Abstract
Invasion by marine species, often considered a grave threat to marine ecosystems, occurs throughout the world as a consequence of many anthropogenic activities. In coastal Paraná, many factors including shipping, aquaculture and the use of artificial substrates provide suitable environments for the establishment and rapid spread of introduced marine species. To better understand this process, the encrusting community was studied on polyethylene plates (n = 120, 10 x 10 cm) that were placed seasonally at fixed locations on the inner continental shelf to detect non-native species. Of the 62 taxa found, 40 were identified to species, 14 of which were native, 9 introduced and 17 cryptogenic. We found a new introduction while most introduced species were previously reported at a nearby estuary with an international port. Possible complementary explanations for these detections are 1) estuaries influence ecological processes on the inner continental shelf, 2) the study area is near the route of cargo and other ships entering the port, 3) other local vectors, such as hulls of fishing and recreational boats, and artificial reefs link the estuary to the offshore areas. Thus, not only are estuaries invaded by exotic species, but also non-indigenous marine species may be present in the open sea where they are likely to colonize artificial substrates.
Artificial substrate; biofouling; marine bioinvasion; sublittoral
BIOLOGY
Detection of introduced sessile species on the near shore continental shelf in southern Brazil
Janaína de Araújo BumbeerI; Rosana Moreira da RochaII
ICorresponding author. Programa de Pós-Graduação em Ecologia e Conservação, Universidade Federal do Paraná. Caixa Postal 19031, 81531-980 Curitiba, PR, Brazil. E-mail: janabumbeer@gmail.com
IIDepartamento de Zoologia, Universidade Federal do Paraná. Caixa Postal 19020, 81531-980 Curitiba, PR, Brazil
ABSTRACT
Invasion by marine species, often considered a grave threat to marine ecosystems, occurs throughout the world as a consequence of many anthropogenic activities. In coastal Paraná, many factors including shipping, aquaculture and the use of artificial substrates provide suitable environments for the establishment and rapid spread of introduced marine species. To better understand this process, the encrusting community was studied on polyethylene plates (n = 120, 10 x 10 cm) that were placed seasonally at fixed locations on the inner continental shelf to detect non-native species. Of the 62 taxa found, 40 were identified to species, 14 of which were native, 9 introduced and 17 cryptogenic. We found a new introduction while most introduced species were previously reported at a nearby estuary with an international port. Possible complementary explanations for these detections are 1) estuaries influence ecological processes on the inner continental shelf, 2) the study area is near the route of cargo and other ships entering the port, 3) other local vectors, such as hulls of fishing and recreational boats, and artificial reefs link the estuary to the offshore areas. Thus, not only are estuaries invaded by exotic species, but also non-indigenous marine species may be present in the open sea where they are likely to colonize artificial substrates.
KEY WORDS: Artificial substrate; biofouling; marine bioinvasion; sublittoral.
The southern Brazilian state of Paraná has a relatively short coast (~100 km long). Here, the port of Paranaguá (25º30'S, 48º30'W), one of Brazil's largest international ports, is an important potential source of species introductions (NEVES et al. 2007). Previous studies in Paranaguá Bay (where the port is located) found colonization by invasive, non-native and cryptogenic species in fixed and floating substrates made of concrete, fiberglass, granite and polyethylene (ROCHA & KREMER 2005, NEVES et al. 2007, NEVES & ROCHA 2008, CANGUSSU et al. 2010, ROCHA et al. 2010). This result was somewhat surprising due to the wide variation in salinity of these waters due to riverine input.
An additional potential source of introductions is oyster cultivation at the two main coastal estuaries of Paranaguá and Guaratuba Bays. Seed oysters are regularly exchanged by farmers between locations where they are cultivated (Leandro A. Pereira, pers. comm. 2011) and so are possible local vectors for introductions of the associated fauna (RUIZ et al. 1997). Both bays also have private marinas and small piers, recreational and fishing boats that travel frequently between the estuaries and the open sea. Thus, these may be important as local vectors for introductions (WASSON et al. 2001, MURRAY et al. 2011). In the open sea the inner continental shelf has both recreational boating and intense small-scale fishing (ANDRIGUETTO-FILHO et al. 2006) that may also provide vectors for introduced species.
In addition to natural rocky substrates in the shallow inner continental shelf of Paraná (< 30 m deep), approximately 2,000 units of artificial reefs, made of concrete blocks have been placed between the islands of Currais and Itacolomis (south) and another 1,200 between the islands of Currais and Galheta (north). These "reefs" were first placed in 1997 to avoid shrimp trawling (BRANDINI & SILVA 2011). The region is naturally poor in hard bottom habitats and these artificial reefs greatly increased habitat availability for sessile organisms. In natural environments, two main factors may prevent species' establishment: biological interactions (STACHOWICZ et al. 2002) and the limited availability of substrates (DAYTON 1971). Nevertheless, the increase in man-made structures in marine areas, such as pilings, pontoons and rock walls, provide new substrates for encrusting organisms (CONNELL 2001) and may be the first step in habitat colonization by exotic species (TYRELL & BYERS 2007). Artificial structures have long been known to be colonized (fouled) by non-indigenous marine species (NIS). Indeed, the association with such structures is one criterion for categorizing a species as non-indigenous (CHAPMAN & CARLTON 1991). These artificial substrates may disproportionately favor exotic species by increasing local sources of exotic propagules to colonize all types of substrates (GLASBY et al. 2007, TYRELL & BYERS 2007). For example, abundance of NIS in estuaries was greater than that on the open coast (WASSON et al. 2005). These man-made structures may provide favorable environments for the establishment and rapid spread of introduced marine species in the coastal regions of the state of Paraná.
Marine biological invasions occur worldwide due to a variety of anthropogenic activities and are considered one of the greatest threats to marine ecosystems (RUIZ et al. 1997). Recently, shipping has been identified as the main source of species introductions in coastal estuarine and marine habitats. Ballast water, ballast water sediments, and hull fouling are the main vectors associated with shipping (MOLNAR et al. 2008), creating unprecedented levels of biological exchange. Since the main vectors for marine bioinvasions are ballast water and ship hulls, mobile and always submerged "habitats" might be more easily colonized by introduced species (NEVES et al. 2007), in contrast to intertidal substrates. Substrate stability, whether fixed or mobile, also constitutes an important regulator of communities (CONNELL 2000, GLASBY & CONNELL 2001, HOLLOWAY & CONNELL 2002). The use of similar artificial surfaces in the sublittoral zone is an important tool for detecting established and reproducing exotic species in a region. Temporal variation is also important for colonization, hence seasonal surveys are recommended to improve detectability of introduced species at different moments throughout the year (CAMPBELL et al. 2007, CANGUSSU et al. 2010).
Although estuaries are better studied for the detection of marine introductions (RUIZ et al. 2000), sublittoral invasions are also important, though less visible, around the world (KELLY et al. 2011, CARLTON et al. 2011). Here, we describe the first study whose objective is to detect introduced invertebrates in marine sublittoral open-sea environments but still influenced by international shipping. In Brazil, few previous studies examined the sublittoral for the purpose of detecting NIS, and most, in shallow waters, focused on specific groups, such as barnacles (FARRAPEIRA 2009) and ascidians (MARINS et al. 2010). A more comprehensive survey was carried out at Ilha Grande Bay, in Rio de Janeiro, where artificial and natural substrates and sessile communities were compared at depths between 0.5 and 5 m (IGNACIO et al. 2010). Here, to examine the possible presence and importance of non-indigenous species, we describe the encrusting community on mobile artificial substrates and its seasonal variation on the inner continental shelf in southern Brazil.
MATERIAL AND METHODS
The state of Paraná has a continental shelf ranging between 175 and 190 km wide. The platform is predominantly covered by sand, mud and clay, except for the few natural hard substrates that border the islands of Paraná. The main coastal islands are Figueira, Mel, Galheta, Currais and Itacolomis, from north to south.
The open inner shelf here (Fig. 1) is characterized by a well-defined seasonal hydrographic regime. The water column is warm and stratified between November and March and vertically mixed in the rest of the year due to strong tidal currents and constant winds (BRANDINI et al. 2007). In spring-summer periods, salinity varies from 29 to 35, and water temperature varies from 21 to 28ºC, while during autumn-winter salinity varies from 32 to 35 and water temperature from 20 to 22ºC (BRANDINI et al. 2007). Due to nearness of the coast, the processes of mixture and circulation of the water in the near shore are mainly determined by onshore runoff (BRANDINI et al. 2007). The study area is under the influence of the large estuarine complex of Paranaguá (H"18 km apart) and Guaratuba Bays (~24 km apart), and continental drainage from the bays transports nutrients, particulate material and invertebrate larvae towards the open sea, mainly in spring and summer (BRANDINI et al. 2007).
Each season, recruitment plates were placed in the water at fixed stations near a group of artificial reefs (Parque dos Meros or Grouper Park) at 18 m depth, 12 km off the coast and ~ 2 km from Currais Islands (Fig. 1). Between October 2008 and November 2009, black polyethylene plates (10 x 10 cm) were attached to ropes in a vertical position and submerged for approximately three months at 10 different depths from 8-17 m. A set of four ropes (40 plates) was tied to the artificial reefs blocks on the seafloor and kept in vertical position by a buoy. Due to damage by shrimp trawlers three were recovered in autumn and one in winter. The time interval of submersion of the plates varied between seasons. The structures were deployed on 1 October (spring, 100 days, 40 plates), 15 January (summer, 75 days, 40 plates), 31 March (autumn, 97 days, 30 plates) and 7 July (winter, 119 days, 10 plates).
Upon collection, plates were treated for two hours with a menthol solution in seawater for relaxation of the fauna and subsequently fixed in 4% formalin. Upon taxonomic identification, species were classified as native, introduced or cryptogenic, based on criteria previously described and observed in other regions, such as original and present geographical distribution, exclusive or increased presence on artificial substrates, discontinuous or restricted regional distribution and rapid dispersion in a region (CHAPMAN & CARLTON 1991, CARLTON 1996). Among the vagile fauna only a few gastropods and polychaetes were examined.
RESULTS
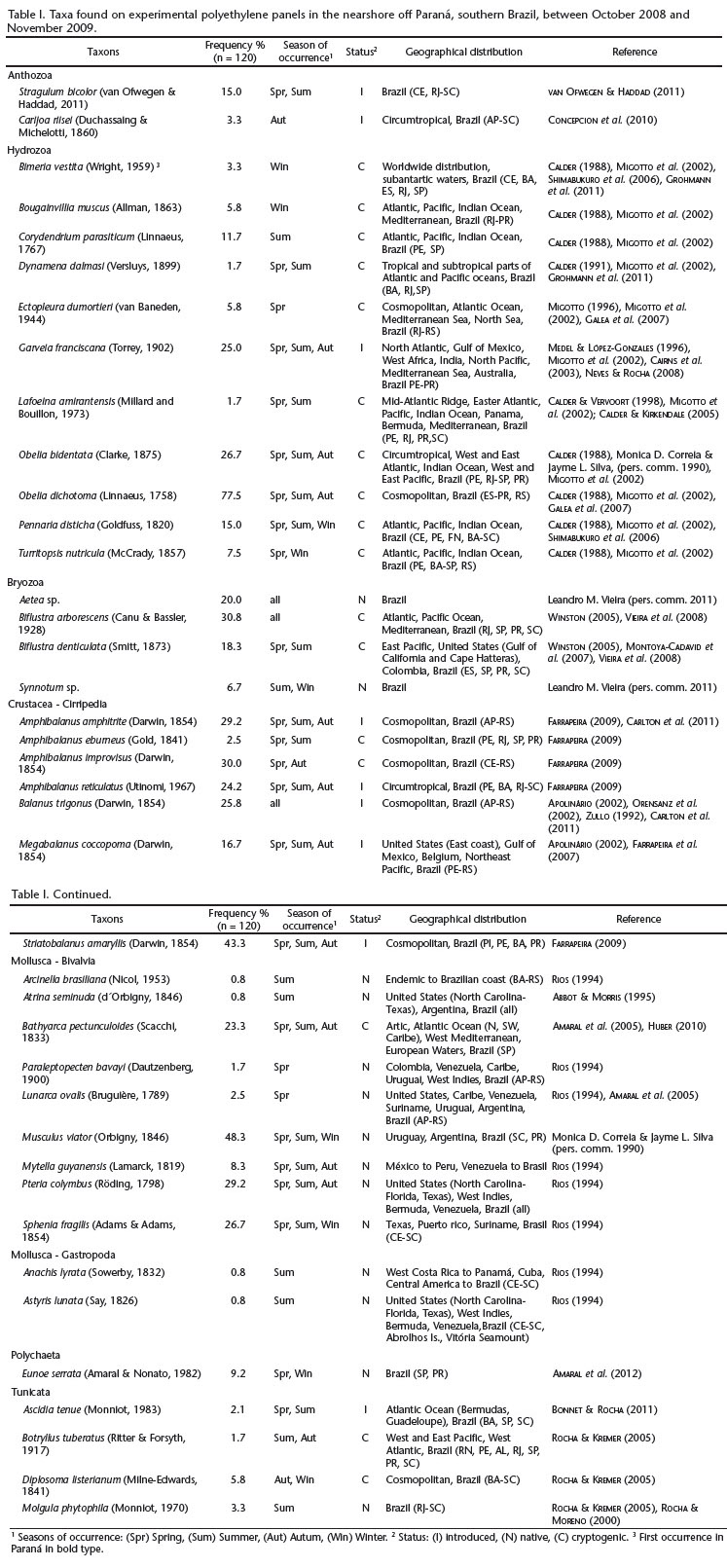
Of the 62 taxa found, 40 were identified to species, 14 of which were native, nine were introduced and 17 were cryptogenic (Tab. I). All species were invertebrates previously reported in Brazil. Macroalgae were not found during the study. The native species were mostly bivalves (Mollusca): Arcinella brasiliana, Atrina seminuda, Paraleptopecten bavayi, Lunarca ovalis, Musculus viator, Mytella guyanensis, Pteria colymbus, Sphenia fragilis; and gastropods: Anachis lyrata, Astyris lunata. Other native species included Molgula phytophila (Ascidiacea), Eunoe serrata (Polychaeta), and the undescribed species Aetea sp. and Synnotum sp. (Bryozoa).
Most of the introduced species have been reported in Paraná, such as the historically introduced octocoral Carijoa riisei, the hydrozoan Garveia franciscana, the barnacles Amphibalanus amphitrite, Amphibalanus reticulatus, Balanus trigonus, Megabalanus coccopoma and Striatobalanus amaryllis. We also found Ascidia tenue which was first recorded in the state of Paraná in BONNET & ROCHA (2011) and has also been found in Santa Catarina, São Paulo, and Bahia (Tab. I). The octocoral Stragulum bicolor belongs to a new, recently reported genus (VAN OFWEGEN & HADDAD 2011, Tab. I).
Most of the species were classified as cryptogenic due to the lack of historical distribution information. Hydrozoans comprised the majority of this category (10 among 11). Several cryptogenic species were found for the first time in Paraná, such as the hydrozoan Turritopsis nutricula and the bivalve Bathyarca pectunculoides as well as the hydrozoans Bimeria vestita, Corydendrium parasiticum and Dynamena dalmasi (Tab. I).
Species composition varied throughout the year, with some species being restricted seasonally while others were not. Eight introduced species occurred during the spring, eight in summer (despite the shorter interval during which panels were in the water), seven in autumn and one in winter. The octocoral C. riisei was restricted to autumn plates, while A. tenue was found during both spring and autumn. Only B. trigonus was found in all seasons. The remaining introduced species were found in all seasons except winter.
The most frequently encountered species was the cryptogenic Obelia dichotoma, found on 78% of the plates, followed by the cryptogenic species, Biflustra arborescens (31%), Amphibalanus improvisus (30%), O. bidentata (27%) and B. pectunculoides (23%). The most frequent native species (> 20%) were M. viator (48%), P. colymbus (29%), S. fragilis (27%) and Aetea sp. (20%), The most common introduced species (> 20% of plates) were S. amaryllis (43%), A. amphitrite (29%), B. trigonus (26%), G. franciscana (25%), and A. reticulatus (24%, Tabl. I). Stragulum bicolor occurred on 15% of the plates, while C. riisei and A. tenue were found on < 5% of the plates.
DISCUSSION
Non-indigenous species are on the inner continental shelf of Paraná and colonize mobile artificial substrates, even though the study area was ~40 km from the port of Paranaguá. Of the species colonizing these plates, 43% were cryptogenic and 23% were introduced. In Paranaguá Bay, the estuarine in which the port is located, 16% of the species were introduced (CANGUSSU et al. 2010). Another comprehensive study found that 16% of species were introduced in Ilha Grande Bay on both artificial and natural substrates (IGNACIO et al. 2010). These proportions of introduced species were greater than those reported in Sepetiba Port in the state of Rio de Janeiro (2%) (CLARKE et al. 2004) and some ports in Australia: Darwin (< 1%), Port Hedland (3%) and Mackey (3%) (HEWITT 2002). The main difference is that the former studies only included sessile communities while the latter included soft sediments, seagrass or macroalgal beds and plankton. Those results suggest that hard substrates may be more easily invaded than other habitats. Introduced species were also among the most common species colonizing the polyethylene plates in our study: of the 14 species occurring in more than 20% of the plates, six were introduced.
The first study of the fouling community in Paranaguá Bay in 1987-88 found 35 species, among which two are now considered historical introductions (the anthozoan C. riisei and the bivalve Perna perna) and two more are recent introductions (the ascidians Diplosoma singulare and Styela plicata) (Monica D. Correia & Jayme L. Silva, pers. comm. 1990). The number of introduced species has continued to rise since that study. Some species probably arrived later as they were first found only after the year 2000: the hydrozoan G. franciscana, the octocoral Stragulum bicolor (VAN OFWEGEN & HADDAD 2011), both found in this study, and the tunicate Sidneioides peregrinus (KREMER et al. 2011). A greater understanding of the historical distribution of the organisms also lead to reclassification, increasing the number of introduced species: the barnacles previously considered cryptogenic A. amphitrite and B. trigonus (CANGUSSU et al. 2010) are now considered introduced here (CARLTON et al. 2011). In contrast, Brazilian records of the bryozoans Aetea anguina and Synnotum aegyptiacum that should be classified as cryptogenic based on their wide geographical distribution, belong to new undescribed species native to Brazil (Leandro M. Vieira, pers. comm. 2011). A better taxonomic resolution of many groups or detailed genetic studies including populations throughout the geographical distribution of the species will be helpful to reveal both introduced and native species where they have been previously considered cryptogenic.
Species classified as cryptogenic in this study comprised mainly hydrozoans and bryozoans, which are usually small cryptic organisms for which certain identification requires experts. In this case, the lack of previous records is usually due to the lack of study rather than being new introductions. Indeed, two of the cryptogenic species in our list are reported for the first time in the state of Paraná (the hydrozoan T. nutricula and the bivalve B. pectunculoides) and three are first records in southern Brazil (the hydrozoans B. vestita, C. parasiticum, and D. dalmasi), indicating either that these species have spread, or they were previously not found due to lack of study.
The recent record of A. tenue in the state of Paraná comes from samples taken in the present study (BONNET & ROCHA 2011) and it was not found in a previous survey on natural substrates in Currais Island (2 km from the experiment) (ROCHA & FARIA 2005, ROCHA & KREMER 2005). Known from Bermuda and Guadeloupe, where it is not very abundant, its occurrence in Brazil is also very spotty, with few individuals found in the states of Bahia, São Paulo, Santa Catarina and Paraná (BONNET & ROCHA 2011). Therefore, this species probably does not suggest a great risk of establishment and invasion of natural communities. The other two ascidians found are also known from Currais Island (ROCHA & FARIA 2005). As in the natural substrates, the cryptogenic Botryllus tuberatus and the native M. phytophila were uncommon on the plates.
The introduced octocoral Stragulum bicolor is a recently described genus and species and has only recently been found in Brazil in shallow waters of the states of Ceará, Rio de Janeiro, São Paulo, Paraná and Santa Catarina (VAN OFWEGEN & HADDAD 2011). The suspected invasive nature of the species has since been supported by past and recent data and many observations collected in benthic environments in coastal Brazil (VAN OFWEGEN & HADDAD 2011). In Paraná S. bicolor was found in Paranaguá Bay either on artificial substrates, such as polyethylene plates and pontoons, as well as natural substrates near Cotinga Island. Also, S. bicolor was found away from the estuaries, on intertidal beach rocks at Itapoá (at the neighbor state of Santa Catarina), nearly 50 km south of Guaratuba Bay (VAN OFWEGEN & HADDAD 2011). The behavior of S. bicolor is that of an invasive species, including rapid colonization on artificial substrates in and near ports, followed by colonization of natural beach rocks along the coast (VAN OFWEGEN & HADDAD 2011). In this study, we often found S. bicolor, in 15% of the plates, during spring and summer. Its frequency on different substrates also suggests recent introduction, as well as a risk of establishment on natural substrates. In contrast, the other octocoral, C. riisei, was introduced in the Atlantic long ago (CONCEPCION et al. 2010) and was present in only 3% of samples and only in autumn. In Paranaguá Bay, C. riisei was first found in 1987 in samples taken from acrylic substrates (Monica D. Correia & Jayme L. Silva, pers. comm. 1990) and, later, on polyethylene plates between 2007-2008 (CANGUSSU et al. 2010). Although not reported in the literature, it also occurs on natural substrates in Currais Island (JAB, unpub. data).
The hydrozoan G. franciscana was first reported in Paranaguá Bay in 2004 (NEVES et al. 2007). In comparisons of submerged concrete and floating structures, G. franciscana may colonize both fixed and floating substrates (ROCHA et al. 2010), but, in either case, the species was very rare. This species was more common on granite plates during warmer periods (CANGUSSU et al. 2010). In this study, it was one of the most common, suggesting that the off shore marine environment favors this hydrozoan.
Barnacles comprised five of the nine introduced species reported. All introduced species may be considered common (>15%) and their occurrence on artificial substrates was expected because barnacles easily colonize different artificial substrates (FARRAPEIRA 2009) and have been extensively introduced to new regions mainly by ship traffic for many years (CARLTON et al. 2011). Amphibalanus amphitrite, A. reticulatus, B. trigonus, A. eburneus and A. improvisus are the most common species reported in shipping records and all have been introduced around the world (CARLTON et al. 2011). The last two are provisionally regarded as cryptogenic in Brazil, but they may be native to the northern Atlantic Ocean and then introduced in the southern Atlantic (CARLTON et al. 2011). Thus, due to the ease of introductions, these species require additional attention, especially the very common (in 30% of our samples) A. improvisus, with the widest range of vectors (CARLTON et al. 2011). Amphibalanus reticulatus has been recently reported in Paraná (NEVES et al. 2007) and, similar to A. amphitrite, is already quite abundant and colonizes many kinds of substrates (CANGUSSU et al. 2010).
Balanus trigonus was first collected in Paranaguá Bay in 1987-88 (Monica D. Correia & Jayme L. Silva, pers. comm. 1990). In a subsequent study using granite and polyethylene plates, it colonized only granite, in low frequencies, restricted to the colder periods (CANGUSSU et al. 2010). In Rio de Janeiro, the species was abundant on natural and even more so on artificial substrates (IGNACIO et al. 2010). In this study, B. trigonus was frequent on the polyethylene plates throughout the year.
The less common introduced barnacle was M. coccopoma, recently found in Paraná colonizing granite and polyethylene surfaces. While less common in Paranaguá Bay, when found, it was mainly on mobile substrates (CANGUSSU et al. 2010, ROCHA et al. 2010). In contrast, in Santa Catarina, it was abundant on polyethylene plates in very shallow water (~1 m), and considered a potential invasion (RMR unpublished data). Elsewhere in Brazil, it is commonly found on exposed rocky shores and on offshore structures (SILVEIRA et al. 2006). We expected M. coccopoma to be more abundant on our plates.
Striatobalanus amaryllis was first reported in northern and northeastern Brazil in 1987 (YOUNG 1989, 1998), and occasionally on artificial substrates in Paranaguá Bay in 2004 and 2007 (NEVES et al. 2007, CANGUSSU et al. 2010). It was the most frequent barnacle in this study (43%) and has been found on natural rocky shores in Paranaguá Bay (CARLTON et al. 2011). Thus, southern range expansion seems to be continuous for this species and requires special attention to its establishment and possible occurrence in southern sites.
It is interesting to note that no native barnacle species was found, which suggests that the artificial substrate favored colonization by non-native barnacles. In contrast, all mollusks were native species, except for the cryptogenic B. pectunculoides that was frequent in the samples (23%).
Most studies of NIS take place in harbors, where many invasive species are expected to be found (WASSON et al. 2005). Today, with the rapid spread of NIS in all marine ecosystems, adjacent open-ocean areas must also be considered to be potentially threatened. In a recent comparison, port and non-port sites in California had very similar NIS and which may be explained by introductions due to local vessels and aquaculture as well as natural dispersal by larvae (COHEN et al. 2005).
The introduced species found in this study are similar to those found in an estuary with an international port (ROCHA & KREMER 2005, NEVES et al. 2007, NEVES & ROCHA 2008, CANGUSSU et al. 2010, ROCHA et al. 2010). These patterns of introduction suggest the following: 1) Ecological processes in the inner shelf off the state of Paraná are influenced by estuaries. Epilithic colonization in the study area is probably a direct consequence of continental drainage, given that it is one of the main processes of larval dispersal. Larval dispersal potential varies among taxonomic groups and very large dispersal distances are common (REED et al. 2000). For example, larvae of the wood boring Teredinidae, typical of mangrove ecosystems, have been found 112 km offshore in Paraná during periods of greater rainfall (Juan Ugaz-Codina, pers. comm. 2003). 2) The study area is near freight ship traffic routes as they enter and leave the port (JAB, pers. obs.). 3) Other local vectors, including fishing and recreational boat hulls, contribute to the regional spread of marine introduced species. Important economic activities include artisanal fishing, aquaculture, and ecological tourism in coastal Paraná. For example, a fleet of of 1,678 fishing boats in Paraná of many kinds of materials, including aluminum, plywood, fiberglass and wood, is working constantly off shore (ANDRIGUETTO-FILHO et al. 2006). In one small part of the region, in the city Pontal do Paraná, there are more than 20 marinas and 1,200 registered recreational boats. These boats travel through both, the estuaries and the open sea, thereby connecting coasts, islands and marinas, increasing the probability that NIS will be transported to an increasing number of habitats (MURRAY et al. 2011). 4) Stationary, artificial, hard substrate reefs may also favor exotic species by increasing local sources of exotic propagules to colonize natural habitats (GLASBY et al. 2007, TYRELL & BYERS 2007, IGNACIO et al. 2010). This possibility is controversial and requires additional study. Although artificial reefs have been built on the shallow continental shelf of Paraná for more than 10 years, no study of the influence of these reefs on NIS has been carried out. The fouling community of concrete modules near Currais Islands was analyzed, but without focusing on introductions (BRANDINI & SILVA 2011). Of the resulting list of species, only Diplosoma listerianum and C. riisei are introduced. That result is incomplete, however, because most taxa were not identified to species. Hence, the artificial reefs of the state of Paraná require particular attention to assess their effect on species introduction.
Here we have shown that, estuaries are not unique in supporting introduced species because NIS may also be common in the open sea where they are likely to colonize mobile substrates. Additional, more extensive study that include natural substrates should be a priority in this region, because of the few records of the benthic fauna of natural rocky substrates in the state of Paraná. It is imperative that we know the encrusting community to monitor the establishment of NIS on natural substrates and possible impacts on the native community.
ACKNOWLEDGMENT
This study was financially supported by Rebimar Program Marbrasil Association (www.marbrasil.org). We are grateful for the support provided by the Graduate program in Ecology and Conservation UFPR, the fellowship from CAPES to JAB, and a research grant from CNPq to RMR. Thanks to James J. Roper for his critical review of the English and to two anonymous reviewers who contributed to improve the quality of the manuscript. We also thank Ana Caroline Cabral (Hydrozoa), Ana Paula Raveduti Rigo (Cirripedia), Halina Linzmeier Heyse (Bryozoa), Heliatrice Hadlich (Polychaeta), Joseane Marques (Bivalvia), Kalina Brauko (Polychaeta), Luciana Altvater (Anthozoa), Nadia Yukiji Koto Bonnet (Tunicata), and Verônica Oliveira (Polychaeta) who helped with the identification of many species.
LITERATURA CITED
Submitted: 23.I.2012; Accepted: 01.III.2012.
Editorial responsibility: Paulo da Cunha Lana
- ABBOT, R.T. & P.A. MORRIS. 1995. A field guide to shells of Atlantic and gulf coasts and the West Indies. New York, Peterson Field Guides, 355p.
- AMARAL, A.C.Z.; A.E. RIZZO & E.P. ARRUDA. 2005. Manual de identificação dos invertebrados marinhos da região Sudeste-Sul do Brasil. São Paulo, Editora da Universidade de São Paulo, 287p.
- AMARAL, A.C.Z.; S.A.H. NALLIN & T.M. STEINER; T.O. FORRONI & D. GOMES-FILHO. 2012. Catálogo das espécies dos Annelida Polychaeta do Brasil. Available online at: http://www.ib.unicamp.br/museu_zoologia/files/lab_museu_zoologia/ Catalogo_Polychaeta_Amaral_et_al_2012_0.pdf [Accessed: 24/IV/2012]
- ANDRIGUETTO-FILHO J.M.; P.T. CHAVES; C. SANTOS & S.A. LIBERATI. 2006. Diagnóstico da pesca no litoral do estado do Paraná, p. 117-140. In: V.J. ISAAC; A.S. MARTINS; M. HAIMOVIC & J.M. ANDRIGUETTO-FILHO (Eds). A pesca marinha e estuarina do Brasil no início do século XXI: recursos, tecnologias, aspectos socioeconômicos e institucionais. Belém, Editoria Universitária da UFPA, 186p.
- APOLINÁRIO, M. 2002. Cracas invasoras no litoral brasileiro. Ciência Hoje 188: 44-48.
- BONNET, N.Y.K. & R.M. ROCHA. 2011. The Ascidiidae (Ascidiacea: Tunicata) of coastal Brazil. Zoological Studies 50 (6): 809-825.
- BRANDINI, F.P. & A.S. SILVA. 2011. Epilithic community development on artificial reefs deployed along a cross-shelf environmental gradient off Parana State, Southern Brazil. Brazilian Journal of Oceanography 59: 43-53.
- BRANDINI, F.P.; A.S. SILVA; E.T. SILVA & H. KOLM. 2007. Sources of nutrients and seasonal dynamics of chlorophyll in the inner shelf off Parana State South Brazil Bight. Journal of Coastal Research 23: 200-226.
- CAIRNS, S.D.; D.R. CALDER; A. BRINCKMANN-VOSS; C.B. CASTRO; D.G. FAUTIN & P.R. PUGH. 2003. Common and Scientific Names of Aquatic Invertebrates from the United States and Canada: Cnidaria and Ctenophore. Bethesda, American Fisheries Society Special Publication, XI+115p.
- CALDER, D.R. 1988. Shallow-water hydroid of Bermuda: The Athecate. Life Sciences Contribution Royal Ontario Museum 148: 1-107.
- CALDER, D.R. 1991. Shallow-water hydroids of Bermuda: The Thecatae, exclusive of Plumularioidea. Life Sciences Contribution Royal Ontario Museum 154: 1-140.
- CALDER, D.R. & L. KIRKENDALE. 2005. Hydroids (Cnidaria, Hydrozoa) from shallow-water environments along the Caribbean Coast of Panama. Caribbean Journal of Science 41 (3): 476-491.
- CALDER, D.R. & W. VERVOORT. 1998. Some hydroids (Cnidaria: Hydrozoa) from the Mid-Atlantic Ridge, in the North Atlantic Ocean. Zoologische verhandelingen 319: 1-65.
- CAMPBELL, M.L.; B. GOULD & C.L. HEWITT. 2007. Survey evaluations to assess marine bioinvasions. Marine Pollution Bulletin 55: 360-378.
- CANGUSSU, L.C.; L. ALTVATER; M.A. HADDAD; A.C. CABRAL; H.L. HEISE & R.M. ROCHA. 2010. Substrate type as a selective tool against colonization by non-native sessile invertebrates. Brazilian Journal of Oceanography 58 (3): 219-231.
- CARLTON, J.T. 1996. Biological invasions and cryptogenic species. Ecology 77: 1653-1655.
- CARLTON, J.T.; W.A. NEWMAN & F.B. PITOMBO. 2011. Barnacle invasions: Introduced, cryptogenic, and range expanding Cirripedia of North and South America, p. 159-214. In: B.A. GALIL; P.F. CLARK & J.T. CARLTON (Eds). In the Wrong Place Alien Marine Crustaceans: Distribution, Biology and Impacts. Dordrecht, Springer Series in Invasion Ecology, XVI+716p.
- CHAPMAN, J.W. & J.T. CARLTON. 1991. A test of criteria for introduced species: The global invasion by the isopod Synidotea laevidorsalis (Miers, 1881). Journal of Crustacean Biology 11: 386-400.
- CLARKE, C.; R. HILLIARD; A.O.R. JUNQUEIRA; J. POLGLAZE & S.RAAYMAKERS. 2004. Ballast Water Risk Assessment, Port of Sepetiba, Federal Republic of Brazil, December 2003. London, IMO Globallast, Monographs Series 14, 63p.
- COHEN, A.N.; L.H. HARRIS; B.L. BINGHAM; J.T. CARLTON; J.W. CHAPMAN; C.C. LAMBERT; G. LAMBERT; J.C. LJUBENKOV; S.N. MURRAY; K. REARDON & E. SCHWINDT. 2005. Rapid assessment survey for exotic organisms in southern California bays and harbors, and abundance in port and non-port areas. Biological Invasions 7: 995-1002.
- CONCEPCION, G.T.; S.E. KAHNG; M.W. CREPEAU; E.C. FRANKLIN; S.L. COLES & R.J. TOONEN. 2010. Resolving natural ranges and marine invasions in a globally distributed octocoral (genus Carijoa). Marine Ecology Progress Series 401: 113-127.
- CONNELL, S.D. 2000. Floating pontoons create novel habitats for subtidal epibiota. Journal of Experimental Marine Biology and Ecology 247: 183-194.
- CONNELL, S.D. 2001. Urban structures as marine habitats: an experimental comparison of the composition and abundance of subtidal epibiota among pilings, pontoons and rocky reefs. Marine Environmental Research 52: 115-125.
- DAYTON, P. K. 1971. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecological Monographs 41: 351-389.
- FARRAPEIRA, C.M.R. 2009. Barnacles (Crustacea: Cirripedia) of the estuarine and marine areas of the port of Recife (Pernambuco, Brazil). Cahiers de Biologie Marine 50: 119-129.
- FARRAPEIRA, C.M.R.; A.V.O.M. MELO; D.F. BARBOSA & K.M.E. SILVA. 2007. Ship hull fouling in the Port of Recife, Pernambuco. Brazilian Journal of Oceanography 55 (3): 207-221.
- GALEA, H.R.; V. HÄUSSERMANN & G. FÖRSTERRA. 2007. Cnidaria, Hydrozoa: latitudinal distribution of hydroids along the fjords region of southern Chile, with notes on the world distribution of some species. Check List 3 (4): 308-320.
- GLASBY, T.M. & S.D. CONNELL. 2001. Orientation and position of substratum have large effects on epibiotic assemblages. Marine Ecology Progress Series 214: 127-135.
- GLASBY, T.M.; S.D. CONNELL; M.G. HOLLOWAY & C.L. HEWITT. 2007. Nonindigenous biota on artificial structures: could habitat creation facilitate biological invasions? Marine Biology 151: 887-895.
- GROHMANN, P.A.; C.C. NOGUEIRA & V.M.A.P. SILVA. 2011. Hidróides (Cnidaria, Hydrozoa) coletados na plataforma continental interna do estado do Rio de Janeiro, Brasil, durante as Operações Oceanográficas GEOCOSTA RIO I e II. Biota Neotropica 11 (3): 193-201.
- HEWITT, C.L. 2002. Distribution and biodiversity of Australian tropical marine invasions. Pacific Science 56: 213-222.
- HOLLOWAY, M.G. & S.D. CONNELL. 2002. Why do floating structures create novel habitats for subtidal epibiota? Marine Ecology Progress Series 235: 43-52.
- HUBER, M. 2010. Compendium of Bivalves. A Full-color Guide to 3'300 of the World's Marine Bivalves. A Status on Bivalvia after 250 Years of Research. Hackenhein, Conchbooks, 901p.
- IGNACIO, B.L.; L.M. JULIO; A.O.R. JUNQUEIRA & M.A.G. FERREIRA-SILVA. 2010. Bioinvasion in a Brazilian Bay: filling gaps in the knowledge of Southwestern Atlantic biota. PLoS ONE 5 (9): 1-9.
- KELLY, J.R.; R.R. SCHEIBLING & T. BALCH. 2011. Invasion-mediated shifts in the macrobenthic assemblage of a rocky subtidal ecosystem. Marine Ecology Progress Series 437: 69-78.
- KREMER, L.P.; R. METRI & R.M. ROCHA. 2011. Description of Sidneioides peregrinus sp. nov. (Tunicata: Ascidiacea: Polyclinidae): a possible exotic species in the Atlantic Ocean. Zoologia 28 (6): 784-788.
- MARINS, F.O.; R.L.M. NOVAES; R.M. ROCHA & A.O.R. JUNQUEIRA. 2010. Zoologia 27 (2): 213-221.
- MEDEL, M.D. & P.J. LÓPEZ-GONZÁLEZ. 1996. Updated catalogue of hydrozoans of the Iberian Peninsula and Balearic Islands, with remarks on zoogeography and affinities. Scientia Marina 60 (1): 183-209.
- MIGOTTO, A.E. 1996. Benthic shallow-water hydroids (Cnidaria, Hydrozoa) of the coast of São Sebastião, Brazil, including a checklist of Brazilian hydroids. Zoologische Verhandelingen 306: 1-125.
- MIGOTTO, A.E.; A.C. MARQUES; A.C. MORANDINI & F.L. SILVEIRA. 2002. Checklist of the Cnidaria Medusozoa of Brazil. Biota Neotropica 2: 1-31.
- MOLNAR, J.L.; R.L. GAMBOA; C. REVENGA & M.D. SPALDING. 2008. Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment 6 (9): 485-492.
- MONTOYA-CADAVID, E.; P. FLÓREZ-ROMERO & J.E. WINSTON. 2007. Checklist of the marine Bryozoa of the Colombian Caribbean. Biota Colombiana 8 (2): 159-184.
- MURRAY, C.C.; E.A. PAKHOMOV & T.W. THERRIAULT. 2011. Recreational boating: a large unregulated vector transporting marine invasive species. Diversity and Distributions 17: 1161-1172.
- NEVES, C.S. & R.M. ROCHA. 2008. Introduced and cryptogenic species and their management in Paranaguá Bay, Brazil. Brazilian Archives of Biology and Technology 51 (3): 623-633.
- NEVES, C.S.; R.M. ROCHA; F.B. PITOMBO & J.J. ROPER. 2007. Use of artificial substrata by introduced and cryptogenic marine species in Paranaguá Bay, southern Brazil. Biofouling 23 (5): 319-330.
- ORENSANZ, J.M.L.; E. SCHWINDT; G. PASTORINO; A. BORTOLUS; G. CASAS; G. DARRIGRAN; R. ELÍAS; J.J.L. GAPPA; S. OBENAT; M. PASCUAL; P. PENCHASZADEH; M.L. PIRIZ; F. SCARABINO; E.D. SPIVAK & E.A. VALLARINO. 2002. No longer pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biological Invasions 4: 115-143.
- REED, D.C.; P.T. RAIMONDI; M.H. CARR & L. GOLDWASSER. 2000. The role of dispersal and disturbance in determining spatial heterogeneity in sedentary organisms. Ecology 81 (7): 20112026.
- RIOS, E.C. 1994. Seashells of Brazil. Rio Grande, Fundação Universidade de Rio Grande, Museu Oceanográfico, 550p.
- ROCHA, R.M. & S.B. FARIA. 2005. Ascidians at Currais islands, Paraná, Brazil: taxonomy and distribution. Biota Neotropica 5 (2): 1-20.
- ROCHA, R.M. & L.P. KREMER. 2005. Introduced ascidians in Paranaguá Bay, Paraná, southern Brazil. Revista Brasileira de Zoologia 22 (4): 1170-1184.
- ROCHA, R.M. & T.R. MORENO. 2000. Ascidians associated with Eudistoma carolinense Van Name, 1945 with description of a new species of Polycarpa Ophelia 52: 9-16.
- ROCHA, R.M.; L.C. CANGUSSU & M.P. BRAGA. 2010. Stationary substrates facilitate bioinvasion in Paranaguá Bay in Southern Brazil. Brazilian Journal of Oceanography 58: 219-231.
- RUIZ, G.M.; J.T. CARLTON; E.D. GROSHOLZ & A.H. HINES. 1997. Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. American Zoologist 37: 621-632.
- RUIZ, G.M.; P.W. FOFONOFF; J.T. CARLTON; M.J. WONHAM & A.H. HINES. 2000. Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annual Review of Ecology and Systematics 31: 481-531.
- SHIMABUKURO, V.; A.C. MARQUES & A.E. MIGOTTO. 2006. Fauna de hidrozoários atecados (Hydrozoa, Anthoathecata) da costa do Estado do Ceará, Brasil. Biota Neotropica 6 (3): 1-13.
- SILVEIRA, N.G.; R.C.C.L. SOUZA; F. FERNANDES & E.P. SILVA. 2006. Occurrence of Perna perna, Modiolus carvalhoi (Mollusca, Bivalvia, Mytilidae) and Megabalanus coccopoma (Crustacea, Cirripedia) off Areia Branca, Rio Grande do Norte State, Brazil. Biociências 14: 89-90.
- STACHOWICZ, J.J.; H. FRIED; R.W. OSMAN & R.B. WHITLACH. 2002. Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology 83 (9): 2575-2590.
- TYRELL, M.C. & J.E. BYERS. 2007. Do artificial substrates favor non-indigenous fouling species over native species? Journal of Experimental Marine Biology and Ecology 342: 54-60.
- VAN OFWEGEN, L.P. & M.A. HADDAD. 2011. A probably invasive new genus and new species of soft coral (Octocorallia: Alcyonacea: Clavulariidae) from Brazil. Zootaxa 3107: 38-46.
- VIEIRA, L.M.; A.E. MIGOTTO & J.E. WINSTON. 2008. Synopsis and annotated checklist of recent marine Bryozoa from Brazil. Zootaxa 1810: 1-39.
- WASSON, K.; K. FENN & J.S. PEARSE. 2005. Habitat differences in marine invasions of central California. Biological Invasions 7: 935-948.
- WASSON, K.; C.J. ZABINC; L. BEDINGER; M. C. DIAZ & J. S. PEARSE. 2001. Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biological Conservation 102: 143-153.
- WINSTON, J.E. 2005. Re-description and revision of Smitt's "Floridan Bryozoa" in the collection of the Museum of Comparative Zoology, Harvard University; with an annotated catalogue of specimens collected by L.F. de Pourtalès in Florida and Carolina, housed at the Museum of Comparative Zoology. Martinsville, Virginia Museum of Natural History Memoir, 148p.
- YOUNG, P.S. 1989. Establishment of an Indo-Pacific barnacle in Brazil. Crustaceana 56: 12-214.
- YOUNG, P.S. 1998. Maxillopoda. Thecostraca, p. 263-285. In: P.S. YOUNG (Ed.). Catalogue of Crustacea from Brazil. Rio de Janeiro, Série Livros, XVII+717p.
- ZULLO, V.A. 1992. Balanus trigonus Darwin (Cirripedia, Balaninae) in the Atlantic basin: an introduced species? Bulletin of Marine Science 50: 66-74.
Publication Dates
-
Publication in this collection
07 May 2012 -
Date of issue
Apr 2012
History
-
Received
23 Jan 2012 -
Accepted
01 Mar 2012