Abstract
The Ocelot (Leopardus pardalis) is the largest species of this genus, despite having broad distribution in the Americas; it is included in the main list of endangered species. Their conservation is widely studied, but there is a lack of studies about their morphology. In order to contribute to the knowledge of its reproductive system, five male and female ocelots were examined macro- and microscopically by histological techniques. Macroscopic analysis of the male reproductive system revealed presence of prostate and bulbourethral gland located caudally to the urinary bladder and a penis with small spicules. Microscopically, the testes were encased by the tunica albuginea and divided it into lobules with 5-10 tubules per lobe. In females, macroscopic analysis demonstrated two ovaries position dorsally in the sublumbar region and caudal to the kidneys. The bicornuate uterus is composed by uterine horns (12 to 14 cm in length), which travels from the ovaries in a caudal direction to form a small uterine body (4 cm in length). The ovary analysis revealed, in longitudinal section, medullary region composed of loose connective tissue, a stroma rich in blood vessels, and an external parenchymal region surrounded by a tunica albuginea. The results of the study confirmed the similarity between ocelot's reproductive system as domestic cat's ones and showing for the first time the complete morphological tool to highlight these organs and tissue in this male and female endangered wild felid specie. The present study open venue for other researchers to consider morphological and preservationist features and aimed to help at long-term conservation of wild felines.
Keywords:
morphology; histology; reproductive system; wild felines
Introduction
The ocelot, Leopardus pardalis (Carnivora: Felidae), is a medium-sized mammal, weighing 8-15 kg, with large legs and a slender body, measuring from 50 cm to 1 m in length, males being larger than females (Oliveira and Cassaro, 2005Oliveira TG, Cassaro K. Guia de campo dos felinos do Brasil. São Paulo: Instituto Pró-Carnívoros/Fundação Parque Zoológico de São Paulo; 2005. 80 p.). Its activity pattern is typically nocturnal-crepuscular (Murray and Gardner, 1997Murray JL, Gardner GL. Leopardus pardalis. Mamm Species. 1997;(548):1-10. http://dx.doi.org/10.2307/3504082.
http://dx.doi.org/10.2307/3504082...
; Di Bitetti et al., 2006Di Bitetti MS, Paviolo A, De Angelo C. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. J Zool. 2006;270(0):153-63. http://dx.doi.org/10.1111/j.1469-7998.2006.00102.x.
http://dx.doi.org/10.1111/j.1469-7998.20...
). Although a good climber, it is a species with terrestrial habits. Its diet includes large and small mammals (Ludlow and Sunquist, 1987Ludlow ME, Sunquist ME. Ecology and behavior of ocelots in Venezuela. Natl Geogr Res. 1987;3(4):447-61.; Moreno et al., 2006Moreno RS, Kays RW, Samudio R Jr. Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J Mammal. 2006;87(4):808-16. http://dx.doi.org/10.1644/05-MAMM-A-360R2.1.
http://dx.doi.org/10.1644/05-MAMM-A-360R...
; Bianchi and Mendes, 2007Bianchi RC, Mendes SL. Ocelot (Leopardus pardalis) predation on primates in Caratinga Biological Station, southeast Brazil. Am J Primatol. 2007;69(10):1173-8. http://dx.doi.org/10.1002/ajp.20415. PMid:17330310.
http://dx.doi.org/10.1002/ajp.20415...
). The most frequent prey are rats, armadillos, opossums and mice, but it can also feed on anteaters, bats, deer, hares, iguanas and birds (Emmons, 1987Emmons LH. Comparative feeding ecology of felids in a Neotropical Rainforest. Behav Ecol Sociobiol. 1987;20(4):4, 271-83. http://dx.doi.org/10.1007/BF00292180.
http://dx.doi.org/10.1007/BF00292180...
; Ludlow and Sunquist, 1987Ludlow ME, Sunquist ME. Ecology and behavior of ocelots in Venezuela. Natl Geogr Res. 1987;3(4):447-61.).
It has a wide distribution from the north of Argentina to the south of Texas (Murray and Gardner, 1997Murray JL, Gardner GL. Leopardus pardalis. Mamm Species. 1997;(548):1-10. http://dx.doi.org/10.2307/3504082.
http://dx.doi.org/10.2307/3504082...
). This species can occur in environments such as flood plains, coniferous forests, fields and wet and dry forests (Emmons and Feer, 1997Emmons LH, Feer F. Neotropical rainforest mammals: a field guide. 2nd ed. Chicago: University of Chicago Press; 1997. 307 p.). In Brazil, it occurs in all states with the probable exception of Rio Grande do Sul, occupying the different biomes of the Pantanal, Caatinga, Cerrado, and especially subtropical and tropical forests (Oliveira and Cassaro, 2005Oliveira TG, Cassaro K. Guia de campo dos felinos do Brasil. São Paulo: Instituto Pró-Carnívoros/Fundação Parque Zoológico de São Paulo; 2005. 80 p.). Due to this wide distribution, during the 1960’s and 1970’s, the ocelot was the cat species most exploited by the fur trade (Nowell and Jackson, 1996Nowell K, Jackson P. Wild cats, status survey and conservation action plan [Internet]. Gland, Switzerland: IUCN/SSC Cat Specialist Group, International Union for Conservation of Nature and Natural Resources; 1996. 382 p. [cited 2020 Jan 6]. Available from: https://portals.iucn.org/library/efiles/documents/1996-008.pdf
https://portals.iucn.org/library/efiles/...
).
The ocelot currently is included in the Red Book of Brazilian Fauna Threatened by Extinction, elaborated by the IUCN (International Union for Conservation of Nature), where it is classified as of Least Concern - LC (IUCN 3.1) (Caso et al., 2008Caso A, Lopez-Gonzalez C, Payan E, Eizirik E, Oliveira TG, Leite-Pitman R, Kelly M, Valderrama C. Panthera onça. In: International Union for Conservation of Nature and Natural Resources, editor. The IUCN Red List of Threatened Species, 2008. e: TI5953A5327466. Gland, Switzerland: IUCN; 2008. http://dx.doi.org./10.2305/IUCN.UK.2008.RLTS.TI5953A5327466.en.
http://dx.doi.org./10.2305/IUCN.UK.2008....
). However, wild felines such as Leopardus pardalis can be considered important for conservation of the ecosystems they live in due to their abundance and ecological significance (Machado et al., 2016Machado LC, Oliveira VC, Paraventi MD, Cardoso RNR, Martins DS, Ambrósio CE. Maintenance of Brazilian biodiversity by germplasm bank. Pesq Vet Bras. 2016;36(1):62-6. http://dx.doi.org/10.1590/S0100-736X2016000100010.
http://dx.doi.org/10.1590/S0100-736X2016...
). In Brazil, a Management Plan for the ocelot was adopted by IBAMA in 1994 (Portaria 106 of 12/26/95). This plan is coordinated by the Mata Ciliar Association (ACM - Jundiaí - São Paulo - Brazil), with the support of the Zoological Society of Brazil (SZB) and of IBAMA itself, and its objective is “[…] the structuring of a permanent committee for the preservation of the species, coordination of all relevant activities and establishment of strategies for study, management and protection of the ocelot, seeking resources to program them” (ACM, 1998ACM: Associação Mata Ciliar. Avaliação das condições veterinárias e de manejo dos pequenos felinos neotropicais em cativeiro no Estado de São Paulo. Revista de Educação Continuada. 1998;1:44-53., p. 44-53). However, this ordinance of IBAMA was revoked in 2004.
Research related to the reproduction biology of wild species, in this case more specifically focusing on morphological and ultrastructural studies, is motivated by the need for a greater understanding of reproductive biology, even of non-threatened species, considering the ecological relevance of the species presented here (Machado et al., 2016Machado LC, Oliveira VC, Paraventi MD, Cardoso RNR, Martins DS, Ambrósio CE. Maintenance of Brazilian biodiversity by germplasm bank. Pesq Vet Bras. 2016;36(1):62-6. http://dx.doi.org/10.1590/S0100-736X2016000100010.
http://dx.doi.org/10.1590/S0100-736X2016...
). Such studies are of great relevance, as they aim to reproduce in captivity more efficiently, reduce neonatal mortality rates in captivity, as well as the constitution and/or enrichment of animal germplasm banks (Mellen, 1991Mellen JD. Factors influencing reproductive success in small captive exotic felids (Felis spp.): a multiple regression analysis. Zoo Biol. 1991;10(2):95-110. http://dx.doi.org/10.1002/zoo.1430100202.
http://dx.doi.org/10.1002/zoo.1430100202...
; Brown et al., 1994Brown JL, Wasser SK, Wildt DE, Graham LH. Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biol Reprod. 1994;51(4):776-86. http://dx.doi.org/10.1095/biolreprod51.4.776. PMid:7819459.
http://dx.doi.org/10.1095/biolreprod51.4...
; Morato and Barnabé, 1998Morato R, Barnabé R. Biotécnicas de reprodução aplicadas à preservação de felídeos selvagens. Clín Vet [serial on the Internet]. 1998 [cited 2020 Jan 6];(12):24-6. Available from: http://www.cbra.org.br/portal/downloads/publicacoes/rbra/v42/n3-4/p141-145%20(RB751).pdf
http://www.cbra.org.br/portal/downloads/...
; Moreira et al., 2001Moreira N, Monteiro‐Filho E, Moraes W, Swanson W, Graham L, Pasquali O, Gomes M, Morais R, Wildt D, Brown J. Reproductive steroid hormones and ovarian activity in felids of the Leopardus genus. Zoo Biol. 2001;20(2):103-16. http://dx.doi.org/10.1002/zoo.1010. PMid:11429781.
http://dx.doi.org/10.1002/zoo.1010...
; Kleiman, 2010Kleiman DG. Reproduction. In: Kleiman DG, Thompson KV, Baer CK, editors. Wild mammals in captivity: principles and techniques for zoo management. Chicago: University of Chicago Press; 2010. p. 377-8. http://dx.doi.org/10.7208/chicago/9780226440118.001.0001.
http://dx.doi.org/10.7208/chicago/978022...
; Micheletti et al., 2012Micheletti T, Cubas ZS, Moraes W, Oliveira MJ, Moreira N. Reprodução natural de felídeos selvagens em cativeiro: dificuldades e orientações. Rev Bras Reprod Anim [serial on the Internet]. 2012 [cited 2020 Jan 6];36(1):39-43. Available from: http://locus.ufv.br/handle/123456789/5115
http://locus.ufv.br/handle/123456789/511...
). Therefore, in view of the countless threats to wildlife, it is essential to have more in-depth knowledge about the reproductive biology of target species, with investments in research and actions aimed at in situ and ex situ conservation.
There are few studies of the ocelot, mainly on its conservation, but there is still a lack of information and data about its morphology with focus in reproductive tools, since structural organization or biobanking (Comizzoli, 2017Comizzoli P. Biobanking and fertility preservation for rare and endangered species. Anim Reprod. 2017;14(1):30-3. http://dx.doi.org/10.21451/1984-3143-AR889.
http://dx.doi.org/10.21451/1984-3143-AR8...
). Thus, the main objective of this study was to describe the morphology and histology of the organs that compose the male and female reproductive system of Leopardus pardalis. Such information could be useful in the reproductive management of the species as well as in comparative studies of wild cat species.
Material and methods
This research was approved by COBEA (Brazilian College of Animal Experimentation) and the University Ethics Committee (CEP - FZEA) Nº 3351210715 and SISBIO Nº. 49271-1. All the animals used in this study were killed in road traffic accidents in Alta Floresta, Mato Grosso, Brazil. A total of five animal’s adults, three males and two females, were assigned to the Laboratório de Zoologia e Morfologia Animal of the Universidade do Estado de Mato Grosso (UNEMAT). Due to the ecological role of great relevance and the difficulty in obtaining corpses of this species, which are rare to be found, we consider that the sample (n = 5) of this study becomes quite representative.
To study the male and female reproductive apparatus of the ocelot, the animals were fixed by perfusion processes, injecting 10% aqueous formaldehyde solution through the external jugular vein, and immersion in the same fixative, where the pieces remained submerged for a minimum period of 48 hours. Subsequently, the animals were placed in the supine position for dissection of the perineal region. After an incision in the Alba line, the skin was retracted and the organs exposed for dissection and photographic recording with a digital camera. For microscopy, small tissue fragments were fixed in 8% buffered paraformaldehyde solution, and then submitted to standard histological procedures for embedding in paraffin. Blocks were cut in 5 µm sections and stained with hematoxylin-eosin (HE) (Banks, 1992Banks WJ. Histologia veterinária aplicada. 2nd ed. São Paulo: Manole; 1992. 560 p.; Bacha and Bacha, 2003Bacha WJ Jr, Bacha LM. Atlas colorido de histologia veterinária. 2nd ed. São Paulo: Roca; 2003 .457 p.).
Results
Male reproductive system of the ocelot
The male reproductive system of Leopardus pardalis consists of the following structures: scrotum, penis, testes, vas deferens and epididymis. Also included in this system are the accessory bulbourethral and prostate glands, with absence of vesicular glands (Figures1and2).
Photograph of the reproductive tract of a male ocelot. (A) the urinary bladder (UB), prostate (Pt), bulbourethral gland (BG), spermatic funiculus (SP) and penis (P); (B) scrotum (Sc) and penis (P); (C) penis (P) and spermatic funiculus (*).
Photograph of the reproductive tract of a male ocelot. (A) penis (P); (B) cavernous body (CB), deep artery (DA) and urethra (U); (C) spicules (Sp); (D) visceral vaginal tunic (VVT) and parietal vaginal tunic (PVT).
Scrotum
The scrotum with a perineal location was positioned as an extension of the skin of the abdominal region in the pelvic region, as a membranous pocket, divided by a median septum, covered by hairs that harbored the testes, epididymides and the vas deferens (Figure 1B).
Testis
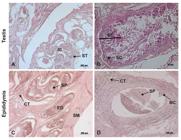
The paired testes were separated by the scrotal septum. With ovoid shape and rounded contours, they presented a concave border laterally and a straight border positioned medially. They were covered by a fibrous outer membrane of whitish pink color, the visceral vaginal tunic (Figure 2D). Microscopically, the testes were encased by the tunica albuginea, a thick capsule of dense connective tissue, especially at the dorsal surface, which is the mediastinum, through which the fibrous septa that penetrate the testis divided it into lobules. Each lobule was occupied by seminiferous tubules, with 5-10 tubules per lobe (Figures 33 B).
Photomicrograph of the testis and epididymis of an ocelot. (A) note the Interstitial cell (IC); Seminiferous tubules (ST); (B) Seminiferous epithelium (SE); Sertoli cells (SC); (C and D) Connective tissue (CT); Epididymal duct (ED); Smooth muscle (SM); Basal cells (BC); Spermatozoids (SP). Hematoxylin/eosin technique, bar 20 and 200µm.
Spermatic cord
The spermatic cord consisted of the vas deferens, cremaster muscle, visceral lamina of the vaginal tunica and the pampiniform plexus, composed of testicular veins and arteries. Stretching ventrally through the inguinal ring, it was positioned laterally to the penis, reaching up to the testes (Figures 11B).
Epididymis
Covered by a thin serous membrane and located on the medial borders of the testes, were the epididymides, comprising head, body and tail. The most prominent portion, the head of the epididymis, was located in the cranial portion of the gonad, firmly attached to the vaginal tunica. The body extended through the ventral region of the testis, reaching the tail at the caudal end of the testis, from which the vas deferens protruded cranially (Figure 2D). Microscopically the epididymal duct and the basal cells with their nuclei were observed. It was also observed that the epididymis consists of basal cells, loose connective tissue, smooth muscle and a cylindrical pseudo-stratified epithelium (Figures 33D).
Prostate and bulbourethral gland
The prostate was composed of a large, irregularly shaped, compact mass located caudal to the urinary bladder and cranial to the bulbourethral gland. It covered almost the entire pelvic portion of the urethra. The paired bulbourethral glands, with a rounded shape, were located posterior to the pelvic urethra and caudal to the prostate. In the image, the respective structures could not be seen (Figure 1A).
Foreskin and penis
The foreskin presented as a thick layer of epithelial tissue, covered by hair, with an orifice facing the ventral region, through which the urine and the semen are ejected. The penis was divided into tail, body and glans, and small spicules were observed. In cross section could be seen the cavernous bodies surrounded by tunica albuginea, erectile tissue and deep artery (Figures 11C and 2A).
Female reproductive system of the ocelot
The female reproductive system of Leopardus pardalis consists of the following structures: ovary, oviducts, uterus, cervix, vagina, clitoris and vulva (Figure 4).
Photograph of female reproductive tract of ocelot. (A) vulvar lips (*), ventral commissure (VC), dorsal commissure (DC) and anus (A); (B) Figure to observe longitudinal folds (LF) of the cervix and vagina and vulvar lips (*); (C) External urethral ostio (C); In (D), note intestine (I), uterine horns (*) and uterine body (U); (E) note the ovary (O), broad ligament of the ovary (L) and intestine (I). In (F), cross section of ovary. Note the parenchymal zone (Pz), medullar zone (Mz), tertiary follicle (F) and mesosalpinx (L).
Ovary
The paired ovaries were positioned dorsally in the sub lumbar region and caudal to the kidneys. They had a curved expanded shape with the concave face downwards, similar to a kidney bean. They were about 1.5 cm in length (Figures 44F). In longitudinal section, there was a medullary region composed of loose connective tissue, a stroma rich in blood vessels, and an external parenchymal region surrounded by a tunica albuginea (Figure 5).
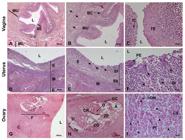
Photomicrograph of female reproductive tract of ocelot. Vagina: (A) Lumen (L), Vaginal epithelium (VE), Muscular layer (ML) and Mucus layer (MU); (B) Lumen (L), Basal cells (BC), Mucus layer (MU) and Blood Vessel (arrow); (C) Lumen (L), Intermediate cells (IC), Transitional Epithelium (TE) and Blood Vessel (arrow). Uterus: (D) Lumen (L), Endometrium (E) and Myometrium (M); (E) Lumen (L), Endometrium (E), Myometrium (M), Stratum basalis (Sb), Stratum functionalis (Sf) and Endometrial gland (arrow); (F) Lumen (L), Pseudostratified epithelium (PE), Endometrial stroma (St) and Endometrial gland (arrow). Ovary: (G) Lumen (L), Cortex (C) and Follicle (F); (H) Oocyte (O), Corona radiata (CR), Zona pellucida (ZP), Antrum (A), Granulosa cells (GC), Cumulus oophofus (CO), Theca interna (Ti), Theca externa (Te) and Primordial follicle (arrow head); (I) Primary follicle (Pf), Cortical stroma (CS), Oocyte nuclei (ON), Atretic follicle (arrow). Hematoxylin/eosin technique, bar 20, 100 and 200µm.
Oviduct
The paired oviducts were pinkish-white in color and measured 6 and 10 cm. The uterine tube is surrounded by a peritoneal tissue derived from the broad ligament of the uterus, called mesosalpinx, which is subdivided into: the infundibulum of the uterine tube, ampulla and isthmus (Figure 4D). The uterine tube makes an opening in the internal uterine ostium, being irrigated by the arteries: ovarian and uterine (Figures 555I).
Uterus
The uterus consists of a cervix, a uterine body and two uterine horns (bicornuated). The uterine body is positioned between the ascending colon and the urinary bladder. The uterine body was small, about 4 cm in length (Figure 4D). The horns extended to the ovarian pouch and measured 12 and 14 cm in length. Microscopically the presence of vascular extract, endometrial glands, glandular epithelium and pseudo-stratified myometrium epithelium could be shown (Figures 555F).
Cervix and vagina
Macroscopically, the cervix presented circular longitudinal folds, in annular shape. Internally, it had a thick wall of smooth muscle. It terminated at the external uterine ostium, communicating with the vagina, where the longitudinal folds of the mucosa formed (Figures4B 4C). The vagina extended to the external urethral ostium a had a thick wall composed of smooth musculature with large lips filled by a dense layer of adipose tissue. Microscopically, the vagina comprised the muscular layer, mucus layer, vaginal epithelium basal cells, transitional epithelium and intermediate cells (Figures 555C).
Vulva and clitoris
The vulva comprised the vulvar lips, which joined together dorsally and ventrally. The ventral clitoris was the most prominent part of this organ. The dorsal part had more rounded contours (Figures 44C).
Discussion
Male reproductive system of the ocelot
A macroscopic description of the male reproductive system of Leopardus pardalis was previously given by Carneiro et al. (2010)Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83.
http://dx.doi.org/10.5007/2175-7925.2010...
, as the penis, scrotum, testis, epididymis, vas deferens and accessory genital glands such as a bulbourethral gland and prostate are present, but not the vesicular glands. The latter are also absent in domestic cats (Godinho, 1999Godinho CL. Análise histométrica do testículo e duração da espermatogênese em gatos (Felis domestica) sexualmente maduros [tese]. Belo Horizonte: Universidade Federal de Minas Gerais; 1999.; Dyce et al., 2010Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p.) and in wild species as Puma yaguaroundii (Rocha et al., 2017Rocha EF, Santos NTA, Dias RFF, Diniz JARA, Dos Santos JRS, De Menezes DJA. Anatomia macroscópica dos órgãos reprodutores de Puma yagouaroundi (Geoffroy, 1803) macho. Pubvet. 2017;(11):744-839. http://dx.doi.org/10.22256/pubvet.v11n8.767-770.
http://dx.doi.org/10.22256/pubvet.v11n8....
). Leopardus pardalis has relatively small testes (Sarti, 2006Sarti P. Avaliação morfométrica do testículo e da espermatogênese de jaguatiricas (Leopardus pardalis, Linnaeus, 1758) adultas [dissertation]. Viçosa: Universidade Federal de Viçosa; 2006.; Carneiro et al., 2010Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83.
http://dx.doi.org/10.5007/2175-7925.2010...
) with the same anatomy as puma (Puma concolor) (Mansfield and Land, 2002Mansfield KG, Land D. Cryptorchidism in Florida panthers: prevalence, features, and influence of genetic restoration. J Wildl Dis. 2002;38(4):693-8. http://dx.doi.org/10.7589/0090-3558-38.4.693. PMid:12528434.
http://dx.doi.org/10.7589/0090-3558-38.4...
; Guião-Leite, 2002Guião-Leite FL. Análise morfológica do testículo e do processo espermatogênico da onça parda (Puma concolor, Wozencraft, 1993) adulta [dissertação]. Viçosa: Universidade Federal de Viçosa; 2002 [cited 2020 Jan 6]. Available from: https://www.locus.ufv.br/bitstream/handle/123456789/7910/texto%20completo.pdf?sequence=1&isAllowed=y
https://www.locus.ufv.br/bitstream/handl...
), jaguar (Panthera onca) (Azevedo, 2004Azevedo MA. Análise morfofuncional do testículo da onça-pintada (Panthera onca) adulta [dissertação]. Viçosa: Universidade Federal de Viçosa; 2004 [cited 2020 Jan 6]. Available from: http://locus.ufv.br/handle/123456789/5115
http://locus.ufv.br/handle/123456789/511...
) and Puma yaguaroundii (Rocha et al., 2017Rocha EF, Santos NTA, Dias RFF, Diniz JARA, Dos Santos JRS, De Menezes DJA. Anatomia macroscópica dos órgãos reprodutores de Puma yagouaroundi (Geoffroy, 1803) macho. Pubvet. 2017;(11):744-839. http://dx.doi.org/10.22256/pubvet.v11n8.767-770.
http://dx.doi.org/10.22256/pubvet.v11n8....
). The scrotum is positioned in the perineal region, lateral to the penis as described by Carneiro et al. (2010)Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83.
http://dx.doi.org/10.5007/2175-7925.2010...
for ocelot and similar to the domestic cat (Getty, 1987Getty R. Anatomia dos animais domésticos. 5th ed. Rio de janeiro: Guanabara Koogan; 1987. p. 147-53.). Microscopically, the albuginea tunica in the examined testes of Leopardus pardalis is thick and consists of the presence of dense connective tissue moderately modeled, with considerable amounts of collagen fibers and discrete elastic fibers, with emission of thin septa into the parenchyma testicular. The findings corroborate the research conducted by Silva et al. (2009)Silva CAO, Perri SHV, Koivisto MB, Silva AM, Carvalho RG, Monteiro CMR. Histological and morphometric evaluation of the testes of cats (Felis catus). Aspectos histológicos e morfométricos dos testículos de gatos domésticos (Felis catus). Pesq Vet Bras. 2009;29(4):312-6. http://dx.doi.org/10.1590/S0100-736X2009000400006.
http://dx.doi.org/10.1590/S0100-736X2009...
on domestic felines. As described by Carneiro et al. (2010)Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83.
http://dx.doi.org/10.5007/2175-7925.2010...
, in Leopardus pardalis the vas deferens is a long and thin structure, similar to that of the domestic cat (Getty, 1987Getty R. Anatomia dos animais domésticos. 5th ed. Rio de janeiro: Guanabara Koogan; 1987. p. 147-53.). As shown by Junqueira and Carneiro (2015)Junqueira LCU, Carneiro J. Histologia básica: texto y atlas. 12nd ed. Barcelona: Editorial Médica Panamericana; 2015. 556 p., the body of the epididymis extends through the ventral region of the testis, reaching the tail at the caudal end of the testis, from which the vas deferens cranially projects, in the same way as in domestic carnivores. Microscopically, the epididymal duct and the basal cells with their nuclei were demonstrated. It was also observed that the epididymis is composed of smooth muscle and a cylindrical pseudo-stratified epithelium, similar to other mammalian species (Wick and Kress, 2002Wick R, Kress A. Ultrastructural changes in the uterine luminal and glandular epithelium during the oestrous cycle of the marsupial Monodelphis domestica (Grey Short‐Tailed Opossum). Cells Tissues Organs. 2002;170(2-3):111-31. http://dx.doi.org/10.1159/000046185. PMid:11731700.
http://dx.doi.org/10.1159/000046185...
; Banks, 1992Banks WJ. Histologia veterinária aplicada. 2nd ed. São Paulo: Manole; 1992. 560 p.; Junqueira and Carneiro, 2015Junqueira LCU, Carneiro J. Histologia básica: texto y atlas. 12nd ed. Barcelona: Editorial Médica Panamericana; 2015. 556 p.). Our findings on the prostate and bulbourethral glands of Leopardus pardalis complement the findings of Carneiro et al. (2010)Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83.
http://dx.doi.org/10.5007/2175-7925.2010...
for the oncilla (Leopardus tigrinus) and Puma yaguaroundii (Rocha et al., 2017Rocha EF, Santos NTA, Dias RFF, Diniz JARA, Dos Santos JRS, De Menezes DJA. Anatomia macroscópica dos órgãos reprodutores de Puma yagouaroundi (Geoffroy, 1803) macho. Pubvet. 2017;(11):744-839. http://dx.doi.org/10.22256/pubvet.v11n8.767-770.
http://dx.doi.org/10.22256/pubvet.v11n8....
). Microscopically, it was possible to observe the internal epithelial layer as well as the prostatic concretion, similar to the findings of Wick and Kress (2002)Wick R, Kress A. Ultrastructural changes in the uterine luminal and glandular epithelium during the oestrous cycle of the marsupial Monodelphis domestica (Grey Short‐Tailed Opossum). Cells Tissues Organs. 2002;170(2-3):111-31. http://dx.doi.org/10.1159/000046185. PMid:11731700.
http://dx.doi.org/10.1159/000046185...
and Junqueira and Carneiro (2015)Junqueira LCU, Carneiro J. Histologia básica: texto y atlas. 12nd ed. Barcelona: Editorial Médica Panamericana; 2015. 556 p..
The penis was divided into three regions: tail, body and glans and the presence of small spicules was confirmed (Queiroz, 2003Queiroz VS. Estudo do efeito das condições de manipulação do sêmen de jaguatiricas (Leopardus pardalis: Linnaeus, 1758) sobre a capacitação e a integridade morfológica e funcional dos espermatozóides [dissertação]. São Paulo: Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo; 2003. https://doi.org/10.11606/d.10.2003.tde-09062004-140805.
https://doi.org/10.11606/d.10.2003.tde-0...
; Rocha et al., 2017Rocha EF, Santos NTA, Dias RFF, Diniz JARA, Dos Santos JRS, De Menezes DJA. Anatomia macroscópica dos órgãos reprodutores de Puma yagouaroundi (Geoffroy, 1803) macho. Pubvet. 2017;(11):744-839. http://dx.doi.org/10.22256/pubvet.v11n8.767-770.
http://dx.doi.org/10.22256/pubvet.v11n8....
). Authors relate the function of these spicules to stimulation of the female reproductive tract to accelerate copulation and enhance peristalsis to move sperm through the female reproductive tract (Harcourt and Gardiner, 1994Harcourt AH, Gardiner J. Sexual selection and genital anatomy of male primates. Proc Biol Sci. 1994;255(1342):47-53. http://dx.doi.org/10.1098/rspb.1994.0007. PMid:8153136.
http://dx.doi.org/10.1098/rspb.1994.0007...
). In cross-section it can be observed that the cavernous bodies are surrounded by tunica albuginea, erectile tissue, deep artery, complementing the findings of Carneiro et al. (2010)Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83.
http://dx.doi.org/10.5007/2175-7925.2010...
for this species.
Female reproductive system of the ocelot
The shape and color of the ovaries was as described generically to the others mammals by (Derivaux, 1980Derivaux J. Reprodução dos animais domésticos, 1. Fisiologia, 2. O macho, inseminação artificial, 3. patologia. 1st ed. Zaragoza: Editorial Acriba; 1980. p. 3-68.), Slatter (2007)Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007. and Fossum (2008)Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74. in domestic felines; Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p. in domestic felines and canines and crab-eating fox (Cerdocyon thous) by Machado et al. (2017)Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
. Microscopic analysis of the ovary revealed a medullary region composed of loose connective tissue, a stroma rich in blood vessels and a parenchyma surrounded by a tunica albuginea, structures described in domestic cats and dogs by Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p., Junqueira and Carneiro (2015)Junqueira LCU, Carneiro J. Histologia básica: texto y atlas. 12nd ed. Barcelona: Editorial Médica Panamericana; 2015. 556 p. and crab-eating fox by Machado et al. (2017)Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
. The oviducts are small with a pink coloration, conformation and anatomical positioning similar to the findings of Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p. in domestic canids and felines and Machado et al. (2017)Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
in crab-eating fox. The importance of this organ and continuous studies must be highlighted (Andrade et al., 2019Andrade GM, del Collado M, Meirelles FV, Silveira JC, Perecin F. Intrafollicular barriers and cellular interactions during ovarian follicle development. Anim Reprod. 2019;16(3):485-96. http://dx.doi.org/10.21451/1984-3143-AR2019-0051. PMid:32435292.
http://dx.doi.org/10.21451/1984-3143-AR2...
).
The uterus of Leopardus pardalis is bicornuate as previously described by Slatter (2007)Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007. and Fossum (2008)Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74. and Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p. in domestic felines. It has characteristics similar to the uterus of the crab-eating fox (Machado et al., 2017Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
) as well as to the uterus of domestic felines and canids (Atwood, 1955Atwood WJ. Comparative anatomy. 2nd ed. St. Louis: The C.V. Mosby Company; 1955. 424 p.; Schwarze and Schroder, 1970Schwarze E, Schröder L. Compêndio de anatomia veterinária: sistema visceral. Zaragoza: Acribia; 1970. p. 277-86. Vol. 2.; Nickel et al., 1979Nickel R, Schummer A, Seiferle E. The viscera of the domestic mammals. 2nd ed. Berlin: Verlag Paul Parey: 1979. p. 351-89. http://dx.doi.org/10.1007/978-1-4757-6814-5.
http://dx.doi.org/10.1007/978-1-4757-681...
; Getty, 1987Getty R. Anatomia dos animais domésticos. 5th ed. Rio de janeiro: Guanabara Koogan; 1987. p. 147-53.; Konig and Liebich, 2004König HE, Liebich HJ. Anatomia dos animais domésticos. 2nd ed. Porto Alegre: Artmed; 2004. 400 p.). The uterine body is small also in agreement with Slatter (2007)Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007. and Fossum (2008)Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74. in domestic felines; Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p. in domestic felines and canids and similar in crab-eating fox (Machado et al., 2017Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
). Miscroscopically, it is possible to observe the presence of a smooth muscle, also called the tunica mucosa or myometrium, which is the innermost muscular layer of the uterus. The presence of uterine glands indicates that the tissue analyzed does not comprise the region of the uterine body, because it is a region absent from glands, previously known from domestic canids and felines (Schwarze and Schroder, 1970Schwarze E, Schröder L. Compêndio de anatomia veterinária: sistema visceral. Zaragoza: Acribia; 1970. p. 277-86. Vol. 2.; Nickel et al., 1979Nickel R, Schummer A, Seiferle E. The viscera of the domestic mammals. 2nd ed. Berlin: Verlag Paul Parey: 1979. p. 351-89. http://dx.doi.org/10.1007/978-1-4757-6814-5.
http://dx.doi.org/10.1007/978-1-4757-681...
; Derivaux, 1980Derivaux J. Reprodução dos animais domésticos, 1. Fisiologia, 2. O macho, inseminação artificial, 3. patologia. 1st ed. Zaragoza: Editorial Acriba; 1980. p. 3-68.; Banks, 1992Banks WJ. Histologia veterinária aplicada. 2nd ed. São Paulo: Manole; 1992. 560 p.; Gartner and Hiatt, 1999Gartner LP, Hiatt LJ. Tratado de histologia. Rio de Janeiro: Guanabara Koogan; 1999. p. 365-8.; Konig and Liebich, 2004König HE, Liebich HJ. Anatomia dos animais domésticos. 2nd ed. Porto Alegre: Artmed; 2004. 400 p.; Slatter, 2007Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007.; Fossum, 2008Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74.; Dyce et al., 2010Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p.; Junqueira and Carneiro, 2015Junqueira LCU, Carneiro J. Histologia básica: texto y atlas. 12nd ed. Barcelona: Editorial Médica Panamericana; 2015. 556 p.) and the crab-eating fox (Machado et al., 2017Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
). Macroscopically, the cervix corresponds to the uterine entry, presenting a musculature thicker than that presented by the uterine body and the vagina, presenting at the junction vaginal uterus, a knotlike aspect, according to the descriptions performed by Slatter (2007)Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007. and Fossum (2008)Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74. in domestic felines. The internal orifice of the cervix is positioned dorsally and the internal orifice ventrally to the floor of the vagina, in the same way as described by Slatter (2007)Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007., Fossum (2008)Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74. and Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p. in domestic felines. The vagina extends to the external urethral ostium and has a thick muscular wall. The vulva comprises vulvar lips and a prominent clitoris. These observations are in agreement with Slatter (2007)Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007. and Fossum (2008)Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74. in domestic felines, Dyce et al. (2010)Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p. in domestic felines and canids and Machado et al. (2017)Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306. PMid:28913836.
http://dx.doi.org/10.1111/ahe.12306...
in the crab-eating fox.
Although there are studies on the macroscopic description of the male reproductive system of Leopardus pardalis, the present study sought to complement previous findings based on the microscopic description by histological technique (HE). Regarding the morphology of the female reproductive system of Leopardus pardalis,it is important to note that there are studies that have not been published so far, which demonstrates the importance of the work presented here.
The key points and specific findings of this study demonstrated not only the importance of the morphological description of this species, based on a new macroscopic study, but also aimed at the ultrastructural description of the reproductive system of males and females of Leopardus pardalis. It is important to note that the microscopic description by classical histological technique (Hematoxylin and Eosin), performed in this study is unprecedented, of great importance and will serve as a complement to the research carried out up to this moment.
Conclusion
This research emphasizes the importance of the macro- and microscopic description of the male and female reproductive system of Leopardus pardalis. The information obtained from the present study made it possible to fill in the gaps that often hinder the reproductive management of this species and also to complement previous macroscopic studies that have been carried out on the species in question. From the insertion of microscopic studies accomplished by classical techniques of histology, it was possible to amplify the knowledge, ultra-structurally. However, we emphasize the importance of further studies on the species in question (scanning electron microscopy, transmission electron microscopy, for example), with contributions to the maintenance of biodiversity and preservation of this species.
Acknowledgements
The authors are grateful to Laboratório de Zoologia e Morfologia Animal of the Universidade do Mato Grosso – UNEMAT (Alta Floresta – Mato Grosso - Brazil), for providing the animals; the Laboratório de Biologia Molecular do Hemocentro of Ribeirão Preto and Sandra Navarro Bresciani for construction of figures; the Laboratório de Anatomia Animal (BDMV/ZMV/FZEA - USP) and PhD. Anthony Michael Carter (Docent Emeritus - University of Southern Denmark) for technical assistance.
-
Financial support: None.
-
How to cite: Machado LC, Orlandin JR, Karam RG, Rós FA, Martins DS, Costa GM, Ambrósio CE. Morphology of male and female reproductive tract of the ocelot (Leopardus pardalis). Anim Reprod. 2020;17(2):e20200010. https://doi.org/10.1590/1984-3143-AR2020-0010
References
- ACM: Associação Mata Ciliar. Avaliação das condições veterinárias e de manejo dos pequenos felinos neotropicais em cativeiro no Estado de São Paulo. Revista de Educação Continuada. 1998;1:44-53.
- Andrade GM, del Collado M, Meirelles FV, Silveira JC, Perecin F. Intrafollicular barriers and cellular interactions during ovarian follicle development. Anim Reprod. 2019;16(3):485-96. http://dx.doi.org/10.21451/1984-3143-AR2019-0051 PMid:32435292.
» http://dx.doi.org/10.21451/1984-3143-AR2019-0051 - Atwood WJ. Comparative anatomy. 2nd ed. St. Louis: The C.V. Mosby Company; 1955. 424 p.
- Azevedo MA. Análise morfofuncional do testículo da onça-pintada (Panthera onca) adulta [dissertação]. Viçosa: Universidade Federal de Viçosa; 2004 [cited 2020 Jan 6]. Available from: http://locus.ufv.br/handle/123456789/5115
» http://locus.ufv.br/handle/123456789/5115 - Bacha WJ Jr, Bacha LM. Atlas colorido de histologia veterinária. 2nd ed. São Paulo: Roca; 2003 .457 p.
- Banks WJ. Histologia veterinária aplicada. 2nd ed. São Paulo: Manole; 1992. 560 p.
- Bianchi RC, Mendes SL. Ocelot (Leopardus pardalis) predation on primates in Caratinga Biological Station, southeast Brazil. Am J Primatol. 2007;69(10):1173-8. http://dx.doi.org/10.1002/ajp.20415 PMid:17330310.
» http://dx.doi.org/10.1002/ajp.20415 - Brown JL, Wasser SK, Wildt DE, Graham LH. Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biol Reprod. 1994;51(4):776-86. http://dx.doi.org/10.1095/biolreprod51.4.776 PMid:7819459.
» http://dx.doi.org/10.1095/biolreprod51.4.776 - Carneiro RM, Branco É, Pinheiro LL, Martins DM, Brígida SDS, Araújo EB, Souza ACB, Pereira LC, Lima AR. Descrição morfológica do sistema reprodutor masculino de jaguatirica (Leopardus pardalis). Biotemas. 2010;23(4):83-9. http://dx.doi.org/10.5007/2175-7925.2010v23n4p83
» http://dx.doi.org/10.5007/2175-7925.2010v23n4p83 - Caso A, Lopez-Gonzalez C, Payan E, Eizirik E, Oliveira TG, Leite-Pitman R, Kelly M, Valderrama C. Panthera onça In: International Union for Conservation of Nature and Natural Resources, editor. The IUCN Red List of Threatened Species, 2008. e: TI5953A5327466. Gland, Switzerland: IUCN; 2008. http://dx.doi.org./10.2305/IUCN.UK.2008.RLTS.TI5953A5327466.en
» http://dx.doi.org./10.2305/IUCN.UK.2008.RLTS.TI5953A5327466.en - Comizzoli P. Biobanking and fertility preservation for rare and endangered species. Anim Reprod. 2017;14(1):30-3. http://dx.doi.org/10.21451/1984-3143-AR889
» http://dx.doi.org/10.21451/1984-3143-AR889 - Derivaux J. Reprodução dos animais domésticos, 1. Fisiologia, 2. O macho, inseminação artificial, 3. patologia. 1st ed. Zaragoza: Editorial Acriba; 1980. p. 3-68.
- Di Bitetti MS, Paviolo A, De Angelo C. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. J Zool. 2006;270(0):153-63. http://dx.doi.org/10.1111/j.1469-7998.2006.00102.x
» http://dx.doi.org/10.1111/j.1469-7998.2006.00102.x - Dyce RM, Sack WO, Wensing CJG. O tratado de anatomia veterinária. 3rd ed. Rio de Janeiro: Elsevier; 2010. 816 p.
- Emmons LH. Comparative feeding ecology of felids in a Neotropical Rainforest. Behav Ecol Sociobiol. 1987;20(4):4, 271-83. http://dx.doi.org/10.1007/BF00292180
» http://dx.doi.org/10.1007/BF00292180 - Emmons LH, Feer F. Neotropical rainforest mammals: a field guide. 2nd ed. Chicago: University of Chicago Press; 1997. 307 p.
- Fossum TW. Cirurgia de pequenos animais. 3rd ed. São Paulo: Elsevier; 2008. Cirurgia dos sistemas reprodutivo e genital; p. 702-74.
- Gartner LP, Hiatt LJ. Tratado de histologia. Rio de Janeiro: Guanabara Koogan; 1999. p. 365-8.
- Getty R. Anatomia dos animais domésticos. 5th ed. Rio de janeiro: Guanabara Koogan; 1987. p. 147-53.
- Godinho CL. Análise histométrica do testículo e duração da espermatogênese em gatos (Felis domestica) sexualmente maduros [tese]. Belo Horizonte: Universidade Federal de Minas Gerais; 1999.
- Guião-Leite FL. Análise morfológica do testículo e do processo espermatogênico da onça parda (Puma concolor, Wozencraft, 1993) adulta [dissertação]. Viçosa: Universidade Federal de Viçosa; 2002 [cited 2020 Jan 6]. Available from: https://www.locus.ufv.br/bitstream/handle/123456789/7910/texto%20completo.pdf?sequence=1&isAllowed=y
» https://www.locus.ufv.br/bitstream/handle/123456789/7910/texto%20completo.pdf?sequence=1&isAllowed=y - Harcourt AH, Gardiner J. Sexual selection and genital anatomy of male primates. Proc Biol Sci. 1994;255(1342):47-53. http://dx.doi.org/10.1098/rspb.1994.0007 PMid:8153136.
» http://dx.doi.org/10.1098/rspb.1994.0007 - Junqueira LCU, Carneiro J. Histologia básica: texto y atlas. 12nd ed. Barcelona: Editorial Médica Panamericana; 2015. 556 p.
- Kleiman DG. Reproduction. In: Kleiman DG, Thompson KV, Baer CK, editors. Wild mammals in captivity: principles and techniques for zoo management. Chicago: University of Chicago Press; 2010. p. 377-8. http://dx.doi.org/10.7208/chicago/9780226440118.001.0001
» http://dx.doi.org/10.7208/chicago/9780226440118.001.0001 - König HE, Liebich HJ. Anatomia dos animais domésticos. 2nd ed. Porto Alegre: Artmed; 2004. 400 p.
- Ludlow ME, Sunquist ME. Ecology and behavior of ocelots in Venezuela. Natl Geogr Res. 1987;3(4):447-61.
- Machado LC, Oliveira VC, Paraventi MD, Cardoso RNR, Martins DS, Ambrósio CE. Maintenance of Brazilian biodiversity by germplasm bank. Pesq Vet Bras. 2016;36(1):62-6. http://dx.doi.org/10.1590/S0100-736X2016000100010
» http://dx.doi.org/10.1590/S0100-736X2016000100010 - Machado LC, Roballo KCS, Cury FS, Ambrósio CE. Female reproductive system morphology of crab-eating fox (Cerdocyon thous) and cryopreservation of genetic material for animal germplasm bank enrichment. Anat Histol Embryol. 2017;46(6):539-46. http://dx.doi.org/10.1111/ahe.12306 PMid:28913836.
» http://dx.doi.org/10.1111/ahe.12306 - Mansfield KG, Land D. Cryptorchidism in Florida panthers: prevalence, features, and influence of genetic restoration. J Wildl Dis. 2002;38(4):693-8. http://dx.doi.org/10.7589/0090-3558-38.4.693 PMid:12528434.
» http://dx.doi.org/10.7589/0090-3558-38.4.693 - Mellen JD. Factors influencing reproductive success in small captive exotic felids (Felis spp): a multiple regression analysis. Zoo Biol. 1991;10(2):95-110. http://dx.doi.org/10.1002/zoo.1430100202
» http://dx.doi.org/10.1002/zoo.1430100202 - Micheletti T, Cubas ZS, Moraes W, Oliveira MJ, Moreira N. Reprodução natural de felídeos selvagens em cativeiro: dificuldades e orientações. Rev Bras Reprod Anim [serial on the Internet]. 2012 [cited 2020 Jan 6];36(1):39-43. Available from: http://locus.ufv.br/handle/123456789/5115
» http://locus.ufv.br/handle/123456789/5115 - Morato R, Barnabé R. Biotécnicas de reprodução aplicadas à preservação de felídeos selvagens. Clín Vet [serial on the Internet]. 1998 [cited 2020 Jan 6];(12):24-6. Available from: http://www.cbra.org.br/portal/downloads/publicacoes/rbra/v42/n3-4/p141-145%20(RB751).pdf
» http://www.cbra.org.br/portal/downloads/publicacoes/rbra/v42/n3-4/p141-145%20(RB751).pdf - Moreira N, Monteiro‐Filho E, Moraes W, Swanson W, Graham L, Pasquali O, Gomes M, Morais R, Wildt D, Brown J. Reproductive steroid hormones and ovarian activity in felids of the Leopardus genus. Zoo Biol. 2001;20(2):103-16. http://dx.doi.org/10.1002/zoo.1010 PMid:11429781.
» http://dx.doi.org/10.1002/zoo.1010 - Moreno RS, Kays RW, Samudio R Jr. Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J Mammal. 2006;87(4):808-16. http://dx.doi.org/10.1644/05-MAMM-A-360R2.1
» http://dx.doi.org/10.1644/05-MAMM-A-360R2.1 - Murray JL, Gardner GL. Leopardus pardalis. Mamm Species. 1997;(548):1-10. http://dx.doi.org/10.2307/3504082
» http://dx.doi.org/10.2307/3504082 - Nickel R, Schummer A, Seiferle E. The viscera of the domestic mammals. 2nd ed. Berlin: Verlag Paul Parey: 1979. p. 351-89. http://dx.doi.org/10.1007/978-1-4757-6814-5
» http://dx.doi.org/10.1007/978-1-4757-6814-5 - Nowell K, Jackson P. Wild cats, status survey and conservation action plan [Internet]. Gland, Switzerland: IUCN/SSC Cat Specialist Group, International Union for Conservation of Nature and Natural Resources; 1996. 382 p. [cited 2020 Jan 6]. Available from: https://portals.iucn.org/library/efiles/documents/1996-008.pdf
» https://portals.iucn.org/library/efiles/documents/1996-008.pdf - Oliveira TG, Cassaro K. Guia de campo dos felinos do Brasil. São Paulo: Instituto Pró-Carnívoros/Fundação Parque Zoológico de São Paulo; 2005. 80 p.
- Queiroz VS. Estudo do efeito das condições de manipulação do sêmen de jaguatiricas (Leopardus pardalis: Linnaeus, 1758) sobre a capacitação e a integridade morfológica e funcional dos espermatozóides [dissertação]. São Paulo: Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo; 2003. https://doi.org/10.11606/d.10.2003.tde-09062004-140805
» https://doi.org/10.11606/d.10.2003.tde-09062004-140805 - Rocha EF, Santos NTA, Dias RFF, Diniz JARA, Dos Santos JRS, De Menezes DJA. Anatomia macroscópica dos órgãos reprodutores de Puma yagouaroundi (Geoffroy, 1803) macho. Pubvet. 2017;(11):744-839. http://dx.doi.org/10.22256/pubvet.v11n8.767-770
» http://dx.doi.org/10.22256/pubvet.v11n8.767-770 - Sarti P. Avaliação morfométrica do testículo e da espermatogênese de jaguatiricas (Leopardus pardalis, Linnaeus, 1758) adultas [dissertation]. Viçosa: Universidade Federal de Viçosa; 2006.
- Schwarze E, Schröder L. Compêndio de anatomia veterinária: sistema visceral. Zaragoza: Acribia; 1970. p. 277-86. Vol. 2.
- Silva CAO, Perri SHV, Koivisto MB, Silva AM, Carvalho RG, Monteiro CMR. Histological and morphometric evaluation of the testes of cats (Felis catus). Aspectos histológicos e morfométricos dos testículos de gatos domésticos (Felis catus). Pesq Vet Bras. 2009;29(4):312-6. http://dx.doi.org/10.1590/S0100-736X2009000400006
» http://dx.doi.org/10.1590/S0100-736X2009000400006 - Slatter D. Manual de cirurgia de pequenos animais. 3rd ed. São Paulo: Manole; 2007.
- Wick R, Kress A. Ultrastructural changes in the uterine luminal and glandular epithelium during the oestrous cycle of the marsupial Monodelphis domestica (Grey Short‐Tailed Opossum). Cells Tissues Organs. 2002;170(2-3):111-31. http://dx.doi.org/10.1159/000046185 PMid:11731700.
» http://dx.doi.org/10.1159/000046185
Publication Dates
-
Publication in this collection
08 July 2020 -
Date of issue
2020
History
-
Received
06 Feb 2020 -
Accepted
09 June 2020