Abstract
In this study we analyzed the diet of the gymnophthalmid lizard Ecpleopus gaudichaudii Duméril & Bibron, 1839, a typical inhabitant of the forest-floor leaf litter, in an Atlantic Forest area in the state of Rio de Janeiro, southeastern Brazil. The 26 individuals sampled during the study had a mean snout-vent length (SVL) of 36.2 ± 4.2 mm and a mean jaw width (JW) of 4.1 ± 0.5 mm. We did not find differences in SVL between males and females, though the sexes differed in JW when the effect of body size was factored out, with females presenting higher values. The diet of the lizards was composed exclusively of arthropods, especially isopods and orthopterans. The similarity in trophic niches among seasons (volumetric and numerical proportions of prey categories consumed) were 0.096 and to 0.43, respectively. There were also no detectable seasonal differences in mean number and mean volume of prey ingested, as well as no significant influence of lizard SVL on prey number and of lizard JW on mean prey volume, which may reflect the tendency of E. gaudichaudii to feed on few, relatively large prey.
Diet; Lacertilia; rainforest; seasonality; Squamata
BIOLOGY
Diet of the lizard Ecpleopus gaudichaudii (Gymnophthalmidae) in Atlantic Rainforest, state of Rio de Janeiro, Brazil
Thiago MaiaI, 1 1 Corresponding author. E-mail: thiagomaianc@gmail.com ; Mauricio Almeida-GomesI; Carla C. SiqueiraII; Davor VrcibradicIII; Mara C. KieferIV; Carlos Frederico D. RochaI
IDepartamento de Ecologia, Universidade do Estado do Rio de Janeiro. Rua São Francisco Xavier 524, 20550-019 Rio de Janeiro, RJ, Brazil
IIPrograma de Pós-Graduação em Ecologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro. Avenida Carlos Chagas Filho 373, Bloco A, Cidade Universitária, 21941-902 Rio de Janeiro, RJ, Brazil
IIIDepartamento de Zoologia, Universidade Federal do Estado do Rio de Janeiro. Avenida Pasteur 458, Urca, 22240-290 Rio de Janeiro, RJ, Brazil
IVDepartamento de Biologia Geral, Instituto de Biologia, Universidade Federal Fluminense. Caixa Postal 100436, Centro, 24020-971 Niterói, RJ, Brazil
ABSTRACT
In this study we analyzed the diet of the gymnophthalmid lizard Ecpleopus gaudichaudii Duméril & Bibron, 1839, a typical inhabitant of the forest-floor leaf litter, in an Atlantic Forest area in the state of Rio de Janeiro, southeastern Brazil. The 26 individuals sampled during the study had a mean snout-vent length (SVL) of 36.2 ± 4.2 mm and a mean jaw width (JW) of 4.1 ± 0.5 mm. We did not find differences in SVL between males and females, though the sexes differed in JW when the effect of body size was factored out, with females presenting higher values. The diet of the lizards was composed exclusively of arthropods, especially isopods and orthopterans. The similarity in trophic niches among seasons (volumetric and numerical proportions of prey categories consumed) were 0.096 and to 0.43, respectively. There were also no detectable seasonal differences in mean number and mean volume of prey ingested, as well as no significant influence of lizard SVL on prey number and of lizard JW on mean prey volume, which may reflect the tendency of E. gaudichaudii to feed on few, relatively large prey.
Key words: Diet; Lacertilia; rainforest; seasonality; Squamata.
Gymnophthalmidae currently contains more than 200 species (UETZ & HALLERMANN 2011) of predominantly small lizards which are restricted to tropical latitudes in the New World (PIANKA & VITT 2003). Most are terrestrial, though some species may be semiaquatic and others semifossorial or fossorial, with a few species partly arboreal (PIANKA & VITT 2003).The family has undergone numerous taxonomic changes within the last decade, including the splitting or lumping of genera (DOAN 2003, DOAN & CASTOE 2005, RODRIGUES et al. 2007), the creation and re-delimitation (i.e., with the exclusion or inclusion of taxa) of subfamilies and tribes (PELLEGRINO et al. 2001, CASTOE et al. 2004), and the erection of new genera to accommodate some newly discovered species (RODRIGUES et al. 2005, 2007, 2009, RODRIGUES & SANTOS 2008, KOK 2005, 2009, PELOSO et al. 2011).
Currently, most of the published information on the ecology, particularly regarding feeding habits, of gymnophtalmid lizards in South America pertains to taxa from Amazonian or Andean forests (e.g., DUELLMAN 1978, ROCHA 1991, VITT & ÁVILAPIRES 1998, VITT & ZANI 1998, VITT et al. 1998a,b, 2003, 2007, DOAN 2008, ANAYA-ROJAS et al. 2010) or from open habitats (e.g., VITT 1995, VITT & CARVALHO 1995, VIEIRA et al. 2000, ROCHA & RODRIGUES 2005, MESQUITA et al. 2006a,b), with few reports from Atlantic Forest species (e.g., TEIXEIRA & FONSECA 2003, EISEMBERG et al. 2004). The gymnophtalmid Ecpleopus gaudichaudii Duméril & Bibron, 1839, the single species in this genus, is endemic to the Atlantic Forest of southeastern and southern Brazil (PETERS et al. 1986, UETZ & HALLERMANN 2011), and although it may occur also in anthropically modified habitats (see COSTA et al. 2009), it inhabits mainly the forest floor leaf litter. At present, the available information on aspects of its biology is restricted to a study on the feeding habits of two populations in the state of Minas Gerais, based on relatively small sample sizes (pooled sample = 17 specimens; EISEMBERG et al. 2004).
In the Reserva Ecológica de Guapiaçu (REGUA), in the state of Rio de Janeiro, individuals of E. gaudichaudii have been recently collected during herpetological surveys, thus providing an opportunity to increase the knowledge of its ecology. In the present study we provide information on the diet of E. gaudichaudii based on data from the population living in the area of REGUA and its surroundings. We also assess whether its diet shows seasonal differences in composition, considering that the availability of potential prey vary seasonally in some areas of southeastern Brazil (e.g., VAN SLUYS 1995, ROCHA 1996, DEVELEY & PERES 2000).
MATERIAL AND METHODS
The lizards were collected in the forest of REGUA (22º24'S, 42º44'W) and in five forest fragments at its surroundings, in the municipality of Cachoeiras de Macacu, state of Rio de Janeiro, southeastern Brazil. The Reserve is covered by Atlantic Forest at different levels of conservation, with regions of undisturbed forests occurring in the steepest and less accessible parts of the Reserve (ROCHA et al. 2007). This area is inserted within one of the largest remnants of Atlantic Forest in the state (total area over 60,000 ha), has a wet and warm climate with annual rainfall varying from 2000 to 2500 mm, and the mean annual temperature is about 24ºC (ROCHA et al. 2007).
Lizards were collected during herpetological surveys carried out between July 2007 and March 2010, at altitudes between 40 and 500 m. Surveys were performed during the dry (April-September) and wet (October-March) seasons, and two sampling methods were employed: visual encounter surveys (hereafter VES; CRUMP & SCOTT 1994), and pitfall traps with drift fences (CORN 1994). The VES method was performed by timeconstrained transects (30 minutes), totaling 1,250 hours of active search. Pitfall-trap systems consisted each of eleven 60-liter buckets buried on the ground and set approximately 10 m apart from each other, with soft plastic drift fences about 50 cm high extended between them. A total of 15 pitfall-trap systems (165 buckets) were established (12 in fragments and three in the reserve) and were surveyed during six days in each season (a total of 30 days) totaling 4950 bucket-days of sampling effort (pitfall trapping was not used during the 2010 wet season).
After preservation in 10% formalin and storage in 70% alcohol, the snout-vent length (SVL) and jaw width (JW) of collected lizards were measured with a digital caliper (precision of 0.1 mm). Specimens were dissected, and their stomach contents were analyzed qualitatively and quantitatively. Prey items found in stomachs were identified under a stereomicroscope to the level of Order (or Family in the case of Formicidae). Diet was quantified using number, volume (mm3) and frequency (%) of occurrence of items. Prey items were counted and measured (greatest length and width) with a digital caliper (precision of 0.1 mm) and their volume was estimated using the formula for a prolate spheroid [V = 4/3.π. (L/2) (W/2) 2], where L is the length and W is the width of the food item (DUNHAM 1983). The frequency of occurrence of each category of prey in the diet was expressed as the proportion of stomachs that contained that category.
To assess the degree of similarity of the trophic niche of E. gaudichaudii between the dry and wet seasons (considering the possibility of seasonal differences in local arthropod availability), we compared the patterns of prey consumption (based on volumetric/numerical proportions of prey categories) among seasons using the niche overlap index of MacArthur and Levins (PIANKA 1973) Ojk = Σ pijpik/√(Σ pijΣ pik), where pij and pik are the volumetric/numerical proportion of prey category i in the diet in seasons j and k, respectively.
We evaluated whether there were differences in the number of prey and in the mean volume of the three largest prey consumed per lizard between seasons (again, assuming the possibility of seasonal variation in prey availability) using One-Way Analysis of Variance (ANOVA) (ZAR 1999); mean prey volume per lizard was estimated as the mean volume of the three largest prey items consumed (or all items, when stomachs contained less than three). To analyze if the number of ingested items and the volume of individual prey are affected as a result of the lizards' body size and mouth width, respectively, we tested for relationships between prey number and lizard body size (expressed as SVL) and between mean prey volume and jaw width (JW) by performing Simple Regression Analyses. To assess if there is sexual dimorphism in body size and head dimensions in this population of E. gaudichaudii, we tested for differences in SVL and in JW between males and females using ANOVA and Analysis of Covariance (ANCOVA, with SVL as covariate; ZAR 1999), respectively.
Descriptive statistics are presented throughout the text as mean ± one standard deviation. All data were tested for homoscedasticity of variances and for the normality of distribution before performing statistical analyses.
RESULTS
A total of 26 individuals of E. gaudichaudii (13 at each season) were collected during the surveys: 18 males, seven females, and one individual whose sex could not be determined. The mean SVL of E. gaudichaudii in our sample was 36.2 ± 4.2 mm (26.7-43.2 mm; N = 26), with females averaging 38.7 ± 3.5 mm (33.1-43.2 mm, N = 7) and males 35.3 ± 4.2 mm (26.7-42.4 mm, N = 18). The mean JW was 4.1 ± 0.5 (3.1-5.0 mm, N = 26), with females averaging 4.3 ± 0.5 mm (4.6-5.0 mm, N = 7) and males 4.0 ± 0.4 mm (3.1-4.9, N = 18). No significant difference was detected between males and females in SVL (ANOVA, F1,23 = 3.509, p = 0.074), but the sexes differed significantly in JW (ANCOVA, F1,1,22 = 19.460, p < 0.001), with females having proportionally wider jaws.
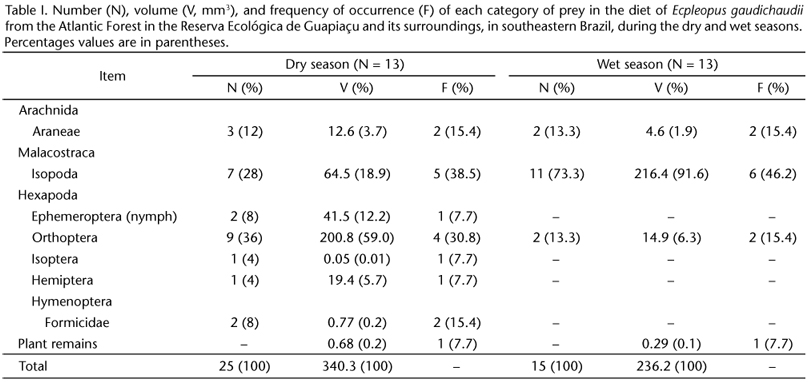
Three individuals (23.1%; two males and one female) of E. gaudichaudii from dry season months and four (30.8%, four males) from wet season months had empty stomachs. A total of seven orders of arthropods were consumed in the dry season, but only three during the wet season (Tab. I). Plant remains had low proportions in the diet in both seasons and usually corresponded to small parts of dry leaves from the leaf litter.
In the dry season, in numerical terms, lizards fed predominantly on orthopterans (36%), followed by isopods (28%). Volumetrically, orthopterans (59% of the total ingested) and isopods (18.9%) were also the most important items. In terms of frequency of occurrence, isopods were the main prey, being found in 38.5% of the specimens analyzed, followed by orthopterans (30.8%) (Tab. I).
In the wet season, lizards fed predominantly on isopods, which were the most important among the three prey categories consumed in that season. Isopods corresponded to 73.3% of the total prey ingested, 91.6% of the total volume consumed, and were found in 46.2% of the stomachs (Tab. I).
The values of trophic niche overlap (Oij) between seasons, in terms of volumetric and numerical proportions of prey categories consumed, were 0.096 and 0.43, respectively. The mean number of prey consumed per individual of E. gaudichaudii was 2.3 ± 1.5 (range = 1-7, N = 18) and did not differ significantly (ANOVA, F1,16 = 1.400, p = 0.254) between dry (2.6 ± 1.7, 1-7, N = 10) and wet (1.9 ± 1.1, 1-4, N = 8) seasons. The mean volume of the three largest items consumed per lizard was 21.3 ± 19.7 mm3 (0.1-65.2 mm3, N = 18) and also did not vary significantly (ANOVA, F1,16 = 0.508, p = 0.490) between dry (17.8 ± 14.8 mm3, 0.1-46.8 mm3, N = 10) and wet (25.7 ± 25 mm3, 1.5-65.2 mm3, N = 8) seasons. The number and mean volume of prey ingested were not significantly related, respectively, to SVL (Regression Analysis, R = 0.216, p = 0.389, N = 18), and to JW (Regression Analysis, R = 0.006, p = 0.770, N = 18).
DISCUSSION
Despite the comparatively intense sampling effort employed in this study, only 26 individuals of E. gaudichaudii were collected. The secretive habits of this species may render it difficult to detect in the habitat (which could lead to an underestimation of its local abundance). EISEMBERG et al. (2004) referred to E. gaudichaudii as a "rare" lizard when reporting data on its diet in two areas in the state of Minas Gerais, southeastern Brazil. Studies carried out in other areas of Atlantic Forest reported E. gaudichaudii as an abundant species (e.g., DIXO & VERDADE 2006, CONDEZ et al. 2009, COSTA et al. 2009). Indeed, CONDEZ et al. (2009) and DIXO & VERDADE (2006) mentioned that E. gaudichaudii was the most abundant lizard species in areas of Atlantic Forest in the state of São Paulo, southeastern Brazil, where they carried out herpetological inventories. Actually, E. gaudichaudi was the second most abundant lizard species found during surveys in REGUA, after the leiosaurid Enyalius brasiliensis (Lesson, 1828) (M. Almeida-Gomes, unpub. data). Hence, E. gaudichaudii seems to be an abundant species in at least some of the areas of Atlantic Forest it inhabits.
Our data did not support evidence for sexual dimorphism in body size in E. gaudichaudii, although relative head width differed between the sexes, with females presenting greater values. The occurrence of sexual dimorphism in body size and/or relative head dimensions has been reported for some gymnophthalmid lizards (e.g., PIANKA & VITT 2003, DOAN 2008), although in those cases males had the larger heads. However, in some other species in this family sexual size dimorphism may be very slight or non-existent (VITT & ÁVILA-PIRES 1998, VITT et al. 2007). Our limited data for this study suggest that sexes may not differ significantly in size in E. gaudichaudii, but our sample size for females was too low and thus we cannot make a definite statement. Analysis of a larger sample would be useful to verify if this species indeed lacks sexual size dimorphism, and if it actually is sexually dimorphic in relative head width.
The results of our study indicated that E. gaudichaudii has a diet exclusively composed of small arthropods, with isopods, orthopterans and spiders being the main prey. We found plant remains in the diet, such as pieces of twigs and leaves, but due to its low volumetric importance and the type of plant matter present in stomachs (small dry pieces of leaves from leaf-litter) we considered it as a result of accidental ingestion during prey capture by the lizards. In another study carried out in the state of Minas Gerais, the diet of E. gaudichaudii was numerically dominated by isopterans and spiders, and the most frequently consumed prey were spiders, followed by orthopterans and isopods (EISEMBERG et al. 2004). In our study, there was high consumption of isopods and low consumption of isopterans, suggesting that the local availability of food items may be an important factor affecting prey ingestion by these lizards. Nevertheless, the diet of E. gaudichaudii was rather similar in both studies, with isopods and orthopterans being among the most frequently consumed items in studied areas in both states of Rio de Janeiro and Minas Gerais. Isopods have also been reported as the main prey item in the diet of other gymnophthalmids such as Leposoma scincoides Spix, 1825 in a Brazilian Atlantic Forest area in the state of Espírito Santo (TEIXEIRA & FONSECA 2003), and Ptychoglossus bicolor (Werner, 1916) in a coffee plantation in Colombia (ANAYA-ROJAS et al. 2010). However, judging from most dietary surveys so far carried out on gymnophthalmids, it seems that spiders and orthopterans are, in general, the predominant prey types in the diets of such lizards (e.g., DUELLMAN 1978, VITT 1995, VITT & ZANI 1998, VITT et al. 1998a,b, 2003, 2007, VIEIRA et al. 2000, ROCHA & RODRIGUES 2005, MESQUITA et al. 2006a,b, DOAN 2008).
The low similarity of trophic niches between the dry and wet seasons was apparently due to the substantial predominance of isopods in the diet during the wet season, as compared to the dry season, when a greater variety of prey types was consumed. Additionally, spiders and orthopterans had a higher proportional contribution to the diet of E. gaudichaudii in the dry season comparing to the wet season. Optimal foraging models predict more generalized diets as a result of food shortage, leading predators to consume more "sub-optimal" prey than in periods of greater resource abundance (SCHOENER 1971). The availability of potential food resources (i.e., arthropods) for lizards may be higher during the wet than during the dry season in some seasonal tropical areas (e.g., VAN SLUYS 1995, ROCHA 1996). Thus, E. gaudichaudii may fit the optimal foraging model in the present study, having fed on more types of prey in the dry season and consuming predominantly one prey type in the wet season. However, as we have no data on arthropod availability in the environment during the period of study we cannot say if a seasonal fluctuation in arthropod abundance actually occurs in the study area and if it is sufficiently pronounced in order to influence the feeding habits of E. gaudichaudii.
The observed lack of seasonal differences in mean number and mean volume of prey per individual may reflect the tendency of E. gaudichaudii to feed on few, relatively large prey (EISEMBERG et al. 2004, this study). We also found no significant relationships between SVL and number of prey and between JW and prey size for E. gaudichaudii. This also may result from the low variation in the size of prey consumed by this lizard (mainly the most consumed, i.e., isopods), whose small body size limits the range of prey sizes that it can swallow. Body size and head dimensions are intrinsic factors that may affect strongly the patterns of prey consumption in lizards, with larger body and head sizes enabling an individual to consume more prey of a given size and prey of larger sizes, respectively (e.g., TOFT 1985, MAGNUSSON & SILVA 1993, MENEZES et al. 2008).
Our results showed that E. gaudichaudii has a diet exclusively composed of arthropods, with isopods and orthopterans being the most consumed prey, and with some variation in prey consumption patterns among seasons. Analysis of a larger sample would be useful to more consistently assess this apparent seasonal trend, as well as verify if E. gaudichaudii indeed lacks sexual size dimorphism, and if females actually tend to have relatively wider heads than males (which is opposite to what has been observed so far in other gymnophtalmid lizards).
ACKNOWLEDGEMENTS
This study was supported by research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq processes 304791/2010-5 and 470265/2010-8) and from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) through "Cientistas do Nosso Estado" Program (process E-26/102.404.2009) to C.F.D. Rocha. We thank Nicholas J. Locke of the Reserva Ecológica de Guapiaçu (REGUA) for making many facilities available during our fieldwork in that area. We also thank T. Moulton for their aid and helpful suggestions. Mara C. Kiefer received a research grant from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (process E-26/171.168/2006). T. Maia received a scientific iniciation grant and C.C. Siqueira received a PhD grant from the CNPq. Thiago Maia received a grant from the FAPERJ. Mauricio A.Gomes received a graduate fellowship from Conservation International do Brasil and FAPERJ.
LITERATURE CITED
Submitted: 05.V.2011; Accepted: 03.VIII.2011.
Editorial responsibility: Diego Astúa de Moraes
- ANAYA-ROJAS, J.M.; V.H. SERRANO-CARDOZO & M.P. RAMÍREZ-PINILLA. 2010. Diet, microhabitat use, and thermal preferences of Ptychoglossus bicolor (Squamata: Gymnophthalmidae) in an organic coffee shade plantation in Colombia. Papéis Avulsos de Zoologia 50 (10): 159-166.
- CASTOE, T.A.; T.M. DOAN & C.L. PARKINSON. 2004. Data partitions and complex models in Bayesian analysis: the phylogeny of gymnophthalmid lizards. Systematic Biology 53 (3): 448 469.
- CONDEZ, T.H.; R.J. SAWAYA & M. DIXO. 2009. Herpetofauna dos remanescentes de Mata Atlântica da região de Tapiraí e Piedade, SP, sudeste do Brasil. Biota Neotropica 9 (1): 157-185.
- CORN, P.S. 1994. Straight-line drift fences and pitfall traps, p. 109-117. In: W.R. HEYER; M.A. DONNELLY; R.W. MCDIARMID; L.A.C. HAYEK & M.S. FOSTER (Eds). Measuring and Monitoring Biological Diversity: Standard methods for amphibians. Washington, DC, Smithsonian Institution Press, 364p.
- COSTA, H.C.; V.D. FERNANDES; A.C. RODRIGUES & R.N. FEIO. 2009. Lizards and Amphisbaenians, municipality of Viçosa, state of Minas Gerais, southeastern Brazil. Check List 5 (3): 732-745.
- CRUMP, M.L. & N.J. SCOTT JR. 1994. Visual encounter surveys, p. 84-92. In: W.R. HEYER; M.A. DONNELLY; R.W. MCDIARMID; L.A.C. HAYEK & M.S. FOSTER (Eds). Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians. Washington, DC, Smithsonian Institution Press, 364p.
- DEVELEY, P.F. & C.A. PERES. 2000. Resource seasonality and the structure of mixed species bird flocks in a coastal Atlantic forest of southeastern Brazil. Journal of Tropical Ecology 16 (1): 33-53.
- DIXO, M. & V.K. VERDADE. 2006. Herpetofauna de serapilheira da Reserva Florestal do Morro Grande, Cotia (SP). Biota Neotropica 6 (2): 1-20.
- DOAN, T.M. 2003. A new phylogenetic classification for the gymnophthalmid genera Cercosaura, Pantodactylus and Prionodactylus (Reptilia: Squamata). Zoological Journal of the Linnean Society 137 (1): 101 115.
- DOAN, T.M. 2008. Dietary variation within the Andean lizard clade Proctoporus (Squamata: Gymnophthalmidae). Journal of Herpetology 42 (1): 16-21.
- DOAN, T.M. & T.A. CASTOE. 2005. Phylogenetic taxonomy of the Cercosaurini (Squamata: Gymnophthalmidae), with new genera for species of Neusticurus and Proctoporus Zoological Journal of the Linnean Society 143 (3): 405 416.
- DUELLMAN, W.E. 1978. The biology of an equatorial herpetofauna in Amazonian Ecuador. Miscellaneous Publication University of Kansas Museum of Natural History 65: 1-352.
- DUNHAM, A.E. 1983. Realized niche overlap, resource abundance, and intensity of interspecific competition, p. 261-280. In: R.B. HUEY; E.R. PIANKA & T.W. SHOENER (Eds). Lizard Ecology: Studies of a model organism. Cambridge, Harvard University Press, 512p.
- EISEMBERG, C.C.; J. CASSIMIRO & J. BERTOLUCI. 2004. Notes on the diet of the rare gymnophthalmid lizard Ecpleopus gaudichaudii from southeastern Brazil. Herpetological Review 35 (4): 336-337.
- KOK, P.J.R. 2005. A new genus and species of gymnophthalmid lizard (Squamata: Gymnophthalmidae) from Kaieteur National Park, Guyana. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique (Biologie) 75: 35-45.
- KOK, P.J.R. 2009. Lizard in the clouds: a new highland genus and species of Gymnophthalmidae (Reptilia: Squamata) from Maringma tepui, western Guyana. Zootaxa 1992: 53-67.
- MAGNUSSON, W.E. & E.V. SILVA. 1993. Relative effects of size, season and species on the diets of some Amazonian Savanna Lizards. Journal of Herpetology 27 (4): 380-385.
- MENEZES, V.A.; G.F. DUTRA & C.F.D. ROCHA. 2008. Feeding habits of the endemic tropical parthenogenetic lizard Cnemidophorus nativo (Teiidae) in a restinga area of northeastern Brazil. Journal of Natural History 42 (39-40): 2575-2583.
- MESQUITA, D.O.; G.R. COLLI; F.G.R. FRANÇA & L.J. VITT. 2006a. Ecology of a Cerrado lizard assemblage in the Jalapão region of Brazil. Copeia 2006: 459-470.
- MESQUITA, D.O.; G.C. COSTA; G.R. COLLI. 2006b. Ecology of an Amazonian savanna lizard assemblage in Monte Alegre, Pará state, Brazil. South American Journal of Herpetology 1: 61-71.
- PELLEGRINO, K.C.M.; M.T. RODRIGUES; Y. YONENAGA-YASSUNA & J.W. SITES JR. 2001. A molecular perspective on the evolution of microteiid lizards (Squamata, Gymnophthalmidae), and a new classification for the family. Biological Journal of the Linnean Society 74: 315-338.
- PELOSO, P.L.V.; K.C.M. PELLEGRINO; M.T.U. RODRIGUES & T. C.S. ÁVILA-PIRES. 2011. Description and phylogenetic relationships of a new genus and species of lizard (Squamata, Gymnophthalmidae) from the Amazonian rainforest of northern Brazil. American Museum Novitates 3713: 1-24.
- PETERS, J.A.; R. DONOSO-BARROS & P.E. VANZOLINI 1986. Catalogue of the Neotropical Squamata. Part II: Lizards and Amphisbaenians. Bulletin of the United States National Museum 297: 1-293.
- PIANKA, E.R. 1973. The structure of lizard communities. Annual Review of Ecology and Systematics 4: 53-74.
- PIANKA, E.R. & L.J. VITT. 2003. Lizards: windows to the study of diversity. Berkeley, University of California Press, 333p.
- ROCHA, C.F.D. 1991. Habitat utilization and feeding habits of Neusticurus ecpleopus in a Brazilian tropical rainforest. Herpetological Review 22 (2): 40-42.
- ROCHA, C.F.D. 1996. Seasonal shift in lizard diet: The seasonality in food resources affecting the diet of Liolaemus lutzae (Tropiduridae). Ciência e Cultura 48 (4): 264-269.
- ROCHA, C.F.D.; D. VRCIBRADIC; M.C. KIEFER; M. ALMEIDA-GOMES; V.N.T. BORGES-JUNIOR; P.C.F. CARNEIRO; R.V. MARRA; P. ALMEIDA-SANTOS; C.C. SIQUEIRA; P. GOYANNES-ARAÚJO; C.G.A. FERNANDES; E.C.N. RUBIÃO & M. VAN SLUYS. 2007. A survey of the leaf-litter frog assembly from an Atlantic forest area (Reserva Ecológica de Guapiaçu) in Rio de Janeiro State, Brazil, with an estimate of frog densities. Tropical Zoology 20: 99-108.
- ROCHA, P.L.B. & M.T. RODRIGUES. 2005. Electivities and resource use by an assemblage of lizards endemic to the dunes of the São Francisco river, northeastern Brazil. Papéis Avulsos de Zoologia 45 (22): 261-284.
- RODRIGUES, M.T. & E.M. SANTOS. 2008. A new genus and species of eyelid-less and limb-reduced gymnophthalmid lizard from northeastern Brazil (Squamata, Gymnophthalmidae). Zootaxa 1873: 50-60.
- RODRIGUES, M.T.; E.M.X. FREIRE; K.C.M. PELLEGRINO & J.W. SITES. 2005. Phylogenetic relationships of a new genus and species of microteiid lizard from the Atlantic forest of north-eastern Brazil (Squamata, Gymnophthalmidae). Zoological Journal of the Linnean Society 144 (4): 543-557.
- RODRIGUES, M.T.; J. CASSIMIRO; D. PAVAN; F.F. CURCIO; V.K. VERDADE & K.C.M. PELLEGRINO. 2009. A new genus of microteiid lizard from the Caparaó mountains, southeastern Brazil, with a discussion of relationships among Gymnophthalminae (Squamata). American Museum Novitates 3673: 1-27.
- RODRIGUES, M.T.; K.C.M. PELLEGRINO; M. DIXO; V.K. VERDADE; D. PAVAN; A.J.S. ARGÔLO & J.W. SITES JR. 2007. A new genus of microteiid lizard from the Atlantic Forests of state of Bahia, Brazil, with a new generic name for Colobosaura mentalis, and a discussion of relationships among the Heterodactylini (Squamata, Gymnophthalmidae). American Museum Novitates 3565: 1-27.
- SCHOENER, T.W. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2: 369-404.
- TEIXEIRA, R.L. & F.R. FONSECA. 2003. Tópicos ecológicos de Leposoma scincoides (Sauria, Gymnophthalmidae) da região de Mata Atlântica de Santa Teresa, Espírito Santo, sudeste do Brasil. Boletim do Museu de Biologia Mello Leitão 15: 17 28.
- TOFT, C.A. 1985. Resource partitioning in amphibians and reptiles. Copeia 1985: 1-20.
- UETZ, P. & J. HALLERMANN. 2011. The TIGR Reptile Database. Rockville, JCVI, Available online at: http://www.reptile-database.org [Accessed: 9/II/2011]
- VAN SLUYS, M. 1995. Seasonal variation in prey choice by the lizard Tropidurus itambere (Tropiduridae) in Southeastern Brazil. Ciência e Cultura 47 (1/2): 61-65.
- VIEIRA, G.H.C.; D.O. MESQUITA; K. KITAYAMA & G.R. COLLI. 2000. Micrablepharus atticolus Natural History. Herpetological Review 31: 241-242.
- VITT, L.J. 1995. The ecology of tropical lizards in the Caatinga of northeast Brazil. Occasional Papers of the Oklahoma Museum of Natural History 1: 1-29.
- VITT, L.J & P.A. ZANI. 1998. Ecological relationships among sympatric lizards in a successional landscape in the northern Amazon of Brazil. Journal of Tropical Ecology 14: 63-86.
- VITT, L.J. & C.M. CARVALHO. 1995. Niche partitioning in a tropical wet season: lizards in the Lavrado area of northern Brazil. Copeia 1995: 305-329.
- VITT, L.J. & T.C.S. AVILA-PIRES. 1998. Ecology of two sympatric species of Neusticurus (Sauria: Gymnophthalmidae) in the western Amazon of Brazil. Copeia 1998: 570-582.
- VITT, L.J.; S.S. SARTORIUS; T.C.S. AVILA-PIRES & M.C. ESPÓSITO. 1998a. Use of time, space, and food by the gymnophthalmid lizard Prionodactylus eigenmanni from the western Amazon of Brazil. Canadian Journal of Zoology 76: 1681 1688.
- VITT, L.J.; P.A. ZANI; T.C.S. ÁVILA-PIRES & M.C. ESPOSITO. 1998b. Geographical ecology of the gymnophthalmid lizard Neusticurus ecpleopus in the Amazon rainforest. Canadian Journal of Zoology 76: 1671-1680.
- VITT, L.J.; T.C.S ÁVILA-PIRES; M.C. ESPÓSITO; S.S. SARTORIUS & P.A. ZANI. 2007. Ecology of Alopoglossus angulatus and A. atriventris (Squamata, Gymnophthalmidae) in western Amazonia. Phyllomedusa 6 (1): 11-21.
- VITT, L.J.; T.C.S. ÁVILA-PIRES; P.A. ZANI; M.C. ESPÓSITO & S.S. SARTORIUS. 2003. Life at the interface: ecology of Prionodactylus oshaughnessyi in the Amazon and comparisons with P. argulus and P. eigenmanni Canadian Journal of Zoology 81: 302-312.
- ZAR, J.H. 1999. Biostatistical Analysis. Englewood Cliffs, Prentice-Hall, 663p.
Publication Dates
-
Publication in this collection
11 Nov 2011 -
Date of issue
Oct 2011
History
-
Accepted
03 Aug 2011 -
Received
05 May 2011