Abstract
Trawl fisheries are associated with catches of swimming crabs, which are an important economic resource for commercial as well for small-scale fisheries. This study evaluated the population biology and distribution of the swimming crab Callinectes ornatus (Ordway, 1863) in the Estuary-Bay of São Vicente, state of São Paulo, Brazil. Crabs were collected from a shrimp fishing boat equipped with a semi-balloon otter-trawl net, on eight transects (four in the estuary and four in the bay) from March 2007 through February 2008. Specimens caught were identified, sexed and measured. Samples of bottom water were collected and the temperature and salinity measured. A total of 618 crabs were captured (332 males, 267 females and 19 ovigerous females), with a sex ratio close to 1:1. A large number of juveniles were captured (77.67%). Crab spatial distributions were positively correlated with salinity (Rs = 0.73, p = 0.0395) and temperature (Rs = 0.71, p = 0.0092). Two peaks of recruitment occurred, in summer and autumn, and ovigerous females were mostly captured during summer, showing a seasonal reproductive pattern. The results showed that C. ornatus uses the bay as a nursery area for juvenile development. Callinectes ornatus is not yet a legally protected species, and the minimum allowed size of crabs caught in the area, although already restricted, should be carefully evaluated since the removal of large numbers of juveniles could negatively impact the local population.
Migration; population biology; seasonal distribution; swimming crab
BIOLOGY
Population biology and distribution of the portunid crab Callinectes ornatus (Decapoda: Brachyura) in an estuary-bay complex of southern Brazil
Timoteo T. WatanabeI,III; Bruno S. Sant'AnnaI; Gustavo Y. HattoriI; Fernando J. ZaraII
IPrograma de Pós-graduação em Ciência e Tecnologia para Recursos Amazônicos, Instituto de Ciências Exatas e Tecnologia, Universidade Federal do Amazonas. 69103-128 Itacoatiara, AM, Brazil
IIInvertebrate Morphology Laboratory, Universidade Estadual Paulista, CAUNESP and IEAMar, Departamento de Biologia Aplicada, Campus de Jaboticabal, 14884-900 Jaboticabal, SP, Brazil
IIICorresponding author. E-mail: timoteotw@gmail.com
ABSTRACT
Trawl fisheries are associated with catches of swimming crabs, which are an important economic resource for commercial as well for small-scale fisheries. This study evaluated the population biology and distribution of the swimming crab Callinectes ornatus (Ordway, 1863) in the Estuary-Bay of São Vicente, state of São Paulo, Brazil. Crabs were collected from a shrimp fishing boat equipped with a semi-balloon otter-trawl net, on eight transects (four in the estuary and four in the bay) from March 2007 through February 2008. Specimens caught were identified, sexed and measured. Samples of bottom water were collected and the temperature and salinity measured. A total of 618 crabs were captured (332 males, 267 females and 19 ovigerous females), with a sex ratio close to 1:1. A large number of juveniles were captured (77.67%). Crab spatial distributions were positively correlated with salinity (Rs = 0.73, p = 0.0395) and temperature (Rs = 0.71, p = 0.0092). Two peaks of recruitment occurred, in summer and autumn, and ovigerous females were mostly captured during summer, showing a seasonal reproductive pattern. The results showed that C. ornatus uses the bay as a nursery area for juvenile development. Callinectes ornatus is not yet a legally protected species, and the minimum allowed size of crabs caught in the area, although already restricted, should be carefully evaluated since the removal of large numbers of juveniles could negatively impact the local population.
Keywords: Migration; population biology; seasonal distribution; swimming crab.
Species of Callinectes (Stimpson, 1860) are extensively investigated around the world (Turner et al. 2003, Fischer & Wolff 2006, Kennedy & Cronin 2007). These crabs are important as a valuable fishery resource (Hines et al. 1995, Secor et al. 2002), indicator of heavy metals (Sastre et al. 1999, Skaggs & Henry, 2002, Andrade et al. 2011) and ecologically (Branco et al. 2002, Lipcius & Stockhausen 2002).
In Brazil, most crustacean fisheries target shrimps, and crabs caught in the trawls have secondary economic importance (Costa & Negreiros-Fransozo 1998) or are mostly consumed by small fishing communities (Sforza et al. 2010). The most economically important crab worldwide is Callinectes sapidus (Rathbun, 1896), but Callinectes danae (Smith, 1869) and Callinectes ornatus (Ordway, 1863) are also caught on the Brazilian coast, which has motivated scientific studies (Santos & Bueno 2002, Keunecke et al. 2009, Sant'Anna et al. 2012a). In the Brazilian trawl fishery for the shrimp Xiphopenaeus kroyeri (Heller, 1862), C. ornatus is one of the most abundant brachyurans discarded in the bycatch (Carvalho et al. 2011).
Because of the economic importance of C. ornatus, several studies have examined its reproduction (Mantellato & Fransozo 1997, 1999, Carvalho et al. 2011, Keunecke et al. 2012,), feeding habits (Mantellato & Christofoletti 2001, Reigada & Negreiros-Fransozo 2001, Branco et al. 2002, Mantellato et al. 2002), growth (Haefner 1990, Keunecke et al. 2008), population structure (Buchanan & Stoner 1988, Branco & Lunardon-Branco 1993, Negreiros-Fransozo et al.1999, Branco & Fracasso 2004, Guerra-Castro et al. 2007, Tudesco et al. 2012) and physiology (Norse 1978, Freire et al. 2011, Garçon et al. 2013). However, no study has assessed its distribution in estuaries as well as along the coast, and these areas are important for its biology and life habits (Melo 1996). In addition, the population structure and other biological aspects such as distribution are less well understood, particularly in Santos-São Vicente Bay, the largest Latin American port (Zangrande et al. 2003, Sant'Anna et al. 2012b).
Callinectes ornatus is widely distributed in the western Atlantic, usually on sand or mud bottoms near rivers, and usually in brackish waters (Melo 1996). Most studies on C. ornatus were conducted along the coast with rivers nearby, but the distribution of this species on the coast as well as in the estuaries was not investigated. This study analyzed the population biology and the seasonal and spatial distribution in the Estuary-Bay Complex of São Vicente, São Paulo, Brazil.
MATERIAL AND METHODS
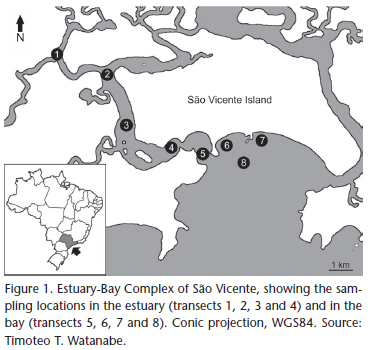
The swimming crabs were collected monthly in the São Vicente Estuary-Bay Complex, state of São Paulo, Brazil, from March 2007 through February 2008. A shrimp fishing boat equipped with an otter-trawl net 4.0 m wide, 2.0 m high and 9.5 long with a 15 mm-mesh body and 10 mm-mesh cod liner was used to capture the crabs during a 20-min sampling on eight transects, four in the estuary and four in the bay (Fig. 1).
On each transect, after the trawl, the bottom water was collected with a Nansen bottle and the temperature (°C) and salinity () were measured.
The crabs were sorted and identified according to Melo (1996), the sex was determined by inspection of abdominal morphology, and the sexual maturity was determined through inspection of the abdomen: adhered for juveniles or loose for adults. The carapace width (CW, measured between the bases of the last and penultimate lateral spines) was recorded using a caliper (0.01 mm). For the size-frequency distribution analysis, size-class intervals of 6 mm of carapace width were used. The size intervals for each class were determined according to the mathematical formula of Sturges (1926). The KolmogorovSmirnov (KS) was used to test the normality of the sizefrequency distribution for each population category (sex). The size was compared between the sexes with the KruskalWallis test. The monthly and overall sex ratios (M:F) were tested with the Chi-square test (x2) and the data for abiotic factors (temperature and salinity) and number of individuals were analyzed by the Spearman's rank correlation coefficient. A significance level of p < 0.05 was adopted for all statistical analyses (Sokal & Rohlf 1995). The reproductive period was determined by the monthly incidence of ovigerous females during the course of the sampling period.
RESULTS
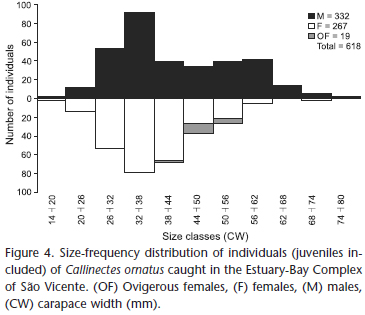
A total of 618 crabs (332 males, 267 females and 19 ovigerous females) were caught; juvenile crabs comprised 77.67% (480 individuals) of this total. Most of the crabs were caught in the bay (84.30%, 521 individuals) (Table I).
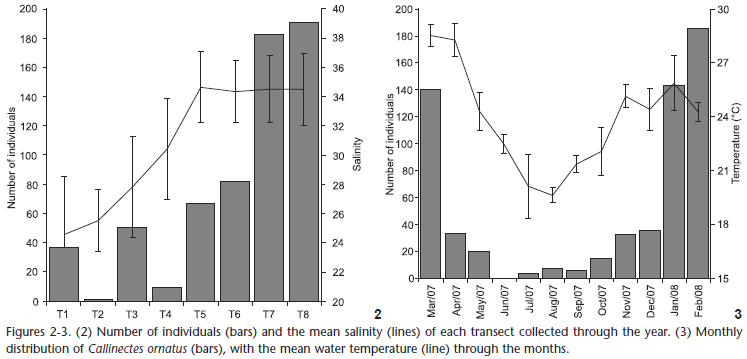
The spatial distribution of the number of individuals (Fig. 2) was positively correlated with salinity (Table II); most individuals occurred in locations with higher salinity.
There was also a significant positive correlation with temperature, where more crabs were found in the warmer months (Table II, Fig. 3).
The high proportion of juveniles decreased the overall mean size, and therefore the ovigerous females were significantly larger than males and non-ovigerous females (Tables III and IV).
The overall size-frequency distribution of C. ornatus (Fig. 4) showed a bimodal distribution for males and a unimodal distribution for females, with the distribution differing from normality for males (KS = 0.1306, p < 0.01), and normal distributions for females and ovigerous females (KS = 0.0701, p = 0.08; KS = 0.1587, p = 0.30, respectively).
The seasonal size-frequency distribution indicated a relationship to the seasons (Fig. 5), with crabs present in larger numbers in the summer and autumn.
The sex ratio of the C. ornatus population did not differ significantly from the expected 1:1 (x2 = 3.576, p = 0.06). This pattern was the same in all months, except in March 2007, when males were more abundant than females (Table V).
Ovigerous females were recorded in higher percentages in the warmer seasons, probably related to seasonal reproduction (Fig. 6).
DISCUSSION
Callinectes ornatus is more euryhaline than other seawater portunids such as Arenaeus cribrarius (Lamarck, 1818) or species of Portunus (Weber, 1795), but less euryhaline than other members of Callinectes such as C. sapidus, C. bocourti or C. danae (Norse 1978). However, this may not in itself explain the low number of adults of C. ornatus in the bay area, where salinities are higher. This species is usually smaller than C. sapidus or C. danae (Buchanan & Stoner 1988), and the large number of adults of C. danae in the region (Sant'Anna et al. 2012a) may lead to agonistic relationships with C. ornatus. Norse (1978) found that C. ornatus living in areas without other, larger species of Callinectes are more tolerant to hyposaline conditions than are individuals found in the presence of other large species of the same genus, although the salinity stress alters the metabolisms of C. ornatus and C. danae metabolisms similarly (Freire et al. 2011). Larger blue crabs behave aggressively toward conspecifics (Clark et al. 1999, Reichmuth et al. 2011), and might also be aggressive toward other species. We speculate that the presence of the other, larger Callinectes species such as C. danae (Sant'Anna et al. 2012a) in the estuary-bay complex may influence the distribution of C. ornatus, combined with the euryhalinity characteristic of the species. Further studies on competition for resources such as territory use or food are needed to test this hypothesis. However, one clue supporting this hypothesis is that the distribution and salinity were positively correlated, indicating that competition with other species could also explain the low number of the adults in both the estuary and bay. For instance, Pinheiro et al. (1997) found that different swimming crabs can show niche overlap, but that the number of individuals of C. ornatus was lower when in the presence of greater numbers of larger crabs such as C. danae or A. cribrarius.
The high proportion of juveniles caught indicates that this species uses the bay for the early part of its life cycle. Our data, which revealed the presence of large numbers of juveniles, mostly in the bay (Table I), indicate that C. ornatus uses the bay as a nursery for the development of the juveniles. These data differ from the findings of Tudesco et al. (2012), of lower numbers of juveniles on the coast near the estuary of Guanabara Bay, Rio de Janeiro, Brazil compared to the number of adults; although the presence of C. ornatus in the estuary was not checked. However, Baptista et al. (2003) also found large numbers of juveniles during the summer near Paranaguá Bay in southern Brazil, outside the estuary, similarly to our data. Another factor that could explain the high proportion of juveniles is the calmer water in the bay; since the juveniles of Callinectes spp. usually prefer calm, shallow waters with more organic matter (Negreiros-Fransozo & Fransozo 1995, Tudesco et al. 2012), it is possible that the conditions in the bay also favor the presence of juveniles. During the larval period, some crustaceans do not have a large capacity for osmoregulation and cannot tolerate sudden changes of salinity, which explains why the juveniles use these areas (Hartnoll 1982).
Species of Callinectes have low tolerance for extreme temperature changes (Leffler 1972, Rome et al. 2005), and C. ornatus is usually found in tropical seas ranging from 18 to 31°C (Williams 1984). The low temperatures during colder seasons in São Vicente Bay could explain the low occurrence of C. ornatus during the study period. The migration pattern of this population showed that the swimming crabs were more abundant during warmer periods; according to Colton et al. (2014), winter temperatures could influence the number of individuals and impact the fluctuation and dynamic synchronization of a population of the blue crab C. sapidus. Some species of Callinectes migrate, which is more evident when correlating their presence with the reproductive period (Millikin & Williams 1984, Hines et al. 1987, Sant'Anna et al. 2012).
During the sampling period, there were high numbers of juveniles during the summer and autumn months, indicating a possible seasonal recruitment. Smaller recruitment peaks were not observed in the other seasons, although C. ornatus has multiple and continuous spawning with peaks in the autumn at Ubatuba (Mantellato & Fransozo 1997), and reproduction was more active during autumn and summer, similar to the present study. The differences between our study and others could be explained by the specific conditions in the different locations, the environment (Schaffner & Diaz 1988, Fernandes et al. 2006), or the differences in sampling effort in each study.
Few crustacean species maintain a sex ratio of 1:1 (Wenner 1972), and C. ornatus shows a segregated distribution according to the local environmental features (Negreiros-Fransozo et al. 1999, Tudesco et al. 2012). Thus, males and females showed different population concentrations as well as varying ratios in different size classes; this is also true for C. ornatus, where the habitats of adults and juveniles differ (Negreiros-Fransozo et al. 1999). In the present study, the data suggest that the São Vicente estuary-bay complex is the main habitat of juveniles, which explains the deviation toward lower individual sizes as well as the trend toward a bimodal distribution for males. The high proportion of juveniles helps to explain the 1:1 sex ratio, since most of the population was sexually immature, and females do not migrate as described for other Callinectes species (Aguilar et al. 2005, Sant'Anna et al. 2012a).
Usually, portunids reproduce throughout the year with peaks in certain seasons, generally winter and summer (Pita et al. 1985, Baptista-Metri et al. 2005). The local population of C. ornatus showed a peak of reproduction only in summer, with the presence of ovigerous females indicating a seasonal rather than a continuous reproduction pattern. Ovigerous females of some Callinectes species prefer higher-salinity waters, which could help the larvae to float and maintain osmotic pressure (Pita et al. 1985, Mantellato 2000). This could explain the low number of females in the study area, which receives a large freshwater inflow from the estuary.
The results of this study revealed that juveniles of C. ornatus use the bay area intensively; and also indicated a regional plasticity in reproduction, since C. ornatus shows different reproductive patterns along the Brazilian southern coast. Although the minimum catch size for C. sapidus and C. danae is regulated (Brazil 1983), the minimum catch size for C. ornatus is not. Despite the similarities in external morphology of these species, C. ornatus is not yet legally protected. The management of C. ornatus should be carefully evaluated, since it is also an economically important species, and trawling in the area, although highly restricted (IBAMA 1994), could still affect the population since most of the individuals were juveniles and this is likely to be an important nursery ground for their development.
ACKNOWLEDGMENTS
FJZ thanks the São Paulo Research Foundation, FAPESP (grants JP#2005/04707-5; Biota #2010/50188-8). The authors thank the Coordination for the Improvement of Higher-Level Personnel (CAPES) (TTW) for scholarship grants, and the National Counsel of Technological and Scientific Development (CNPq) #486337/2013-8 and CIMAR #23038.004309/2014-51(FJZ) and Alvaro Reigada for help during the field collections and laboratory analysis. We also thank Janet W. Reid (JWR Associates) for revising the English text. This research was conducted according to Brazilian laws (MMA-SISBIO, FJZ permanent license #34587-1).
LITERATURE CITED
Submitted: 24.XI.2013
Accepted: 04.VI.2014
- Aguilar, R.; A.H. Hines; T.G. Wolcott; D.L. Wolcott; M.A. Kramer & R.N. Lipcius. 2005. The timing and route of movement and migration of post-copulatory female blue crabs, Callinectes sapidus Rathbun, from the upper Chesapeake Bay. Journal of Experimental Marine Biology and Ecology 319: 117-128. doi: 10.1016/j.jembe.2004.08.030
- Andrade, S.F.; T.B. Matos & C.E.V. Carvalho. 2011. Seasonal variation of heavy metals in muscles of the crab Callinectes ornatus (Ordway, 1863) from a tropical coastal lagoon, Brazil. Revista Virtual de Química 3 (2): 129-137.
- Baptista, C.; M.A.A. Pinheiro; A. Blankensteyn & C.A. Borzone. 2003. Estrutura populacional de Callinectes ornatus Ordway (Crustacea, Portunidae) no Balneário Shangri-Lá, Pontal do Paraná, Paraná, Brasil. Revista Brasileira de Zoologia 20 (4): 661-666. doi: 10.1590/S0101-81752003000400018
- Baptista-Metri, C.; M.A.A. Pinheiro; A. Blankensteyn & C.A. Borzone. 2005. Biologia populacional e reprodutiva de Callinectes danae Smith (Crustacea, Portunidae), no Balneário Shangri-Lá, Pontal do Paraná, Paraná, Brasil. Revista Brasileira de Zoologia 22 (2): 446-453. doi: 10.1590/S0101-81752005000200022
- Branco, J.O. & H.A.A. Fracasso. 2004. Biologia populacional de Callinectes ornatus (Ordway) na Armaçăo de Itapocoroy, Penha, Santa Catarina, Brasil. Revista Brasileira de Zoologia 21 (1): 91-96. doi: 10.1590/S0101-81752004000100016
- Branco, J.O. & M.J. Lunardon-Branco. 1993. Aspectos da biologia de Callinectes ornatus Ordway, 1863 (Decapoda, Portunidae) da regiăo de Matinhos, Paraná, Brazil. Arquivos de Biologia e Tecnologia 36 (3): 489-496.
- Branco, J.O.; M.J. Lunardon-Branco; J.R. Verani; R. Schveitzer; F.X. Souto & W.G Vale. 2002. Natural diet of Callinectes ornatus Ordway, 1863 (Decapoda, Portunidae) in the Itapocoroy Inlet, Penha, SC, Brazil. Brazilian Archives of Biology and Technology 45 (1): 35-40. doi: 10.1590/S1516-89132002000100006
- Brazil, 1983. Dispõe sobre tamanho mínimo e práticas de captura de duas espécies de siris do gênero Callinectes (C. sapidus e C. danae). Portaria SUDEPE N-24, de 26 de julho de 1983.
- Buchanan, B.A. & A.W. Stoner. 1988. Distributional Patterns of Blue Crabs (Callinectes sp.) in a Tropical Estuarine Lagoon. Estuaries 11 (4): 231-239.
- Carvalho, E.A.S.; F.L. Carvalho & E.C.G. Couto. 2011. Maturidade sexual em Callinectes ornatus Ordway, 1893 (Crustacea: Decapoda: Portunidae) no litoral de Ilhéus, BA, Brasil. Papéis Avulsos de Zoologia 51 (24): 367-372. doi: 10.1590/S0031-10492011002400001
- Clark, M.E.; T.G. Wolcott; D.L. Wolcott & A.H. Hines. 1999. Intraspecific interference among foraging blue crabs Callinectes sapidus: interactive effects of predator density and prey patch distribution. Marine Ecology Progress Series 178: 69-78. doi: 10.3354/meps178069
- Colton, A.R.; M.J. Wilberg; V.J. Coles & T.J. Miller. 2014. An evaluation of the synchronization in the dynamics of blue crab (Callinectes sapidus) populations in the western Atlantic. Fisheries Oceanography 23 (2): 132-146. doi: 10.1111/fog.12048.
- Costa, T.M. & M.L. Negreiros-Fransozo. 1998. The reproductive cycle of Callinectes danae Smith, 1869 (Decapoda, Portunidae) in the Ubatuba Region, Brazil. Crustaceana 71: 615-27. doi: 10.1163/156854098X00617
- Fernandes, J.M.; D.M. Rosa; C.C.V. Araujo; L.V. Ripole & H.S. Santos. 2006. Biologia e distribuiçăo temporal de Callinectes ornatus Ordway, 1863 (Crustacea, Portunidae) em uma praia arenosa da Ilha do Frade, Vitória-ES. Boletim do Museu de Biologia Mello Leităo 20: 59-71.
- Fischer, S. & M. Wolff. 2006. Fisheries assessment of Callinectes arcuatus (Brachyura, Portunidae) in the Gulf of Nicoya, Costa Rica. Fisheries Research 77 (3): 301-311. doi: 10.1016/j.fishres.2005.11.009
- Freire, C.A.; V.G. Togni & M. Hermes-Lima. 2011. Responses of free radical metabolism to air exposure and salinity stress in crabs (Callinectes danae and C. ornatus) with different estuarine distributions. Comparative Biochemistry and Physiology, Part A 160: 291-300. doi: 10.1016/j.cbpa.2011.06.024
- Garçon, D.P.; M.N. Lucena; M.R. Pinto; C.F.L. Fontes; J.C. McNamara & F.A. Leone. 2013. Synergistic stimulation by potassium and ammonium of K+-phosphatase activity in gill microsomes from the crab Callinectes ornatus acclimated to low salinity: Novel property of a primordial pump. Archives of Biochemistry and Biophysics 530: 55-63. doi: 10.1016/j.abb.2012.12.006
- Guerra-Castro, E.; C.A. Carmona-Suárez & J.E. Conde. 2007. Activity patterns and zonation of the swimming crabs Arenaeus cribrarius and Callinectes ornatus Journal of Crustacean Biology 27 (1): 49-58. doi: 10.1651/S-2651.1
- Haefner Jr, P.A. 1990. Morphometry and size at maturity of Callinectes ornatus (Brachyura, Portunidae) in Bermuda. Bulletin of Marine Science 46 (2): 274-286.
- Hartnoll, R.G. 1982. Growth, p. 11-185. In: D.E. Bliss & L.G. Abele (Eds). The Biology of Crustacea. New York, Academic Press.
- Hines, A.H.; R.N. Lipcius & A.M. Haddon. 1987. Population dynamics and habitat partitioning by size, sex, and molt stage of blue crabs Callinectes sapidus in a subestuary of central Chesapeake Bay. Marine Ecology Progress Series 36: 55-64.
- Hines, A.H.; T.G. Wolcott; E. González-Gurriarán & J.L. González-Escalante. 1995. Movement patterns and migrations in crabs: Telemetry of juvenile and adult behavior in Callinectes sapidus and Maja squinado Journal of the Marine Biological Association of the UK 75: 27-42. doi: 10.1017/S0025315400015174
- IBAMA. 1994. Portaria IBAMA/SUPES/SP No. 5, de 27 de Setembro de 1994. Available online at: http://www.icmbio.gov.br/cepsul/images/stories/legislacao/Portaria/1994/p_ibama_05_ 1994_sp_areaexclusaopescacomplexobaiaestuariodesantos.pdf [Accessed: 11/XI/2013.
- Kennedy, V.S. & L.E. Cronin. 2007. The Blue Crab: Callinectes sapidus. College Park, Maryland Sea Grant College, University of Maryland, 774 pages.
- Keunecke, K.A.; F. D'Incao; F.N. Moreira; D.R. Silva Jr & J.R. Verani. 2008. Idade e crescimento de Callinectes danae e C. ornatus (Crustacea, Decapoda) na Baía de Guanabara, Rio de Janeiro, Brasil. Iheringia 98 (2): 231-235. doi: 10.1590/S0073-47212008000200011
- Keunecke, K.A.; D.R. da Silva; M. Vianna; J.R. Verani & F. D'Incao. 2009. Ovarian development stages of Callinectes danae and Callinectes ornatus (Brachyura, Portunidae). Crustaceana 82 (6): 753-761. doi: 10.1163/156854009X423175
- Keunecke, K.A.; F. D'Incao; J.R. Verani & M. Vianna. 2012. Reproductive strategies of two sympatric swimming crabs Callinectes danae and Callinectes ornatus (Crustacea: Portunidae) in an estuarine system, south-eastern Brazil. Journal of the Marine Biological Association of the UK 92 (2): 343-347. doi: 10.1017/S0025315411000397
- Leffler, C.W. 1972. Some effects of temperature on the growth and metabolic rate of juvenile blue crabs, Callinectes sapidus, in the laboratory. Marine Biology 14: 104-110. doi: 10.1007/BF00373209
- Lipcius, R.N. & W.T Stockhausen. 2002. Concurrent decline of the spawning stock, recruitment, larval abundance, and size of the blue crab Callinectes sapidus in Chesapeake Bay. Marine Ecology Progress Series 226: 45-61.
- Mantelatto, F.L.M. 2000. Allocation of the portunid crab Callinectes ornatus (Decapoda: Brachyura) in the Ubatuba Bay, northern coast of Săo Paulo State, Brazil. Crustacean Issues 12: 431-443.
- Mantellato, F.L.M. & R.A. Christofoletti. 2001. Natural feeding activity of the crab Callinectes ornatus (Portunidae) in Ubatuba Bay (Săo Paulo, Brazil): influence of season, sex, size and molt stage. Marine Biology 138: 585-594. doi: 10.1007/s002270000474
- Mantellato, F.L.M & A. Fransozo. 1997. Fecundity of the Crab Callinectes ornatus Ordway, 1863 (Decapoda, Brachyura, Portunidae) from the Ubatuba Region, Săo Paulo, Brazil. Crustaceana 70 (2): 214-226.
- Mantellato, F.L.M. & A. Fransozo. 1999. Reproductive Biology and Moulting Cycle of the Crab Callinectes ornatus (Decapoda, Portunidae) from the Ubatuba Region, Săo Paulo, Brazil. Crustaceana 72 (1): 63-76. doi: 10.1163/156854099502871
- Mantellato, F.L.M.; R.A. Christofoletti & P.B. Camargo. 2002. A food source analysis for the swimming crab Callinectes ornatus (Portunidae) in Ubatuba Bay (Brazil), using carbon isotopes. Nauplius 10 (1): 61-66.
- Melo, G.A.S. 1996. Manual de identificaçăo dos Brachyura (Caranguejos e Siris) do litoral brasileiro Plęiade/FAPESP, Săo Paulo, 603p.
- Millikin, M.R. & A.B. Williams. 1984. Synopsis of biological data on the blue crab, Callinectes sapidus Rathbun. FAO Fisheries Synopsis No. 138, NOAA Technical Reports NMF 51: 1-38.
- Negreiros-Fransozo, M.A. & A. Fransozo. 1995. On the distribution of Callinectes ornatus Ordway, 1863 and Callinectes danae Smith, 1869 (Brachyura, Portunidae) in the Fortaleza Bay, Ubatuba, Brazil. Iheringia 29 (79): 13-25.
- Negreiros-Fransozo, M.L.; F.L.M. Mantelatto & A. Fransozo. 1999. Population biology of Callinectes ornatus Ordway. 1863 (Decapoda, Portunidae) from Ubatuba (SP), Brazil. Scientia Marina 63 (2): 157-163. doi: 10.3989/scimar.1999.63n2157
- Norse, E.A. 1978. An experimental gradient analysis: hyposalinity as an "upstress" distributional determinant for Caribbean portunid crabs. Biological Bulletin 155: 586-598.
- Pinheiro, M.A.A.; A. Fransozo & M.L. Negreiros-Fransozo. 1997. Dimensionamento e sobreposiçăo de nichos dos portunídeos (Decapoda, Brachyura), na Enseada da Fortaleza, Ubatuba, Săo Paulo, Brasil. Revista Brasileira de Zoologia 14 (2): 371-378. doi: 10.1590/S0101-81751997000200010
- Pita, J.B.; E.S. Rodrigues; R. Graça-Lopes & J.A.P. Coelho. 1985. Levantamento da família Portunidae (Crustacea, Decapoda, Brachyura) no complexo baía-estuário de Santos, Săo Paulo, Brasil. Boletim do Instituto de Pesca 12 (3): 153-162.
- Reichmuth, J.M.; J. MacDonald; J. Ramirez & J.S. Weis. 2011. Fight or flight: an investigation of aggressive behavior and predator avoidance in two populations of blue crabs (Callinectes sapidus Rathbun) in New Jersey. Hydrobiologia 658 (1): 173-182. doi: 10.1007/s10750-010-0460-z
- Reigada, A.L.D. & M.L. Negreiros-Fransozo. 2001. Feeding activity of Callinectes ornatus Ordway, 1863 and Callinectes danae Smith, 1869 (Crustacea, Brachyura, Portunidae) in Ubatuba, SP, Brazil. Hydrobiologia 449: 249-252. doi: 10.1023/A:1017563119813
- Rome, M.S.; A.C. Young-Williams; G.R. Davis & A.H. Hines. 2005. Linking temperature and salinity tolerance to winter mortality of Chesapeake Bay blue crabs (Callinectes sapidus). Journal of Experimental Marine Biology and Ecology 319: 129-145. doi: 10.1016/j.jembe.2004.06.014
- Sant'Anna, B.S.; A. Turra & F.J. Zara. 2012a. Reproductive migration and population dynamics of the blue crab Callinectes danae in an estuary in southeastern Brazil. Marine Biology Research 8: 354-362. doi: 10.1080/17451000.2011.637563
- Sant'Anna, B.S.; T.T. Watanabe; A. Turra & F.J. Zara. 2012b. First record of the non-indigenous portunid crab Charybdis variegata from the western Atlantic coast. BioInvasion Records 1:11-16. doi: 10.3391/bir.2012.1.1.03
- Santos, C. & S.L. Bueno. 2002. Infestation by Octolasmis lowei (Cirripedia: Poecilasmatidae) in Callinectes danae and Callinectes ornatus (Decapoda: Portunidae) from Sao Sebastiao, Brazil. Journal of Crustacean Biology 22 (2): 241-248. doi: 10.1651/0278-0372(2002)022[0241:IBOLCP]2.0.CO;2
- Sastre, M.P.; P. Reyes; H. Ramos; R. Romero & J. Rivera. 1999. Heavy metal bioaccumulation in Puerto Rican blue crabs (Callinectes spp.). Bulletin of Marine Science 64 (2): 209-217.
- Schaffner, L.C. & R.J. Diaz. 1988. Distribution and Abundance of Overwintering Blue Crabs, Callinectes sapidus, in the Lower Chesapeake Bay. Estuaries 11 (1): 68-72. doi: 10.2307/1351719
- Secor, D.H.; A.H. Hines & A.R. Place. 2002. Japanese Hatchery-based Stock Enhancement: Lessons for the Chesapeake Bay Blue Crab. College Park, Maryland Sea Grant College, 46p.
- Sforza, R.; R.C. Nalesso & J.C. Joyeux. 2010. Distribution and population structure of Callinectes danae (Decapoda: Portunidae) in a tropical Brazilian estuary. Journal of Crustacean Biology 30 (4): 597-606. doi: 10.1651/09-3223.1
- Skaggs, H.S. & R.P. Henry. 2002. Inhibition of carbonic anhydrase in the gills of two euryhaline crabs, Callinectes sapidus and Carcinus maenas, by heavy metals. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 133 (4): 605-612. doi: 10.1016/S1532-0456(02)00175-8
- Sokal, R.R. & F.J. Rohlf. 1995. Biometry: The Principles and Practice of Statistics in Biological Research. New York, W.H. Freeman and Company, 887p.
- Sturges, H.A. 1926. The Choice of a Class Interval. Journal of the American Statistical Association 21 (153): 65-66.
- Tudesco, C.C.; L.P. Fernandes & A.P.M. Di Beneditto. 2012. Population structure of the crab Callinectes ornatus Ordway, 1863 (Brachyura: Portunidae) bycatch in shrimp fishery in northern Rio de Janeiro State, Brazil. Biota Neotropica 12 (1): 93-98. doi: 10.1590/S1676-06032012000100007
- Turner, H.V.; D.W. Wolcott; T.G. Wolcott & A.H. Hines. 2003. Post-mating behavior, intramolt growth, and onset of migration to Chesapeake Bay spawning grounds by adult female blue crabs, Callinectes sapidus Rathbun. Journal of Experimental Marine Biology and Ecology 295: 107-30. doi: 10.1016/S0022-0981(03)00290-9
- Wenner, A.M. 1972. Sex-ratio as a function of size in marine Crustacea. American Naturalist 106: 321-350.
- Williams, A.B. 1984. Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida. Washington, DC, Smithsonian Institution Press, 550p.
- Zangrande, C.M.; B.S. Sant'anna & A.L.D. Reigada. 2003. Distribuiçăo de Arenaeus cribrarius (Lamarck, 1818), (Decapoda, Brachyura), no Complexo Baía-Estuário de Săo Vicente, (SP), Brasil. Boletim do Instituto de Pesca 29 (2): 133-138.
Publication Dates
-
Publication in this collection
08 Sept 2014 -
Date of issue
Aug 2014
History
-
Received
24 Sept 2013 -
Accepted
04 Apr 2014