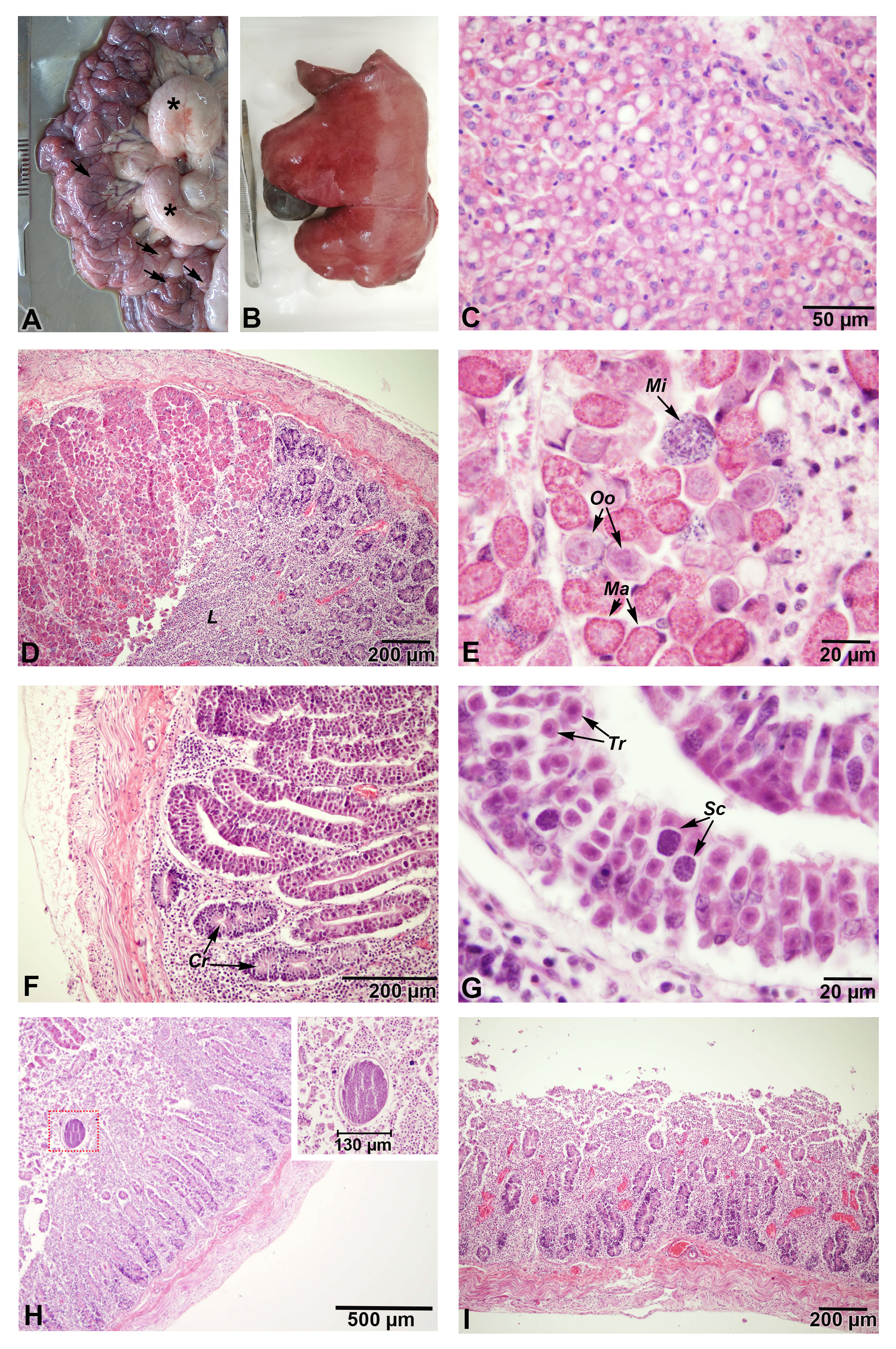
Whitish yellowish foci were noted through the jejunal serosa with severe enlargement of the mesenteric lymph nodes (Figure 2A). Fatty livers with rounded borders and yellowish brown colour were observed (Figure 2B). Examined sections from hepatic tissues revealed periportal diffuse macrovesicular steatosis (fatty liver) (Figure 2C). Examined jejunal tissue sections showed replacement of the intestinal villi and the crypts with lymphocytes and different sexual developmental stages of Eimeria spp. Mature microgamonts containing and shedding microgametes, mature macrogamont and, young oocysts were seen. The majority of the crypts of Lieberkühn revealed various asexual developmental stages of Eimeria spp. Moreover, myriads of trophozoites and multiple first generation schizonts were detected. The jejunum exhibited complete loss of the intestinal villi and the presence of macroschizonts in the intestinal lumen. Severe necrotic enteritis with lymphocytic infiltrations and multiple dilated blood vessels were observed in the terminal part of the jejunum (Figure 2DI).
Pathological findings in naturally infected goat kid due to E. arloingi (Stain: H&E): (A) severely enlarged mesenteric lymph nodes (*) with presence of whitish yellowish patches (arrows) in the jejunum that can be seen through the serosa; (B) fatty liver with rounded borders and yellowish brown colour; (C) micrograph of (B) revealing periportal diffuse macrovesicular steatosis; (D) jejunum with complete replacement of the intestinal villi and the majority of the crypts with lymphocytes (L) and different sexual developmental stages of E. arloingi; (E) higher magnification of (D) showing mature microgamonts (Mi) containing and shedding microgametes, mature macrogamont (Ma) and young oocysts (Oo); (F) few normal crypts of Lieberkühn (Cr) with the majority of the crypts invaded with the asexual developmental stages of E. arloingi; (G) higher magnification of (F) showing myriads of trophozoites (Tr) and multiple first generation schizonts (Sc); (H) jejunum with complete loss of the intestinal villi and the presence of a macroschizonts in the intestinal lumen (inset: higher magnification of the macroschizonts); (I) terminal part of the jejunum showing severe necrotic enteritis with complete loss of the intestinal villi, lymphocytic infiltrations and multiple dilated blood vessels.
The average serum vitamin B12 measured in group A kids (with diarrhea and reduced weight gains) was 188.4 pg/ml (range; 109-294 pg/ml). This result was significantly lower (P<0.05) than the mean values of the group B goat kids which was >2000 pg/ml.
Amplification of Eimeria at the 18S rRNA and COI genes
PCR amplification of the Eimeria microscopically positive samples resulted in successfully generating 1280 bp identical products which were confirmed by sequencing in both directions. Distance, Parsimony and ML analyses were used to construct the phylogeny. Two Iranian and Australian goat-derived E. arloingi 18S rRNA sequences were retrieved from GenBank (KC507792) (Khodakaram-Tafti et al., 2013Khodakaram-Tafti A, Hashemnia M, Razavi SM, Sharifiyazdi H, Nazifi S. Genetic characterization and phylogenetic analysis of Eimeria arloingi in Iranian native kids. Parasitol Res 2013; 112(9): 3187-3192. http://dx.doi.org/10.1007/s00436-013-3494-0. PMid:23779225.
http://dx.doi.org/10.1007/s00436-013-349...
) and (KX845686) (Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
) with a length of 637 and 1270 bp respectively. Eimeria arloingi from Omani goats (MK830054) grouped in one clade with E. arloingi (KX845686) which was derived from captured rangeland goats (Capra hircus) in Western Australia (Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
) with 99.4% homology (Figure 3A).
Evolutionary relationships of Eimeria spp. inferred by distance analysis of 18S rRNA gene (A) and cytochrome c oxidase subunit I (COI) gene (B). Accession numbers of samples follow the species name. Bootstrap values (> 50%) based on 1000 replicates are indicated at the left of each supported node. The scale bar is the proportion of base substitutions per site.
At the cytochrome c oxidase I (COI) locus, 677 bp PCR products were successfully amplified from all the microscopy positive isolates. Single ovine E. ahsata (KT184373) and caprine E. arloingi (KX857470) (Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
) cytochrome c oxidase I (COI) sequences were available in the GenBank database. Phylogenetic analysis of the 677 bp COI sequence grouped E. arloingi from Omani goats (MK861046) of the present study in one clade with E. arloingi isolate from rangeland goats of western Australia (Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
), with 99.8% similarity (Figure 3B).
Discussion
The present study revealed that Omani goat kids with low levels of serum vitamin B12 (<200 pg/ml) had severe clinical manifestations as a result of infection with E. arloingi. Ten of the animals had severe diarrhea and showed poor growth compared to the five healthy ones. Two of the ten goats that succumbed to the infection had histopathological inflammatory alterations in the jejunum consistent with Eimeria infection with different developmental stages of the parasite seen. In addition these animals had enlarged pale livers with macroscopic and microscopic evidence of hepatic lipidosis; pathological lesions that we have also previously experimentally reproduced by inducing low levels of serum vitamin B12 in newly weaned goats by feeding them a diet with low levels of cobalt (<0.1 ppm Co per kg DM) (Johnson et al., 2004Johnson EH, Al-Habsi K, Kaplan E, Srikandakumar A, Kadim IT, Annamalai K, et al. Caprine hepatic lipidosis induced through the intake of low levels of dietary cobalt. Vet J 2004; 168(2): 174-179. http://dx.doi.org/10.1016/j.tvjl.2003.10.012. PMid:15301766.
http://dx.doi.org/10.1016/j.tvjl.2003.10...
). In contrast to these clinically sick group A goat kids, the group B goat kids had normal levels of vitamin B12 and showed no clinical signs, despite being affected with E. arloingi.
Previous studies have suggested that there might be a causal relationship between low levels of serum vitamin B12 and susceptibility to infection. Al-Zadjali et al. (2004)Al-Zadjali A, Johnson EH, Srikandakumar A. Serum vitamin B12 levels in Omani goats. Trop Anim Health Prod 2004; 36(5): 473-482. http://dx.doi.org/10.1023/B:TROP.0000035009.66980.2b. PMid:15449837.
http://dx.doi.org/10.1023/B:TROP.0000035...
reported that goats with low levels of serum vitamin B12 at the field level under a variety of management conditions had significantly higher coccidia counts. Paterson & MacPherson (1990)Paterson JE, MacPherson A. The influence of a low cobalt intake on the neutrophil function and severity of Ostertagia infection in cattle. Br Vet J 1990; 146(6): 519-530. http://dx.doi.org/10.1016/0007-1935(90)90055-8. PMid:2271909.
http://dx.doi.org/10.1016/0007-1935(90)9...
reported that cobalt-deficient calves had significantly higher Ostertagia egg counts than cobalt-supplemented calves. Our study provides evidence of clinical severity due to E. arloingi infection associated with vitamin B12 deficiency.
Morphological characterization of sporulated oocysts and host specificity were conventionally used as the basis for Eimeria species identification. Based on microscopic examination, a total of 17 Eimeria species have been described worldwide in goats (Al-Habsi, 2017bAl-Habsi K. Molecular characterisation of selected enteric pathogens and antimicrobial resistance of Salmonella in rangeland goats in Western Australia [dissertation]. Perth: Murdoch University; 2017b.). However, microscopy has limitations of time consumption and possible human error (Kawahara et al., 2010Kawahara F, Zhang G, Mingala CN, Tamura Y, Koiwa M, Onuma M, et al. Genetic analysis and development of species-specific PCR assays based on ITS-1 region of rRNA in bovine Eimeria parasites. Vet Parasitol 2010; 174(1-2): 49-57. http://dx.doi.org/10.1016/j.vetpar.2010.08.001. PMid:20817404.
http://dx.doi.org/10.1016/j.vetpar.2010....
; Carvalho et al., 2011Carvalho FS, Wenceslau AA, Teixeira M, Matos Carneiro JAM, Melo ADB, Albuquerque GR. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet Parasitol 2011; 176(2-3): 95-100. http://dx.doi.org/10.1016/j.vetpar.2010.11.015. PMid:21167646.
http://dx.doi.org/10.1016/j.vetpar.2010....
; Khodakaram-Tafti et al., 2013Khodakaram-Tafti A, Hashemnia M, Razavi SM, Sharifiyazdi H, Nazifi S. Genetic characterization and phylogenetic analysis of Eimeria arloingi in Iranian native kids. Parasitol Res 2013; 112(9): 3187-3192. http://dx.doi.org/10.1007/s00436-013-3494-0. PMid:23779225.
http://dx.doi.org/10.1007/s00436-013-349...
; Nahavandi et al., 2016Nahavandi KH, Mahvi AH, Mohebali M, Keshavarz H, Rezaei S, Mirjalali H, et al. Molecular typing of Eimeria ahsata and E. crandallis isolated from slaughterhouse wastewater. Jundishapur J Microbiol 2016; 9(4): e34140. http://dx.doi.org/10.5812/jjm.34140. PMid:27303617.
http://dx.doi.org/10.5812/jjm.34140...
).
More recently, to address these limitations, molecular tools have been employed to accurately identify Eimeria spp. from goats (Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
; Mohamaden et al., 2018Mohamaden WI, Sallam NH, Abouelhassan EM. Prevalence of Eimeria species among sheep and goats in Suez Governorate, Egypt. Int J Vet Sci Med 2018; 6(1): 65-72. http://dx.doi.org/10.1016/j.ijvsm.2018.02.004. PMid:30255081.
http://dx.doi.org/10.1016/j.ijvsm.2018.0...
). The 18S rRNA locus has been used extensively as a molecular marker in phylogenetic analysis. However, as it is a conservative gene, the 18S rRNA locus may not be the ultimate compatible gene for differentiating closely related Eimeria species and therefore it is best utilized simultaneously with other gene loci (e.g., COI locus) which are acceptable for discriminating morphologically similar Eimeria species in small ruminants (Ogedengbe et al., 2011Ogedengbe JD, Hanner RH, Barta JR. DNA barcoding identifies Eimeria species and contributes to the phylogenetics of coccidian parasites (Eimeriorina, Apicomplexa, Alveolata). Int J Parasitol 2011; 41(8): 843-850. http://dx.doi.org/10.1016/j.ijpara.2011.03.007. PMid:21515277.
http://dx.doi.org/10.1016/j.ijpara.2011....
; Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
).
In the present study, the morphological characteristics of oocysts and sporocysts of the identified E. arloingi were consistent with those of previous reports (Levine et al., 1962Levine ND, Ivens V, Fritz TE. Eimeria christenseni sp. n. and other coccidia (Protozoa: Eimeriidae) of the goat. J Parasitol 1962; 48(2): 255-269. http://dx.doi.org/10.2307/3275578. PMid:14464632.
http://dx.doi.org/10.2307/3275578...
; Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
) and these findings were confirmed by molecular tools and sequencing analysis retrieved from GenBank database. Employing both of these tools increases the accuracy of differentiation of those species sharing nearly identical morphological characteristics (Kawahara et al., 2010Kawahara F, Zhang G, Mingala CN, Tamura Y, Koiwa M, Onuma M, et al. Genetic analysis and development of species-specific PCR assays based on ITS-1 region of rRNA in bovine Eimeria parasites. Vet Parasitol 2010; 174(1-2): 49-57. http://dx.doi.org/10.1016/j.vetpar.2010.08.001. PMid:20817404.
http://dx.doi.org/10.1016/j.vetpar.2010....
; Nahavandi et al., 2016Nahavandi KH, Mahvi AH, Mohebali M, Keshavarz H, Rezaei S, Mirjalali H, et al. Molecular typing of Eimeria ahsata and E. crandallis isolated from slaughterhouse wastewater. Jundishapur J Microbiol 2016; 9(4): e34140. http://dx.doi.org/10.5812/jjm.34140. PMid:27303617.
http://dx.doi.org/10.5812/jjm.34140...
). The phylogenetic analysis at the 18S rRNA and COI loci revealed that E. arloingi from the newly weaned clinically positive and control goats were almost identical to pathogenic E. arloingi (KX845686 and KX857470) from captured rangeland goats in Australia (Al-Habsi et al., 2017aAl-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001. PMid:31014847.
http://dx.doi.org/10.1016/j.vprsr.2017.0...
).
Our lab in previous studies provided evidence that neutrophils from goats with low levels of serum vitamin B12 exhibit impaired oxygen-dependent respiratory bursts during the phagocytic process (Johnson et al., 2010Johnson EH, Al-Habsi K, Al-Busaidy R, Khalaf SK. The effect of low levels of dietary cobalt on the chemiluminescence response of polymorphonuclear leukocytes of goats. Res Vet Sci 2010; 88(1): 61-63. http://dx.doi.org/10.1016/j.rvsc.2009.06.008. PMid:19679325.
http://dx.doi.org/10.1016/j.rvsc.2009.06...
, 2016Johnson EH, Al-Habsi K, Al-Busaidi R, Al-Abri M. Impaired antibody response and phagocytosis in goats fed a diet low in cobalt. Small Rumin Res 2016; 140: 27-31. http://dx.doi.org/10.1016/j.smallrumres.2016.05.013.
http://dx.doi.org/10.1016/j.smallrumres....
) when compared to neutrophils from goats with normal levels of serum vitamin B12. Neutrophils are the first line of immunological defense utilized by the host to fight infections and have potentially a broad repertoire of mechanisms that they can employ. They produce reactive oxygen (superoxide anion and hydrogen peroxide) during the respiratory burst and release antimicrobial peptides. Recently, a study demonstrated the role of NETosis, a mechanism employed by neutrophils to immobilize pathogens by releasing neutrophil extracellular traps (NETs) which are protein-labeled DNA matrices capable of extracellular trapping and killing of invasive sporozoites and oocysts of E. arloingi (Silva et al., 2014Silva LMR, Muñoz Caro T, Gerstberger R, Vila-Viçosa MJM, Cortes HCE, Hermosilla C, et al. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol Res 2014; 113(8): 2797-2807. http://dx.doi.org/10.1007/s00436-014-3939-0. PMid:24849865.
http://dx.doi.org/10.1007/s00436-014-393...
). Bovine neutrophils have also been recognized to cast NETs against Neospora caninum (Villagra-Blanco et al., 2017Villagra-Blanco R, Silva LMR, Muñoz-Caro T, Yang Z, Li J, Gärtner U, et al. Bovine polymorphonuclear neutrophils cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front Immunol 2017; 8: 606. http://dx.doi.org/10.3389/fimmu.2017.00606. PMid:28611772.
http://dx.doi.org/10.3389/fimmu.2017.006...
).
Conclusions
This study provides evidence that low levels of serum vitamin B12 in goat kids increases susceptibility to clinical infection with pathogenic E. arloingi. Goat kids with normal levels of vitamin B12 shed E. arloingi oocysts, however, they did not present clinical signs of weakness and diarrhea. This study also provides further support to our in vitro studies which demonstrated that low levels of serum vitamin B12 leads to an impairment of neutrophil function and thereby has the potential to lower immunity to pathogens. As there are only a few goat-derived sequences available in the GenBank for both 18S rRNA and COI loci, molecularly characterized E. arloingi from the present study will serve as an invaluable addition to the database to be used in future caprine studies.
Acknowledgements
This study was funded by Sultan Qaboos University grant number RF/AGR/ANVS/18/02. We thank Mr. Yunis Al-Balushi, Mr. Matar Al-Maney, Ms. Zayana Al-Dahmani and Mr. Mohmmed Al-Kindi, Sultan Qaboos University and Hospital for their cooperation.
-
How to cite: Al-Habsi K, Ali H, Al-Kharousi K, Elshafie EI, Al-Busaidi R, Muhiuddin A, et al. Vitamin B12 deficiency in newly weaned goat kids associated with clinical infection with Eimeria arloingi. Braz J Vet Parasitol 2020; 29(4): e005920. https://doi.org/10.1590/S1984-29612020078
References
- Al-Habsi K, Yang R, Ryan U, Miller DW, Jacobson C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Rep 2017a; 9: 75-83. http://dx.doi.org/10.1016/j.vprsr.2017.05.001 PMid:31014847.
» http://dx.doi.org/10.1016/j.vprsr.2017.05.001 - Al-Habsi K. Molecular characterisation of selected enteric pathogens and antimicrobial resistance of Salmonella in rangeland goats in Western Australia [dissertation]. Perth: Murdoch University; 2017b.
- Al-Zadjali A, Johnson EH, Srikandakumar A. Serum vitamin B12 levels in Omani goats. Trop Anim Health Prod 2004; 36(5): 473-482. http://dx.doi.org/10.1023/B:TROP.0000035009.66980.2b PMid:15449837.
» http://dx.doi.org/10.1023/B:TROP.0000035009.66980.2b - Carvalho FS, Wenceslau AA, Teixeira M, Matos Carneiro JAM, Melo ADB, Albuquerque GR. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet Parasitol 2011; 176(2-3): 95-100. http://dx.doi.org/10.1016/j.vetpar.2010.11.015 PMid:21167646.
» http://dx.doi.org/10.1016/j.vetpar.2010.11.015 - Chartier C, Paraud C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res 2012; 103(1): 84-92. http://dx.doi.org/10.1016/j.smallrumres.2011.10.022
» http://dx.doi.org/10.1016/j.smallrumres.2011.10.022 - ClustalW [online]. 2020 [cited 2020 Mar 17]. Available from: https://www.genome.jp/tools-bin/clustalw
» https://www.genome.jp/tools-bin/clustalw - Dolnik OV, Palinauskas V, Bensch S. Individual oocysts of Isospora (Apicomplexa: coccidia) parasites from avian faeces: from photo to sequence. J Parasitol 2009; 95(1): 169-174. http://dx.doi.org/10.1645/GE-1873.1 PMid:19245285.
» http://dx.doi.org/10.1645/GE-1873.1 - Johnson EH, Al-Habsi K, Al-Busaidi R, Al-Abri M. Impaired antibody response and phagocytosis in goats fed a diet low in cobalt. Small Rumin Res 2016; 140: 27-31. http://dx.doi.org/10.1016/j.smallrumres.2016.05.013
» http://dx.doi.org/10.1016/j.smallrumres.2016.05.013 - Johnson EH, Al-Habsi K, Al-Busaidy R, Khalaf SK. The effect of low levels of dietary cobalt on the chemiluminescence response of polymorphonuclear leukocytes of goats. Res Vet Sci 2010; 88(1): 61-63. http://dx.doi.org/10.1016/j.rvsc.2009.06.008 PMid:19679325.
» http://dx.doi.org/10.1016/j.rvsc.2009.06.008 - Johnson EH, Al-Habsi K, Kaplan E, Srikandakumar A, Kadim IT, Annamalai K, et al. Caprine hepatic lipidosis induced through the intake of low levels of dietary cobalt. Vet J 2004; 168(2): 174-179. http://dx.doi.org/10.1016/j.tvjl.2003.10.012 PMid:15301766.
» http://dx.doi.org/10.1016/j.tvjl.2003.10.012 - Johnson EH, Muirhead DE, Annamalai K, King GJ, Al-Busaidy R, Shahul Hameed M. Hepatic lipidosis associated with cobalt deficiency in Omani goats. Vet Res Commun 1999; 23(4): 215-221. http://dx.doi.org/10.1023/A:1006244925482 PMid:10461798.
» http://dx.doi.org/10.1023/A:1006244925482 - Kadim IT, Johnson EH, Mahgoub O, Srikandakumar A, Al-Ajmi D, Ritchie A, et al. Effect of low levels of dietary cobalt on apparent nutrient digestibility in Omani goats. Anim Feed Sci Technol 2003; 109(1-4): 209-216. http://dx.doi.org/10.1016/S0377-8401(03)00174-3
» http://dx.doi.org/10.1016/S0377-8401(03)00174-3 - Kadim IT, Mahgoub O, Al-Ajmi D, Al-Habsi KR, Johnson EH. Comparative effects of low levels of dietary cobalt and parenteral injections of Vitamin B12 on body dimensions in different breeds of Omani goats. Small Rumin Res 2006; 66(1-3): 244-252. http://dx.doi.org/10.1016/j.smallrumres.2005.09.018
» http://dx.doi.org/10.1016/j.smallrumres.2005.09.018 - Kadim IT, Mahgoub O, Srikandakumar A, Al-Ajmi DS, Al-Maqbaly RS, Al-Saqri NM, et al. Comparative effect of low levels of dietary cobalt and parenteral injection of vitamin B12 on carcass and meat quality characteristics in Omani goats. Meat Sci 2004; 66(4): 837-844. http://dx.doi.org/10.1016/j.meatsci.2003.08.003 PMid:22061016.
» http://dx.doi.org/10.1016/j.meatsci.2003.08.003 - Kawahara F, Zhang G, Mingala CN, Tamura Y, Koiwa M, Onuma M, et al. Genetic analysis and development of species-specific PCR assays based on ITS-1 region of rRNA in bovine Eimeria parasites. Vet Parasitol 2010; 174(1-2): 49-57. http://dx.doi.org/10.1016/j.vetpar.2010.08.001 PMid:20817404.
» http://dx.doi.org/10.1016/j.vetpar.2010.08.001 - Khodakaram-Tafti A, Hashemnia M, Razavi SM, Sharifiyazdi H, Nazifi S. Genetic characterization and phylogenetic analysis of Eimeria arloingi in Iranian native kids. Parasitol Res 2013; 112(9): 3187-3192. http://dx.doi.org/10.1007/s00436-013-3494-0 PMid:23779225.
» http://dx.doi.org/10.1007/s00436-013-3494-0 - Levine ND, Ivens V, Fritz TE. Eimeria christenseni sp. n. and other coccidia (Protozoa: Eimeriidae) of the goat. J Parasitol 1962; 48(2): 255-269. http://dx.doi.org/10.2307/3275578 PMid:14464632.
» http://dx.doi.org/10.2307/3275578 - Mohamaden WI, Sallam NH, Abouelhassan EM. Prevalence of Eimeria species among sheep and goats in Suez Governorate, Egypt. Int J Vet Sci Med 2018; 6(1): 65-72. http://dx.doi.org/10.1016/j.ijvsm.2018.02.004 PMid:30255081.
» http://dx.doi.org/10.1016/j.ijvsm.2018.02.004 - Nahavandi KH, Mahvi AH, Mohebali M, Keshavarz H, Rezaei S, Mirjalali H, et al. Molecular typing of Eimeria ahsata and E. crandallis isolated from slaughterhouse wastewater. Jundishapur J Microbiol 2016; 9(4): e34140. http://dx.doi.org/10.5812/jjm.34140 PMid:27303617.
» http://dx.doi.org/10.5812/jjm.34140 - National Center for Biotechnology Information – NCBI [online]. 2020 [cited 2020 Mar 17]. Available from: http://blast.ncbi.nlm.nih.gov/Blast.cgi
» http://blast.ncbi.nlm.nih.gov/Blast.cgi - Ogedengbe JD, Hanner RH, Barta JR. DNA barcoding identifies Eimeria species and contributes to the phylogenetics of coccidian parasites (Eimeriorina, Apicomplexa, Alveolata). Int J Parasitol 2011; 41(8): 843-850. http://dx.doi.org/10.1016/j.ijpara.2011.03.007 PMid:21515277.
» http://dx.doi.org/10.1016/j.ijpara.2011.03.007 - Paterson JE, MacPherson A. The influence of a low cobalt intake on the neutrophil function and severity of Ostertagia infection in cattle. Br Vet J 1990; 146(6): 519-530. http://dx.doi.org/10.1016/0007-1935(90)90055-8 PMid:2271909.
» http://dx.doi.org/10.1016/0007-1935(90)90055-8 - Ruiz A, Guedes AC, Muñoz MC, Molina JM, Hermosilla C, Martín S, et al. Control strategies using diclazuril against coccidiosis in goat kids. Parasitol Res 2012; 110(6): 2131-2136. http://dx.doi.org/10.1007/s00436-011-2746-0 PMid:22193521.
» http://dx.doi.org/10.1007/s00436-011-2746-0 - Silva LMR, Muñoz Caro T, Gerstberger R, Vila-Viçosa MJM, Cortes HCE, Hermosilla C, et al. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol Res 2014; 113(8): 2797-2807. http://dx.doi.org/10.1007/s00436-014-3939-0 PMid:24849865.
» http://dx.doi.org/10.1007/s00436-014-3939-0 - Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. http://dx.doi.org/10.1093/molbev/mst197 PMid:24132122.
» http://dx.doi.org/10.1093/molbev/mst197 - Technelysium [online]. 2020 [cited 2020 Mar 17]. Available from: http://www.technelysium.com
» http://www.technelysium.com - Villagra-Blanco R, Silva LMR, Muñoz-Caro T, Yang Z, Li J, Gärtner U, et al. Bovine polymorphonuclear neutrophils cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front Immunol 2017; 8: 606. http://dx.doi.org/10.3389/fimmu.2017.00606 PMid:28611772.
» http://dx.doi.org/10.3389/fimmu.2017.00606 - Yang R, Brice B, Ryan U. Morphological and molecular characterization of Eimeria haematodi, coccidian parasite (Apicomplexa: Eimeriidae) in a rainbow lorikeet (Trichoglossus haematodus). Exp Parasitol 2015; 153: 123-128. http://dx.doi.org/10.1016/j.exppara.2015.03.005 PMid:25795281.
» http://dx.doi.org/10.1016/j.exppara.2015.03.005 - Yang R, Brice B, Ryan U. Morphological and molecular characterization of Eimeria purpureicephali n. sp. (Apicomplexa: Eimeriidae) in a red-capped parrot (Purpureicephalus spurius, Kuhl, 1820) in Western Australia. Int J Parasitol Parasites Wildl 2016; 5(1): 34-39. http://dx.doi.org/10.1016/j.ijppaw.2016.01.003 PMid:26977403.
» http://dx.doi.org/10.1016/j.ijppaw.2016.01.003 - Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004; 36(5): 808-812. http://dx.doi.org/10.2144/04365ST04 PMid:15152600.
» http://dx.doi.org/10.2144/04365ST04 - Zajac AZ, Conboy GA. Veterinary clinical parasitology 8th ed. Ames: John Wiley & Sons; 2012.
Publication Dates
-
Publication in this collection
09 Oct 2020 -
Date of issue
2020
History
-
Received
17 Mar 2020 -
Accepted
04 Aug 2020