ABSTRACT
Restinga occurs as a narrow band of coastal habitats throughout the Atlantic Forest, although it presents considerable variation in vegetation structure, which likely contributes to heterogeneity in species inhabiting this endangered ecosystem. The goal of this study is to examine how variation in vegetation and abiotic conditions in the restinga ecosystem may contribute to heterogeneity of bird communities in Restinga de Jurubatiba, Brazil. Temperature, relative humidity, and vegetation structure were sampled to characterize four sites (dry forest, flooded forest, open scrub and closed scrub). Birds were sampled using observations, mist-netting and voice recordings. Results indicate that major differences of all variables occur between forest and scrub in both vegetation and birds. In addition, differences also exist within forests and within scrub, resulting in considerable heterogeneity among sampled areas. Scrub sites were richer in bird species (n = 58) than forest sites (n = 41), while closed scrub had the most species (n = 49). Also, 64% (47 of 73) of bird species were exclusive to forest or scrub habitats. Scrub habitats were more similar to each other than forest habitats. Normalized Difference Vegetation Index (NDVI) calculated from satellite images distinguished scrub sites and may be useful to monitor changes in vegetation patches through time. The restinga ecosystem is quite heterogeneous with considerable turnover in bird species composition and differences in vegetation structure. Forest strips may serve as connectors on the landscape and to help maintain species diversity and conservation of forest species. Also, this highly dynamic ecosystem, which includes a mosaic of habitat types, likely promotes resilience of bird populations under changing conditions.
KEY WORDS:
Avian community; NDVI; restinga forest; scrub; vegetation structure
Marginal ecosystems in tropical forests, such as restinga in the Atlantic Forest biome, are often neglected in conservation strategies (Scarano 2002Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic Forest. Annals of Botany 90: 517-524. doi: 10.1093/aob/mcf189
https://doi.org/10.1093/aob/mcf189...
). Restinga habitats are mosaics of vegetation physiognomies that occur throughout the Brazilian coast. These habitats vary in vegetation structure as a result of different physical, chemical and biological conditions that occur both regionally and locally (Lacerda et al. 1993Lacerda LD, Araújo DSD, Maciel NC (1993) Dry coastal ecosystems of the tropical Brazilian coast, p. 477-93. In: Van Der Maarel E (Ed.). Dry Coastal Ecosystems: Africa, America and Oceania. Amsterdam, Elsevier, 616p.). Restinga sites with similar physiognomies may be floristically distinct (Marques et al. 2011Marques MC, Swaine MD, Liebsch D (2011) Diversity distribution and floristic differentiation of the coastal lowland vegetation: implications for the conservation of the Brazilian Atlantic Forest. Biodiversity & Conservation 20: 153-168. doi: 10.1007/s10531-010-9952-4
https://doi.org/10.1007/s10531-010-9952-...
), and the edaphic heterogeneity can favor the coexistence of plant species, increasing the regional richness (Oliveira et al. 2014Oliveira AA, Vicentini A, Chave J, Castanho CDT, Davies SJ, Martini AMZ, Lima RAF, Ribeiro RR, Iribar A, Souza VC (2014) Habitat specialization and phylogenetic structure of tree species in a coastal Brazilian white-sand forest. Journal of Plant Ecology 7: 134-144. doi: 10.1093/jpe/rtt073
https://doi.org/10.1093/jpe/rtt073...
).
A mosaic of habitats on the landscape is likely essential to provide resources for animals that move across the components in the landscape (Law & Dickman 1998Law BS, Dickman CR (1998) The use of habitat mosaics by terrestrial vertebrate fauna: implications for conservation and management. Biodiversity & Conservation 7: 323-333. doi: 10.1023/A:1008877611726
https://doi.org/10.1023/A:1008877611726...
). The heterogeneity promoted by different habitats in a landscape mosaic may ensure the presence of habitat-restricted taxa and increase community diversity (Stein & Kreft 2014Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters 17: 866-880. doi: 10.1111/ele.12277
https://doi.org/10.1111/ele.12277...
, Stein et al. 2014Stein A, Kreft H (2014) Terminology and quantification of environmental heterogeneity in species-richness research. Biological Reviews 90: 815-836. doi: 10.1111/brv.12135
https://doi.org/10.1111/brv.12135...
). Also, heterogeneity in vegetation structure and composition within habitats may also be important to maintain species richness and enhance ecological resilience (Tews et al. 2004Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography 31: 79-92. doi: 10.1046/j.0305-0270.2003.00994.x
https://doi.org/10.1046/j.0305-0270.2003...
, Ye et al. 2013Ye X, Skidmore AK, Eang T (2013) Within-patch habitat quality determines the resilience of specialist species in fragmented landscapes. Landscape Ecology 28: 135-147. doi: 10.1007/s10980-012-9826-0
https://doi.org/10.1007/s10980-012-9826-...
, Fleishman et al. 2002Fleishman E, Ray C, Sjögren-Gulve P, Boggs CL, Murphy DD (2002) Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conservation Biology 16: 706-716. doi: 10.1046/j.1523-1739.2002.00539.x
https://doi.org/10.1046/j.1523-1739.2002...
, Franzén & Nilsson 2010Franzén M, Nilsson SG (2010) Both population size and patch quality affect local extinctions and colonizations. Proceedings of the Royal Society of London B 277: 79-85. doi: 10.1098/rspb.2009.1584
https://doi.org/10.1098/rspb.2009.1584...
). Previous studies have shown that habitat characteristics may strongly influence bird diversity and distribution in Atlantic Forests (Goerck 1999Goerck JM (1999) Distribution of birds along an elevational gradient in the Atlantic forest of Brazil: Implications for the conservation of endemic and endangered species. Bird Conservation International 9: 235-253. doi: 10.1017/S0959270900003439
https://doi.org/10.1017/S095927090000343...
, Gomes & Silva 2002Gomes VSM, Silva WR (2002) Spatial variation in understory frugivorous birds in an Atlantic Forest fragment of southeastern Brazil. Ararajuba 10: 219-225. Available online at: http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/1713
http://www4.museu-goeldi.br/revistabrorn...
, Hasui et al. 2007Hasui E, Gomes VSM, Silva WR (2007) Effects of vegetation traits on habitat preferences of frugivorous birds in Atlantic Rain Forest. Biotropica 39: 502-509. doi: 10.1111/j.1744-7429.2007.00299.x
https://doi.org/10.1111/j.1744-7429.2007...
). More complex scrub-like habitats in restinga were richer in bird species than dune vegetation, marshes and grasslands in southern Brazil (Pedroso-Junior 2003Pedroso-Junior NN (2003) Microhabitat occupation by birds in a restinga fragment of Paraná coast, PR, Brazil. Brazilian Archives of Biology and Technology 46: 83-90. doi: 10.1590/S1516-89132003000100013
https://doi.org/10.1590/S1516-8913200300...
) and even richer than restinga forest in northeast Brazil (Motta et al. 2011Mota JVL, Carvalho AAF, Tinoco MS (2011) Distribuição e uso de habitat da avifauna na restinga da Reserva Imbassaí, litoral norte da Bahia. Revista Brasileira de Ornitologia 19: 364-375. Available online at: http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/4407
http://www4.museu-goeldi.br/revistabrorn...
); yet, studies within restinga habitats in southeastern Brazil are lacking.
Relative to other Atlantic forest habitats, restinga vegetation contains low levels of endemism in flora and faunal communities (Rizzini 1979Rizzini CT (1979) Tratado de fitogeografia do Brasil: aspectos sociológicos e florísticos. São Paulo, Universidade de São Paulo, 375p., Cerqueira 2000Cerqueira R (2000) Biogeografia das restingas, p. 65-75. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p., Reis & Gonzaga 2000Reis HBR, Gonzaga LP (2000) Análise da distribuição geográfica das aves das restingas do Estado do Rio de Janeiro, p. 165-178. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.). Restinga avifauna, specifically, shares species with the Amazon, Caatinga, Cerrado and Chaco, although is most similar to the Atlantic Forest (Reis & Gonzaga 2000Reis HBR, Gonzaga LP (2000) Análise da distribuição geográfica das aves das restingas do Estado do Rio de Janeiro, p. 165-178. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.), especially open habitats in this biome (Sick 1997Sick H (1997) Ornitologia Brasileira. Rio de Janeiro, Nova Fronteira, 912p.). Therefore, Gonzaga et al. (2000Gonzaga LP, Castiglioni GDA, Reis HBR (2000) Avifauna das restingas do Sudeste: estado do conhecimento e potencial para futuros estudos, p. 151-163. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.) suggested that bird species in restinga were probably generalists in habitat, as they occur in different biomes. Despite this resemblance with other biomes, the fauna is known to vary among the different restinga habitats, not only in birds (Alves et al. 2004Alves MAS, Storni A, Almeida EM, Gomes VSM, Oliveira CHP, Marques RV, Vecchi MB (2004) A comunidade de aves na Restinga de Jurubatiba, p. 199-214. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.), but also other vertebrates (Bergallo et al. 2004Bergallo HG, Martins-Hatano F, Raíces DS, Ribeiro TTL, Alves AG, Luz JL, Mangolin R, Mello MAR (2004) Os mamíferos da Restinga de Jurubatiba, p. 215-230. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p., Van Sluys et al. 2004Van Sluys M, Rocha CFD, Hatano FH, Boquimpani-Freitas L, Marra RV (2004) Anfíbios na Restinga de Jurubatiba: composição e história natural, p. 165-178. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.) and insects (Monteiro et al. 2004Monteiro RF, Esperanço AP, Becker VO, Otero LS, Herkenhoff EV, Soares A (2004) Mariposas e borboletas na Restinga de Jurubatiba, p. 143-164. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.).
Located within Rio de Janeiro state, the Parque Nacional da Restinga de Jurubatiba (PNRJ) is considered the most preserved area of restinga in Brazil (Rocha et al. 2005Rocha CFD, Sluys MV, Bergallo HG, Alves MAS (2005) Endemic and threatened tetrapods in the restingas of the biodiversity corridors of Serra do Mar and of the Central da Mata Atlântica in eastern Brazil. Brazilian Journal of Biology 65: 159-168. doi: 10.1590/S1519-69842005000100019
https://doi.org/10.1590/S1519-6984200500...
, 2007Rocha CFD, Bergallo HG, Sluys MV, Alves MAS, Jamel CE (2007) The remnants of restinga habitats in the Brazilian Atlantic forest of Rio de Janeiro state, Brazil: habitat loss and risk of disappearance. Brazilian Journal of Biology 67: 631-637. doi: 10.1590/S1519-69842007000200011
https://doi.org/10.1590/S1519-6984200700...
). Restinga de Jurubatiba is surrounded by agriculture and cattle-raising activities, with a few isolated Atlantic forest fragments (Jamel 2004Jamel CEG (2004) Caracterização da vegetação da restinga de Jurubatiba com base em sensoriamento remoto e sistema de informação geográfico: estado atual e perspectivas, p. 25-42. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p., Caris et al. 2013Caris EAP, Kurtz BC, Cruz CBM, Scarano FB (2013) Vegetation cover and land use of a protected coastal area and its surroundings, southeast Brazil. Rodriguésia 64: 747-755. doi: 10.1590/S2175-78602013000400006
https://doi.org/10.1590/S2175-7860201300...
). As it is located within a largely disturbed landscape, this restinga park is an important wildlife refuge that harbors non-breeding (i.e., migrants) and threatened bird species (Alves et al. 2004Alves MAS, Storni A, Almeida EM, Gomes VSM, Oliveira CHP, Marques RV, Vecchi MB (2004) A comunidade de aves na Restinga de Jurubatiba, p. 199-214. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p., Gomes et al. 2010Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS (2010) Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora Morphology, Distribution, Functional Ecology of Plants 205: 647-655. doi: 10.1016/j.flora.2010.04.014
https://doi.org/10.1016/j.flora.2010.04....
).
The objective of the present study was to examine heterogeneity in bird communities within the two dominant vegetation types of restinga ecosystem in Restinga de Jurubatiba, forest and open Clusia scrub, using satellite imagery as a descriptor for restinga habitats. We expect these results contribute to identify tools to track changes in restinga habitats that might predict correspondent bird responses and help to establish conservation measures.
MATERIAL AND METHODS
Data were collected in the PNRJ (22º17'S, 41º41'W), created in 1998 on the north shore of Rio de Janeiro state, included in Quissamã, Carapebus and Macaé municipalities (Fig. 1). The landscape in this region is marked by the presence of many coastal lagoons with various salinity levels. Climate is characterized by a wet season between October and April and a drier season between May and September. Mean annual rainfall is 1200 mm and temperature is 22.6°C. The dominant vegetation type is Clusia scrub, formed by patches of vegetation that cover 20 to 48% of the soil and reach 5 m height, with few small plants in between (Henriques et al. 1986Henriques RPB, Araujo DSD, Hay JD (1986) Descrição e classificação dos tipos de vegetação da restinga de Carapebus, Rio de Janeiro. Revista Brasileira de Botânica 9: 173-189., Araujo et al. 1998Araujo DSD, Scarano FR, Sá CFC, Kurtz BC, Zaluar HLT, Montezuma RCM, Oliveira RC (1998) Comunidades Vegetais do Parque Nacional da Restinga de Jurubatiba, p. 39-62. In: Esteves FA (Ed.) Ecologia das Lagoas Costeiras do Parque Nacional da Restinga de Jurubatiba e do Município de Macaé. Rio de Janeiro, UFRJ, 442p.). PNRJ contains a mosaic of habitat types; ten different plant communities have been recognized in the park and are associated with different nutrient concentrations and levels of water in the soil. Two communities - Clusia and Ericaceae Shrub Formations - dominate the park, covering more than 50% of the area, followed by two forest plant communities (40%) and other communities constituted by smaller-statured plants (Hernriques et al. 1986Henriques RPB, Araujo DSD, Hay JD (1986) Descrição e classificação dos tipos de vegetação da restinga de Carapebus, Rio de Janeiro. Revista Brasileira de Botânica 9: 173-189., Araujo et al. 1998Araujo DSD, Scarano FR, Sá CFC, Kurtz BC, Zaluar HLT, Montezuma RCM, Oliveira RC (1998) Comunidades Vegetais do Parque Nacional da Restinga de Jurubatiba, p. 39-62. In: Esteves FA (Ed.) Ecologia das Lagoas Costeiras do Parque Nacional da Restinga de Jurubatiba e do Município de Macaé. Rio de Janeiro, UFRJ, 442p., Caris et al. 2013Caris EAP, Kurtz BC, Cruz CBM, Scarano FB (2013) Vegetation cover and land use of a protected coastal area and its surroundings, southeast Brazil. Rodriguésia 64: 747-755. doi: 10.1590/S2175-78602013000400006
https://doi.org/10.1590/S2175-7860201300...
). The present study was developed in Clusia scrub and forest vegetation on four sites: two sites next to a lagoon called "Lagoa Comprida" (22°16'41"S, 41°39'41"W) and two sites approximately two kilometers away (22°16'13"S, 41°38'50"W). Sites are hereafter called: site I - "closed scrub" (CS), site II - "flooded forest" (FF), site III - "open scrub" (OS) and site IV - "dry forest" (DF) (Figs. 2-5).
Location of the study area in Brazil. The arrow in Rio de Janeiro state indicates the Parque Nacional da Restinga de Jurubatiba. The four sampled sites are included in the white rectangle (Study area). Bottom map modified from Google Earth.
View of the study sites: (2) closed scrub (CS - site I); (3) open scrub (OS - site III). Forest sites in the background. Photos by VSMG.
Detailed view of the: (4) dry forest (DF - site II), (5) flooded forest (FF - site IV). Photos by VSMG.
Clusia scrub on site III is more open, and has shorter vegetation than on site I, although the two sites belong to the same plant formation (Pimentel et al. 2007Pimentel MCP, Barros MJ, Cirne P, Mattos EA, Oliveira RC, Pereira MCA, Scarano FR, Zaluar HLT, Araujo DSD (2007) Spatial variation in the structure and floristic composition of "restinga" vegetation of southeastern Brazil. Brazilian Journal of Botany 30: 543-351. doi: 10.1590/S0100-84042007000300018
https://doi.org/10.1590/S0100-8404200700...
). Forest in site II is seasonally flooded (flooded for ten months, in the second year of study), while site IV is a forest that was never known to be flooded (Fig. 6).
General view of the restinga and the sampled sites in the Parque Nacional da Restinga de Jurubatiba, Rio de Janeiro state, Brazil. Aerial photo by Romulo Campus, reproduced and ammended with his permission. I = closed scrub (CS), II = flooded forest (FF), III = open scrub (OS), IV = dry forest (DF).
Temperature, relative humidity, and vegetation structure were measured in each of the four sites.
In each site, one data logger was placed inside a wood house (20 cm high x 15cm x 15cm, without walls to allow air circulation) to prevent rain and direct sun which could alter data. At any one time, two data loggers were set simultaneously in pairs of sites (one per site): closed scrub and flooded forest or open scrub and dry forest. Data loggers measured temperature and humidity every five minutes for a 24 hour period per month during nine months in each site between February 2003 and August 2004, resulting in eighteen 24-hour periods for grouped forest and eighteen for grouped scrub sites.
We used both on the ground field measurements and satellite images to characterize vegetation structure. Remote sensing has been used previously as a tool to measure vegetation characteristics (Gould 2000Gould W (2000) Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecological Applications 10: 1861-1870. doi: 10.2307/2641244
https://doi.org/10.2307/2641244...
), as well as identify change in vegetation patterns, such as deforestation (Kerr & Ostrovsky 2003Kerr JT, Ostrovsky M (2003) From space to species: ecological applications for remote sensing. Trends in Ecology & Evolution 18: 299-305. doi: 10.1016/S0169-5347(03)00071-5
https://doi.org/10.1016/S0169-5347(03)00...
, Turner et al. 2003Turner W, Spector S, Gardiner N, Fladeland M, Sterling E, Steininger M (2003) Remote sensing for biodiversity, science and conservation. Trends in Ecology & Evolution 18: 306-314. doi: 10.1016/S0169-5347(03)00070-3
https://doi.org/10.1016/S0169-5347(03)00...
) and guide design of biological corridors and other conservation actions (Taulman & Smith 2002Taulman JF, Smith KG (2002) Habitat mapping for bird conservation in North America. Bird Conservation International 12: 281-309. doi: 10.1017/S0959270902002186
https://doi.org/10.1017/S095927090200218...
).
Satellite image processing
China-Brazil Earth Resource Satellite (CBERS) satellite ima ge was obtained from Instituto Nacional de Pesquisas Espaciais (INPE), with the following specifications: Locality (scen) - Macaé, Date - 7 April 2004 (when flooded forest was flooded), Spatial resolution - 20 m, Sensor - CCD, Spectral Bands - 2, 3 and 4 (i.e., green, red and near infrared) (Fig. S1). The image was geocoded using Spring 3.6.02(r) and points obtained in the field were then recognized. Based on those points and on the knowledge of distribution of various vegetation types on the ground, a rectangle with fixed dimensions was established for each site on the image, using Spring 3.6.02(r). From each rectangle, we obtained the level of gray (LG) of the darker 30 pixels in red and infrared spectral bands, and then calculated NDVI (Normalized Difference Vegetation Index) (Kerr & Ostrovsky 2003Kerr JT, Ostrovsky M (2003) From space to species: ecological applications for remote sensing. Trends in Ecology & Evolution 18: 299-305. doi: 10.1016/S0169-5347(03)00071-5
https://doi.org/10.1016/S0169-5347(03)00...
). NDVI = (near infrared - red)/(near infrared + red). NDVI is an index of vegetation vigor as it is based on the reflectance of chlorophyll and foliar structure in red and infrared bands (Kerr & Ostrovsky 2003Kerr JT, Ostrovsky M (2003) From space to species: ecological applications for remote sensing. Trends in Ecology & Evolution 18: 299-305. doi: 10.1016/S0169-5347(03)00071-5
https://doi.org/10.1016/S0169-5347(03)00...
). NDVI has been shown to be a good predictor of green biomass in dry Atlantic Forest (Freitas et al. 2005Freitas SR, Mello MCS, Cruz CBM (2005) Relationships between forest structure and vegetation indices in Atlantic Rainforest. Forest Ecology and Management 218: 353-362. doi: 10.1016/j.foreco.2005.08.036
https://doi.org/10.1016/j.foreco.2005.08...
) and is being used in this study for the first time in restinga.
Field work
The points-quadrant method was used to measure vegetation physical structure in the field (Sylvestre & Rosa 2002Sylvestre LS, Rosa MMT (2002) Manual metodológico para estudos botânicos na Mata Atlântica. Seropédica, Universidade Federal Rural do Rio de Janairo, 123p.), aiming to calculate vegetation spacing, cover and height. Between 3 and 4 July 2004, two transects (200 m long each) were established in each site, each containing 16 to 25 points and four quadrants per point. Distance between two adjacent points in the same transect was 10 m, and transects were separated from each other by 10 m in forest and 50 m in scrub, as forests were spatially restricted to smaller areas. In each point, the following variables were collected from the nearest four plants >50 cm high (one in each quadrant): distance to point, total height, and circumference at 50 cm above soil. In Clusia scrub sites, the only species isolated from vegetation patches that fit this criterion (>50 cm high) were Marcetia taxifolia (A.St.-Hil.) DC. (Melastomataceae) and Waltheria aspera K. Schum. (Malvaceae). In forests, a different criterion had to be applied due to the highly different vegetation architecture: trees and shrubs were measured when they had DBH at least 2.5 cm. Number of plants sampled: FF = 140, DF = 136, CR = 200, OR = 200.
We focused on terrestrial and diurnal birds (excluding swifts and raptors) in this study using observations, mist-netting and voice recordings. Differences in sampling design among scrub and forest sites occurred due to differences in the spatial dispersion of forests, which were composed of thin strips of vegetation on the landscape, as seen in Fig. 6, i.e., pairs of nets and observation transects were more distant between each other in scrubs than in forests. Birds were identified using field guides (De Schauensee 1982De Schauensee RM (1982) A guide to the birds of South America. Philadelphia, The Academy of Natural Science of Philadelphia, 498p., Dunning 1989Dunning JS (1989) South American Birds. A photographic aid to identification. Newton Square, Harrowood Books, 315p., Ridgely & Tudor 1994aRidgely RS, Tudor G 1994a. The birds of South America. Austin, University of Texas Press, vol. 1, 516p., bRidgely R S, Tudor G 1994b. The birds of South America. Austin, University of Texas Press, vol. 2, 814p., Sick 1997Sick H (1997) Ornitologia Brasileira. Rio de Janeiro, Nova Fronteira, 912p.). Vocalizations of some species were deposited at the Arquivo Sonoro Prof. Elias Coelho (ASEC), Universidade Federal do Rio de Janeiro. In addition, for Elaenia species, some specimens were collected and compared to bird collections in Museu de Zoologia da Universidade de São Paulo (MUZUSP) and Museu Nacional do Rio de Janeiro (MNRJ).
Birds were captured under permits P029/03 (Centro Nacional de Pesquisa e Conservação de Aves Silvestres - Cemave/Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis - IBAMA), 106/2003 and 116/2004 (Diretoria de Ecossistemas/IBAMA - Parque Nacional da Restinga de Jurubatiba). Those permits included permission for capture, transport and collection of specified bird species/families and for conduct of these activities inside the national park.
Birds were observed by VSMG walking along three parallel transects in each site between 06:00 and 12:00 h and between 13:00 and 18:00 h, totaling 50 hours in each site. Observations were conducted between September 2002 and July 2004. Each transect was approximately 200 m long and 50 m distant from any other transect in scrub sites and 10 m distant in forest sites. Birds perched or foraging in the vegetation were registered within a maximum horizontal distance of approximately 15 m in scrub sites and a maximum vertical distance of approximately 15 m in forest sites, where vegetation limited maximum horizontal distance to approximately 5 m.
Birds were captured with 10 mist nets (2.5 x 12 m, 36 mm mesh) set every two months, between August 2002 and June 2004. Nets were opened from 06:00 to10:00 h and from 14:00 to 17:00 h, for two consecutive days, totaling 1680 net-hours in each of the four sites.
Bird songs and calls were recorded between November 2003 and July 2004 for four sessions of 15 minutes in the early morning in each site, using a directional microphone (Sennheiser ME 67) and a cassette tape-recorder (Sony TCM-5000EV), resulting in a total of one hour per site and four hours of recordings. The researcher (VSMG) arrived at the site before dawn and started recording when the first bird started singing, pointing the microphone to each distinct call or song for approximately 60 s (adapted from Parker III 1991Parker III TA (1991) On the use of tape recorders in avifaunal surveys. The Auk 108: 443-444.). During the recording inside forest sites, whenever birds were heard singing outside the forest, an observation was noted down. Voices were identified afterwards by one of us (MBV) using personal experience and comparison with a sound database (Xeno-canto: http://www.xeno-canto.org).
Data analysis
We compared plant height, plant basal area, distance from the point to the nearest plant and NDVI among the four sites using box plots (Wilkinson 1990Wilkinson L (1990) SYSTAT: the system for statistics. Evanston, Systat Software.). In order to examine differences in vegetation structure between habitat types and among sites, we used analysis of similarity (ANOSIM) with Primer 5 for Windows software package (Clarke & Gorley 2002Clarke KR, Gorley RN (2002) PRIMER 5 for Windows. Plymouth, Primer E-Ltd, v. 5.2.9.). The analysis was run once with habitat type as a factor (forest vs. scrub) and another time with site as a factor (flooded forest, dry forest, closed scrub, open scrub); in both cases we used Euclidean distances for similarity matrices. The variables included in the analysis were: maximum distance from a plant to the point, maximum plant height at each point and sum of basal areas at each point; the samples were each point from the point-quadrant method, grouping data from the two transects in each site (n: FF = 19, DF = 18, CS = 25, OS = 25). Satellite data were not included in these ANOSIM analyses due to differences in sampling.
To test whether environmental variables predict captures for each habitat type, we used Stepwise Multiple Regression Analysis with SYSTAT software package (Wilkinson 1990Wilkinson L (1990) SYSTAT: the system for statistics. Evanston, Systat Software.). Only the first day of capture in each month was used to avoid the influence of consecutive days in capture. Using whole-day data, plots of residuals displayed a nested design, i.e., residuals were arranged in five groups, corresponding to the five periods of sampling. Both capture and abiotic data were split into five capture periods (06:00-07:40 h, 07:41-09:20 h, 09:21h-11:00 h, 14:00-15:30 h, 15:31-17:00 h). For each period, we used the number of captures as the response variable and median of temperature, maximum temperature, minimum temperature, and median of humidity (%) as predictor variables. The number of samples is 18 for each period for each habitat type, and nine for each site of the same habitat type.
We used Hierarchical Cluster Analysis to group sites based on vegetation structure and bird species composition (PC-Ord, McCune & Mefford 1999McCune B, Mefford MJ (1999) Multivariate Analysis of Ecological Data. Gleneden Beach, MjM Software, v. 4.10.). Presence/absence of each species was used in the bird community matrix. In the vegetation matrix, the median values of the following variables were used: plant height, basal area, distance, and NDVI. We used Group average (UPGMA) as the linkage method and Bray-Curtis (Sorensen) distance measure as the similarity index for both data matrices, as they deal adequately with both qualitative and quantitative data.
RESULTS
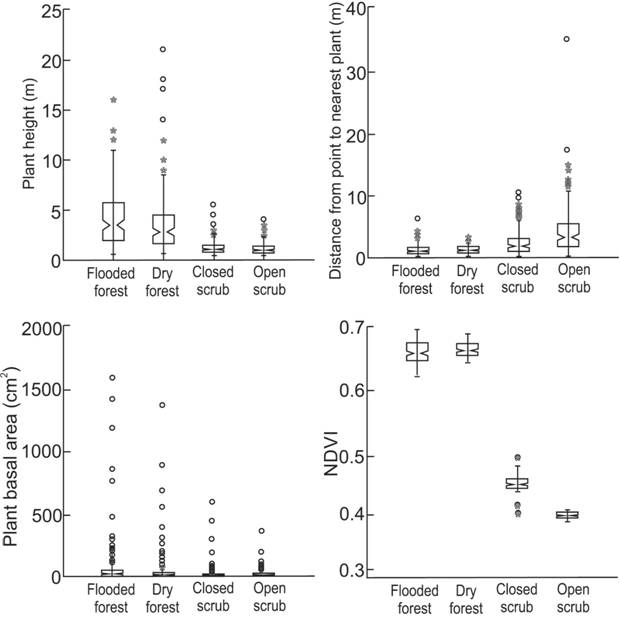
Forest and scrub habitats are primarily distinguished by plant height (Fig. 7). Between forest sites, the dry forest has the tallest plants, though the median plant height is lower due to greater density of plants in the understory. Plant basal area also reflects a more complex understory in dry forest, as smaller stemmed plants appear in the sample. Scrub habitats are separated from each other primarily based on distance among plants; not surprisingly, in open scrub plants are more sparsely distributed as shown by greater distances from point to nearest plant. In addition, there are taller and larger diameter plants in closed scrub than in open scrub. NDVI distinguished scrub sites from each other but not forest sites, reflecting a denser foliage in forest sites and the least dense foliage in open scrub site.
Box plots represent vegetation structure in the four study sites in the Parque Nacional da Restinga de Jurubatiba: vegetation index (NDVI), plant heights, basal area and distances from point-quadrant and nearest plant (internal horizontal line = median; whiskers = minimum and maximum values; box horizontal limits: inferior = 25% quartile, superior = 75% quartile; asterisks and circles = two levels of outliers. Boxes are notched at the median and return to full width at the lower and upper 95% confidence limits of the median). NDVI was obtained from 30 pixels in each physiognomy. Height, basal area and distance from point to plant - number of sampled plants: flooded forest = 140, dry forest = 136, open scrub = 200, closed scrub = 200). Significant differences may be observed by absence of overlay among confidence intervals.
Vegetation structure of forest sites grouped together and differed from scrub sites, which also clustered together (ANOSIM, p = 0.001) although not expressive - Global R = 0.242; high values are greater than 0.3, according to McCune & Grace (2002McCune B, Grace JB (2002) Analysis of ecological communities. Gleneden Beach, MjM Software Design.). This indicates that although there is some variation between samples, there is a significant difference between the structure of the vegetation found in forest and scrub sites. Similarly, when the four sites are compared separately, all comparisons between a forest site and a scrub site are significant, while comparisons within scrub or within forest habitats are not. Additionally, the only expressive result was obtained comparing the site with dense foliage and more complex understory (flooded forest) to the site with more sparse plants and least dense understory (open scrub) (Table 1, Fig. 7).
Pairwise results from AnoSim based on vegetation structure among the four study sites in the Parque Nacional da Restinga de Jurubatiba. FF = flooded forest, DF = dry forest, OS = open scrub, CS = closed scrub. In bold, both significant (p < 0.05) and expressive (R > 0.3) differences.
Although scrub sites may have sparser vegetation than forest sites, inside these scrub sites weather conditions were similar to forest sites (Table 2). In fact, in the middle of the day bird activity was extremely low at all sites, when they probably hide inside the patches (VSMG and MASA pers. obs.). Despite this, it was possible to detect a correlation between climatic variables and bird activity. In scrub sites, early in the morning, humidity and temperature were correlated with the number of captures with higher humidity and higher temperature leading to more captures. In contrast, during mid-day, captures declined with increasing temperatures (Table 3). In the afternoon, higher wind intensities (CPTEC 2000CPTEC (2000) Data from Macaé Airport, in 2000. Centro de Previsão de Tempo e Estudos Climáticos, Instituto Nacional de Pesquisas Espaciais, MCTi. Available online at: Available online at: http://sonda.cptec.inpe.br/basedados/vento_aeroportos.html
[Accessed 10/06/2010]
http://sonda.cptec.inpe.br/basedados/ven...
) likely made nets more visible in scrub sites, reducing captures and changing relationship among variables. In the forests, environmental variables were not correlated with the number of captures (n = 17 for each period; R2 multiple < 0.4, p > 0.2).
Summary of temperature and humidity data collected with data loggers in the Parque Nacional Restinga de Jurubatiba (n = 18 for each site). Medians were obtained per time period each day.
Stepwise Multiple Regression among number of captures (log) and temperature and humidity data in each of five periods in open scrub and closed scrub in the Parque Nacional da Restinga de Jurubatiba (n = 18 for each period).
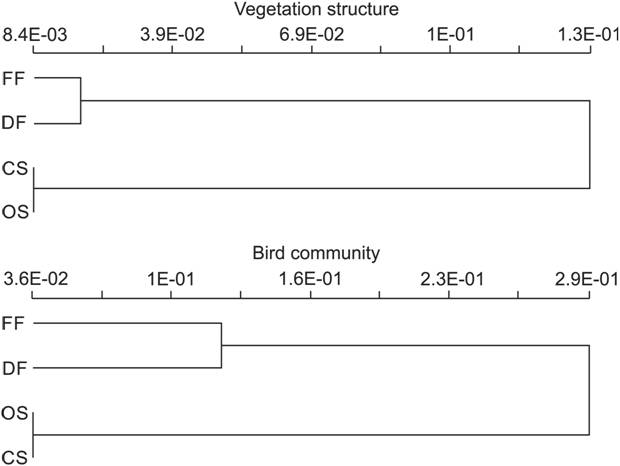
Scrub sites were richer in bird species than forest sites, and closed scrub was the richest, with the same trend for site-exclusive species (Appendix 1). Voice recordings was the sole method responsible that accounted for 16% of the species in DF, 9% in FF, 6% in CS and 2% in OS. We found that 64% (47 of 73) of bird species were exclusive to forest or scrub habitats. Forest and scrub sites separated clearly in both vegetation structure and bird composition, while within forest or scrub, similarity values exceeded 65% (Fig. 8). Also, scrub sites were more similar to each other than were forest sites.
Cluster analysis considering vegetation structure and bird species composition in the Parque Nacional da Restinga de Jurubatiba. Scale represents dissimilarity (1-Sorensen). (FF) flooded forest, (DF) dry forest, (OS) open scrub, (CS) closed scrub.
DISCUSSION
Results from our bird and vegetation surveys indicate that major differences exist between forest and scrub habitats, as well as between sites within forest and scrub habitats. These results suggest that there is significant habitat heterogeneity in the restinga ecosystem. The highest bird species richness found in scrub habitats contrasts with other studies which found that habitats with denser understory and greater vegetation height contain higher number of bird species (Cody 1981Cody ML (1981) Habitat selection in birds: The roles of vegetation structure, competitors, and productivity. BioScience 31: 107-113. doi: 10.2307/1308252
https://doi.org/10.2307/1308252...
, Rotenberry 1985Rotenberry JT (1985) The role of habitat in avian community composition: Physiognomy or floristics? Oecologia 67: 213-217. doi: 10.1007/BF00384286
https://doi.org/10.1007/BF00384286...
, Terborgh 1985Terborgh J (1985) Habitat selection in Amazon birds, p. 311-338. In: Cody ML (Ed.) Habitat selection in birds. New York, Academic Press, 558p.). However, results similar to ours have been found for other ecosystems as well. In a Venezuelan arid/semiarid gradient from coast to mountains (one of the driest areas of the Caribbean region) species richness was higher in the scrub vegetation than in woodland and deciduous forest (Poulin et al. 1993Poulin B, Lefebvre G, McNeil R (1993) Variations in bird abundance in tropical arid and semi-arid habitats. Ibis 135: 432-441. doi: 10.1111/j.1474-919X.1993.tb02116.x
https://doi.org/10.1111/j.1474-919X.1993...
). Similar unexpected results between vegetation structure and species richness have been observed in an African forest and farmland comparison, where farmland open areas contain more bird species than forest, likely due to increased heterogeneity formed by mixed agricultural crops (Mulwa et al. 2012Mulwa RK, Böhning-Gaese K, Schleuning M (2012) High Bird Species Diversity in Structurally Heterogeneous Farmland in Western Kenya. Biotropica 44: 801-809. doi: 10.1111/j.1744-7429.2012.00877.x
https://doi.org/10.1111/j.1744-7429.2012...
).
For restinga, Pedroso-Junior (2003Pedroso-Junior NN (2003) Microhabitat occupation by birds in a restinga fragment of Paraná coast, PR, Brazil. Brazilian Archives of Biology and Technology 46: 83-90. doi: 10.1590/S1516-89132003000100013
https://doi.org/10.1590/S1516-8913200300...
) showed that more complex scrub-like habitats were richer in bird species than dune vegetation, marshes and grasslands; however, forest habitats within restinga were not included in this study. On the other hand, Mota et al. (2011Mota JVL, Carvalho AAF, Tinoco MS (2011) Distribuição e uso de habitat da avifauna na restinga da Reserva Imbassaí, litoral norte da Bahia. Revista Brasileira de Ornitologia 19: 364-375. Available online at: http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/4407
http://www4.museu-goeldi.br/revistabrorn...
) found similar results to the present study, with scrub vegetation holding more species and individuals than restinga forest on northeastern Brazil.
In the present study, the lower richness of birds in forest sites may be related to at least three factors: 1) Biogeography: restinga is composed mostly of open habitats, favoring colonization by generalist species (Gonzaga et al. 2000Gonzaga LP, Castiglioni GDA, Reis HBR (2000) Avifauna das restingas do Sudeste: estado do conhecimento e potencial para futuros estudos, p. 151-163. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.); 2) Floristics/Phenology: although the most important plant species in flooded forest include ornithochoric ones (Marx de Jesus Barros unpublished data, Rodrigo Corrêa de Oliveira unpublished data), physical factors might negatively influence phenology. Only 27% (4 of 15) of forest exclusive bird species were predominately frugivorous and 40% (6 of 15) were insectivorous, while in scrub the tendency was reverse, with 38% (12 of 32) predominately frugivorous and 31% (10 of 32) insectivorous (see Appendix 1); 3) Sampling biases related to habitat structural differences: nets, observations and recordings in open and closed scrub may sample a larger area, including birds that fly over larger distances, than inside forests.
Despite the greater species richness in scrub sites, 15 species were found only in forests, highlighting the importance of such habitats for the restinga bird community as a whole. Recent studies in PNRJ have shown that forests harbor exclusive species that do not occur in open scrub in lepidopterans (Monteiro et al. 2004Monteiro RF, Esperanço AP, Becker VO, Otero LS, Herkenhoff EV, Soares A (2004) Mariposas e borboletas na Restinga de Jurubatiba, p. 143-164. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.), amphibians (Van Sluys et al. 2004Van Sluys M, Rocha CFD, Hatano FH, Boquimpani-Freitas L, Marra RV (2004) Anfíbios na Restinga de Jurubatiba: composição e história natural, p. 165-178. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.) and mammals (Bergallo et al. 2004Bergallo HG, Martins-Hatano F, Raíces DS, Ribeiro TTL, Alves AG, Luz JL, Mangolin R, Mello MAR (2004) Os mamíferos da Restinga de Jurubatiba, p. 215-230. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.). Similarly, exclusive forest bird species have been found (seven species) in two other restinga sites (Porto & Teixeira 1984Porto FCS, Teixeira DM (1984) Um estudo comparativo preliminar sobre as avifaunas das restingas do leste do Brasil, p. 343-349. In: Lacerda LD, Araujo DSD, Cerqueira R, Turcq B (Eds.) Restingas: origens, estruturas, processos. Niterói, CEUFF, 477p.). Although the forests in our study are submitted to an intense edge effect, being thin strips on the landscapes, they represent the majority of forests in PNRJ (Caris et al. 2013Caris EAP, Kurtz BC, Cruz CBM, Scarano FB (2013) Vegetation cover and land use of a protected coastal area and its surroundings, southeast Brazil. Rodriguésia 64: 747-755. doi: 10.1590/S2175-78602013000400006
https://doi.org/10.1590/S2175-7860201300...
). These elongated shapes may actually favor connectivity among forest habitats on the landscape level, which may be extremely important to maintain populations of understory bird species in the Atlantic Forest (Martensen et al. 2008Martensen AC, Pimentel RG, Metzger JP (2008) Relative effects of fragment size and connectivity on bird community in the Atlantic Rain Forest: Implications for conservation. Biological Conservation 141: 2184-2192. doi: 10.1016/j.biocon.2008.06.008
https://doi.org/10.1016/j.biocon.2008.06...
). Our data from marked individuals indicate that three species are especially associated with forest sites in restinga: White-crowned Manakin, Dixiphia pipra (Linnaeus, 1758), (all 11 individuals were captured in forests; a threatened species for Rio de Janeiro state; Alves et al. 2000Alves MAS, Pacheco JF, Gonzaga LAP, Cavalcanti RB, Raposo MA, Yamashita C, Maciel NC, Castanheira M (2000) Aves, p. 113-124. In: Bergallo HG, Rocha CFD, Alves MAS, Van Sluys M (Eds.) A Fauna Ameaçada de Extinção do Estado do Rio de Janeiro. Rio de Janeiro, EdUerj, 166p.), White-flanked Antwren, Myrmotherula axillaris (Vieillot, 1817), (all 21 individuals were captured in forests) and Sooretama Slaty-Antshrike, Thamnophilus ambiguus Swainson, 1825, (17 out of 18 individuals were captured in forests). Those three species also occur in lowland Atlantic Forest, inside two other reserves in the region (Poço das Antas Biological Reserve and União Biological Reserve) which are respectively 50 km and 80 km away from PNRJ (MMA/ICMBio 2008MMA/ICMBio (2008) Plano de Manejo - Reserva Biológica União. Encarte 3 - Análise da Unidade de Conservação. Brasília, Ministério do Meio Ambiente, Instituto Chico Mendes de Conservação da Biodiversidade, 189p., Pacheco et al. 2010Pacheco JF, Castro-Astor IN, Bauer C (2010) Avifauna da Reserva Biológica de Poço das Antas, Silva Jardim, RJ. Atualidades Ornitológicas 157: 55-74.). This lowland region is highly fragmented and all forest remnants may be important to conserve forest-dependent species.
Amazonian flooded forest (Igapó) also contained fewer bird species than the surrounding natural matrix (non-flooded forest - Terra firme), although the former had a higher number of restricted species (Borges & Carvalhaes 2000Borges SH, Carvalhaes A (2000) Bird species of black water inundation forests in the Jaú National Park (Amazonas state, Brazil): their contribution to regional species richness. Biodiversity and Conservation 9: 201-214. doi: 10.1023/A:1008902306499
https://doi.org/10.1023/A:1008902306499...
). These results suggest that similarly to restinga, habitat heterogeneity that results from biotic and abiotic processes promote overall increased species richness via the occurrence of habitat-restricted taxa (see also Stein & Kreft 2014Stein A, Kreft H (2014) Terminology and quantification of environmental heterogeneity in species-richness research. Biological Reviews 90: 815-836. doi: 10.1111/brv.12135
https://doi.org/10.1111/brv.12135...
and Stein et al. 2014Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters 17: 866-880. doi: 10.1111/ele.12277
https://doi.org/10.1111/ele.12277...
).
Between forests in restinga, higher richness in flooded forest may be due to its shorter canopy (up to 15 m) when compared to dry forest (20 m). The shorter stature of flooded forest may result in greater number of sub-canopy birds being captured in mist-nets (Bell 1982Bell HL (1982) A bird community of lowland rainforest in New Guinea. 4. Birds of secondary vegetation. Emu - Austral Ornithology 82: 217-224. doi: 10.1071/MU9820217
https://doi.org/10.1071/MU9820217...
). These results point to the importance of including voice recordings to more effectively sample dry forest bird community. In fact, species recorded by vocalization alone in DF accounted for approximately twice the percentage of species registered in FF. The presence of water in flooded forest also attracted species to the understory, such as the American Pygmy Kingfisher, Chloroceryle aenea (Pallas, 1764) and the Great Kiskadee, Pitangus sulphuratus (Linnaeus, 1766), which were probably searching for fish and tadpoles (Sick 1997Sick H (1997) Ornitologia Brasileira. Rio de Janeiro, Nova Fronteira, 912p.). In contrast, the three most forest-dependent species cited above (D. pipra , M. axillaris and T. ambiguus ) were captured more frequently in DF than in FF (see Appendix 2). Consequently, it appears that sub-canopy birds occasionally used FF understory (foraging in water), while DF contained greater abundance of forest species, likely due to its more structured architecture and apparent higher connectivity with other forests on the landscape.
Restinga forests are apparently prone to disturbances that modify their structure and likely impact their bird communities. For example, the dry forest site experienced winds of 116 km/h on 20 June 2005 and many canopy trees fell or broke during this event. Treefalls in a forest fragment close to our study area as a result of this event were attributed to the instability of peaty soils and the shallow root systems of the trees (Kurtz et al. 2013Kurtz BC, Gomes JC, Scarano FB (2013) Structure and phytogeographic relationships of swamp forests of Southeast Brazil. Acta Botanica Brasilica 27: 647-660. doi: 10.1590/S0102-33062013000400002
https://doi.org/10.1590/S0102-3306201300...
). In addition, flooded forest can vary among years in the amount of time flooded: January to February 2003 (two months), October 2003 to July 2004 (10 months), and February to October 2005 (nine months). Prolonged floods likely result in death of some plants, altering vegetation structure randomly. In fact, flooded forests are considered soil-limited climaxes, as soil conditions limit the vegetation community to reach regional climax (Scarano 2002Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic Forest. Annals of Botany 90: 517-524. doi: 10.1093/aob/mcf189
https://doi.org/10.1093/aob/mcf189...
) and are similar to secondary forests and climax forests gaps Souza & Martins 2005Souza AF, Martins FR (2005) Spatial variation and dynamics of flooding, canopy openness, and structure in a Neotropical swamp forest. Plant Ecology 180: 161-173. doi: 10.1007/s11258-004-7811-7
https://doi.org/10.1007/s11258-004-7811-...
). Araujo et al. (1998Araujo DSD, Scarano FR, Sá CFC, Kurtz BC, Zaluar HLT, Montezuma RCM, Oliveira RC (1998) Comunidades Vegetais do Parque Nacional da Restinga de Jurubatiba, p. 39-62. In: Esteves FA (Ed.) Ecologia das Lagoas Costeiras do Parque Nacional da Restinga de Jurubatiba e do Município de Macaé. Rio de Janeiro, UFRJ, 442p.) suggest that flooded forests are at particular risk to human impacts, due to dispersal of pollutants through water and the delicate equilibrium between plant species and soil water content. They are in fact sensitive to changes in the flood regime (Scarano et al. 1998Scarano FR, Rios RI, Esteves FA (1998) Tree species richness, diversity and flooding regime: case studies of recuperation after anthropic impact in Brazilian flood-prone forests. International Journal of Ecology and Environmental Sciences 24: 223-235.).
Most of the studies dealing with habitat heterogeneity and bird richness have focused on the anthropogenic influence on landscape structure, with few studies focusing on natural mosaic patterns (Bierregaard et al. 1992Bierregaard Jr RO, Lovejoy TE, Kapos V, Santos AA, Hutchings RW (1992) The biological dynamics of tropical rainforest fragments. Bioscience 42: 859-866. doi: 10.2307/1312085
https://doi.org/10.2307/1312085...
, Stein & Kreft 2014Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters 17: 866-880. doi: 10.1111/ele.12277
https://doi.org/10.1111/ele.12277...
). The restinga forest patches of the present study may behave as artificial fragments, due to their susceptibility to disturbances and impacts; these patches may gradually lose bird species.
The restinga is a unique and fragile ecosystem. It is quite heterogeneous and highly dynamic, with considerable turnover in bird species composition and vegetation structure, which demonstrates its importance to fauna species diversity and forest species preservation on the landscape. While scrub habitats show temporal importance to birds (Gomes et al. 2010Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS (2010) Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora Morphology, Distribution, Functional Ecology of Plants 205: 647-655. doi: 10.1016/j.flora.2010.04.014
https://doi.org/10.1016/j.flora.2010.04....
), restinga forests apparently play an important role by connecting habitats and conserving forest species on the landscape. As the restinga environment is a mosaic of habitat types, they complement each other to contribute to regional patterns of diversity. Further, these environments might also help to maintain populations of plants and animals over time, including birds, which may move among habitat types while tracking resources or environmental conditions. Consequently, the heterogeneity of habitats found within restinga might offer some degree of ecological resilience under changing conditions. We suggest that Clusia patches may be considered keystone structures in restinga scrubs, while restinga forests may be keystone structure ecosystems on the landscape level (sensu Tews et al. 2004Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography 31: 79-92. doi: 10.1046/j.0305-0270.2003.00994.x
https://doi.org/10.1046/j.0305-0270.2003...
), both promoting bird species diversity.
The present work shows that remote sensing may be useful to infer vegetation structure and guide management measures in different habitats of a heterogeneous landscape, as restinga. Even a mid-resolution sensor could furnish useful information on vegetation structure in such a mosaic of habitat types. NDVI may also be interesting to monitor changes in vegetation patches through time. Further work is needed to understand how resource conditions might vary over space and time and whether or not this heterogeneity in habitat conditions may actually help to maintain animal populations in this highly dynamic and threatened ecosystem.
ACKNOWLEDGMENTS
To the members of Laboratório de Ecologia de Aves, Departamento de Ecologia, Universidade do Estado do Rio de Janeiro (UERJ), and Marx J. Barros, Carlos H. Oliveira, Barbara L. Ignacio, and Renata P.N. Lima for helping in the field work. To Ricardo Gagliardi for helping with identification of part of the recorded voices. To Dorothy S.D. Araujo for helping with plant identification and important comments on the manuscript and to Luiz Antonio P. Gonzaga for helping with bird identification. To Leidemar M. Gomes for providing wood houses for data loggers and Erly S. Gomes cloth bags for birds (E.S. Gomes passed away on 9 February 2015, a beloved mother who always provided support for her daughter VSMG, this paper is dedicated to her). To Christiano Pinheiro who prepared Fig. 1. Romulo Campus kindly provided the aerial photo of the study area, and Ana Petry helped us to find and get this image. To Idea Wild for providing data loggers and mist nets. To Instituto Nacional de Pesquisas Espaciais (INPE) for supplying CBERS satellite images free of charge. To Instituto Biomas and UERJ for the vehicles used in the field. To Núcleo de Pesquisas de Macaé (NUPEM) for the logistic facilities. VSMG: Ph.D. scholarships by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ). MASA: research grant by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process number 302718/03-6), and by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (process number E-26/102837/2012). This work was also a sub-project of Grupo de Vertebrados/CNPq and PELD - site 5/CNPq.
LITERATURE CITED
- Alves MAS, Pacheco JF, Gonzaga LAP, Cavalcanti RB, Raposo MA, Yamashita C, Maciel NC, Castanheira M (2000) Aves, p. 113-124. In: Bergallo HG, Rocha CFD, Alves MAS, Van Sluys M (Eds.) A Fauna Ameaçada de Extinção do Estado do Rio de Janeiro. Rio de Janeiro, EdUerj, 166p.
- Alves MAS, Storni A, Almeida EM, Gomes VSM, Oliveira CHP, Marques RV, Vecchi MB (2004) A comunidade de aves na Restinga de Jurubatiba, p. 199-214. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.
- Araujo DSD, Scarano FR, Sá CFC, Kurtz BC, Zaluar HLT, Montezuma RCM, Oliveira RC (1998) Comunidades Vegetais do Parque Nacional da Restinga de Jurubatiba, p. 39-62. In: Esteves FA (Ed.) Ecologia das Lagoas Costeiras do Parque Nacional da Restinga de Jurubatiba e do Município de Macaé. Rio de Janeiro, UFRJ, 442p.
- Bell HL (1982) A bird community of lowland rainforest in New Guinea. 4. Birds of secondary vegetation. Emu - Austral Ornithology 82: 217-224. doi: 10.1071/MU9820217
» https://doi.org/10.1071/MU9820217 - Bergallo HG, Martins-Hatano F, Raíces DS, Ribeiro TTL, Alves AG, Luz JL, Mangolin R, Mello MAR (2004) Os mamíferos da Restinga de Jurubatiba, p. 215-230. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.
- Bierregaard Jr RO, Lovejoy TE, Kapos V, Santos AA, Hutchings RW (1992) The biological dynamics of tropical rainforest fragments. Bioscience 42: 859-866. doi: 10.2307/1312085
» https://doi.org/10.2307/1312085 - Borges SH, Carvalhaes A (2000) Bird species of black water inundation forests in the Jaú National Park (Amazonas state, Brazil): their contribution to regional species richness. Biodiversity and Conservation 9: 201-214. doi: 10.1023/A:1008902306499
» https://doi.org/10.1023/A:1008902306499 - Caris EAP, Kurtz BC, Cruz CBM, Scarano FB (2013) Vegetation cover and land use of a protected coastal area and its surroundings, southeast Brazil. Rodriguésia 64: 747-755. doi: 10.1590/S2175-78602013000400006
» https://doi.org/10.1590/S2175-78602013000400006 - Cerqueira R (2000) Biogeografia das restingas, p. 65-75. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.
- Clarke KR, Gorley RN (2002) PRIMER 5 for Windows. Plymouth, Primer E-Ltd, v. 5.2.9.
- Cody ML (1981) Habitat selection in birds: The roles of vegetation structure, competitors, and productivity. BioScience 31: 107-113. doi: 10.2307/1308252
» https://doi.org/10.2307/1308252 - CPTEC (2000) Data from Macaé Airport, in 2000. Centro de Previsão de Tempo e Estudos Climáticos, Instituto Nacional de Pesquisas Espaciais, MCTi. Available online at: Available online at: http://sonda.cptec.inpe.br/basedados/vento_aeroportos.html [Accessed 10/06/2010]
» http://sonda.cptec.inpe.br/basedados/vento_aeroportos.html - CBRO (2014) Listas das aves do Brasil. Comitê Brasileiro de Registros Ornitológicos, 11th ed. Available online at: Available online at: http://www.cbro.org.br/CBRO/listabr.htm [Accessed 21/01/2014]
» http://www.cbro.org.br/CBRO/listabr.htm - De Schauensee RM (1982) A guide to the birds of South America. Philadelphia, The Academy of Natural Science of Philadelphia, 498p.
- Dunning JS (1989) South American Birds. A photographic aid to identification. Newton Square, Harrowood Books, 315p.
- Fleishman E, Ray C, Sjögren-Gulve P, Boggs CL, Murphy DD (2002) Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conservation Biology 16: 706-716. doi: 10.1046/j.1523-1739.2002.00539.x
» https://doi.org/10.1046/j.1523-1739.2002.00539.x - Franzén M, Nilsson SG (2010) Both population size and patch quality affect local extinctions and colonizations. Proceedings of the Royal Society of London B 277: 79-85. doi: 10.1098/rspb.2009.1584
» https://doi.org/10.1098/rspb.2009.1584 - Freitas SR, Mello MCS, Cruz CBM (2005) Relationships between forest structure and vegetation indices in Atlantic Rainforest. Forest Ecology and Management 218: 353-362. doi: 10.1016/j.foreco.2005.08.036
» https://doi.org/10.1016/j.foreco.2005.08.036 - Goerck JM (1999) Distribution of birds along an elevational gradient in the Atlantic forest of Brazil: Implications for the conservation of endemic and endangered species. Bird Conservation International 9: 235-253. doi: 10.1017/S0959270900003439
» https://doi.org/10.1017/S0959270900003439 - Gomes VSM, Silva WR (2002) Spatial variation in understory frugivorous birds in an Atlantic Forest fragment of southeastern Brazil. Ararajuba 10: 219-225. Available online at: http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/1713
» http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/1713 - Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS (2010) Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora Morphology, Distribution, Functional Ecology of Plants 205: 647-655. doi: 10.1016/j.flora.2010.04.014
» https://doi.org/10.1016/j.flora.2010.04.014 - Gonzaga LP, Castiglioni GDA, Reis HBR (2000) Avifauna das restingas do Sudeste: estado do conhecimento e potencial para futuros estudos, p. 151-163. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.
- Gould W (2000) Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecological Applications 10: 1861-1870. doi: 10.2307/2641244
» https://doi.org/10.2307/2641244 - Hasui E, Gomes VSM, Silva WR (2007) Effects of vegetation traits on habitat preferences of frugivorous birds in Atlantic Rain Forest. Biotropica 39: 502-509. doi: 10.1111/j.1744-7429.2007.00299.x
» https://doi.org/10.1111/j.1744-7429.2007.00299.x - Henriques RPB, Araujo DSD, Hay JD (1986) Descrição e classificação dos tipos de vegetação da restinga de Carapebus, Rio de Janeiro. Revista Brasileira de Botânica 9: 173-189.
- Jamel CEG (2004) Caracterização da vegetação da restinga de Jurubatiba com base em sensoriamento remoto e sistema de informação geográfico: estado atual e perspectivas, p. 25-42. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.
- Kerr JT, Ostrovsky M (2003) From space to species: ecological applications for remote sensing. Trends in Ecology & Evolution 18: 299-305. doi: 10.1016/S0169-5347(03)00071-5
» https://doi.org/10.1016/S0169-5347(03)00071-5 - Kurtz BC, Gomes JC, Scarano FB (2013) Structure and phytogeographic relationships of swamp forests of Southeast Brazil. Acta Botanica Brasilica 27: 647-660. doi: 10.1590/S0102-33062013000400002
» https://doi.org/10.1590/S0102-33062013000400002 - Lacerda LD, Araújo DSD, Maciel NC (1993) Dry coastal ecosystems of the tropical Brazilian coast, p. 477-93. In: Van Der Maarel E (Ed.). Dry Coastal Ecosystems: Africa, America and Oceania. Amsterdam, Elsevier, 616p.
- Law BS, Dickman CR (1998) The use of habitat mosaics by terrestrial vertebrate fauna: implications for conservation and management. Biodiversity & Conservation 7: 323-333. doi: 10.1023/A:1008877611726
» https://doi.org/10.1023/A:1008877611726 - Marques MC, Swaine MD, Liebsch D (2011) Diversity distribution and floristic differentiation of the coastal lowland vegetation: implications for the conservation of the Brazilian Atlantic Forest. Biodiversity & Conservation 20: 153-168. doi: 10.1007/s10531-010-9952-4
» https://doi.org/10.1007/s10531-010-9952-4 - Martensen AC, Pimentel RG, Metzger JP (2008) Relative effects of fragment size and connectivity on bird community in the Atlantic Rain Forest: Implications for conservation. Biological Conservation 141: 2184-2192. doi: 10.1016/j.biocon.2008.06.008
» https://doi.org/10.1016/j.biocon.2008.06.008 - McCune B, Mefford MJ (1999) Multivariate Analysis of Ecological Data. Gleneden Beach, MjM Software, v. 4.10.
- McCune B, Grace JB (2002) Analysis of ecological communities. Gleneden Beach, MjM Software Design.
- MMA/ICMBio (2008) Plano de Manejo - Reserva Biológica União. Encarte 3 - Análise da Unidade de Conservação. Brasília, Ministério do Meio Ambiente, Instituto Chico Mendes de Conservação da Biodiversidade, 189p.
- Mota JVL, Carvalho AAF, Tinoco MS (2011) Distribuição e uso de habitat da avifauna na restinga da Reserva Imbassaí, litoral norte da Bahia. Revista Brasileira de Ornitologia 19: 364-375. Available online at: http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/4407
» http://www4.museu-goeldi.br/revistabrornito/revista/index.php/BJO/article/view/4407 - Monteiro RF, Esperanço AP, Becker VO, Otero LS, Herkenhoff EV, Soares A (2004) Mariposas e borboletas na Restinga de Jurubatiba, p. 143-164. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.
- Mulwa RK, Böhning-Gaese K, Schleuning M (2012) High Bird Species Diversity in Structurally Heterogeneous Farmland in Western Kenya. Biotropica 44: 801-809. doi: 10.1111/j.1744-7429.2012.00877.x
» https://doi.org/10.1111/j.1744-7429.2012.00877.x - Oliveira AA, Vicentini A, Chave J, Castanho CDT, Davies SJ, Martini AMZ, Lima RAF, Ribeiro RR, Iribar A, Souza VC (2014) Habitat specialization and phylogenetic structure of tree species in a coastal Brazilian white-sand forest. Journal of Plant Ecology 7: 134-144. doi: 10.1093/jpe/rtt073
» https://doi.org/10.1093/jpe/rtt073 - Pacheco JF, Castro-Astor IN, Bauer C (2010) Avifauna da Reserva Biológica de Poço das Antas, Silva Jardim, RJ. Atualidades Ornitológicas 157: 55-74.
- Parker III TA (1991) On the use of tape recorders in avifaunal surveys. The Auk 108: 443-444.
- Pedroso-Junior NN (2003) Microhabitat occupation by birds in a restinga fragment of Paraná coast, PR, Brazil. Brazilian Archives of Biology and Technology 46: 83-90. doi: 10.1590/S1516-89132003000100013
» https://doi.org/10.1590/S1516-89132003000100013 - Pimentel MCP, Barros MJ, Cirne P, Mattos EA, Oliveira RC, Pereira MCA, Scarano FR, Zaluar HLT, Araujo DSD (2007) Spatial variation in the structure and floristic composition of "restinga" vegetation of southeastern Brazil. Brazilian Journal of Botany 30: 543-351. doi: 10.1590/S0100-84042007000300018
» https://doi.org/10.1590/S0100-84042007000300018 - Porto FCS, Teixeira DM (1984) Um estudo comparativo preliminar sobre as avifaunas das restingas do leste do Brasil, p. 343-349. In: Lacerda LD, Araujo DSD, Cerqueira R, Turcq B (Eds.) Restingas: origens, estruturas, processos. Niterói, CEUFF, 477p.
- Poulin B, Lefebvre G, McNeil R (1993) Variations in bird abundance in tropical arid and semi-arid habitats. Ibis 135: 432-441. doi: 10.1111/j.1474-919X.1993.tb02116.x
» https://doi.org/10.1111/j.1474-919X.1993.tb02116.x - Reis HBR, Gonzaga LP (2000) Análise da distribuição geográfica das aves das restingas do Estado do Rio de Janeiro, p. 165-178. In: Esteves FA, Lacerda LD (Eds.) Ecologia de restingas e lagoas costeiras. Rio de Janeiro, NUPEM\UFRJ, 394p.
- Ridgely RS, Tudor G 1994a. The birds of South America. Austin, University of Texas Press, vol. 1, 516p.
- Ridgely R S, Tudor G 1994b. The birds of South America. Austin, University of Texas Press, vol. 2, 814p.
- Rizzini CT (1979) Tratado de fitogeografia do Brasil: aspectos sociológicos e florísticos. São Paulo, Universidade de São Paulo, 375p.
- Rocha CFD, Sluys MV, Bergallo HG, Alves MAS (2005) Endemic and threatened tetrapods in the restingas of the biodiversity corridors of Serra do Mar and of the Central da Mata Atlântica in eastern Brazil. Brazilian Journal of Biology 65: 159-168. doi: 10.1590/S1519-69842005000100019
» https://doi.org/10.1590/S1519-69842005000100019 - Rocha CFD, Bergallo HG, Sluys MV, Alves MAS, Jamel CE (2007) The remnants of restinga habitats in the Brazilian Atlantic forest of Rio de Janeiro state, Brazil: habitat loss and risk of disappearance. Brazilian Journal of Biology 67: 631-637. doi: 10.1590/S1519-69842007000200011
» https://doi.org/10.1590/S1519-69842007000200011 - Rotenberry JT (1985) The role of habitat in avian community composition: Physiognomy or floristics? Oecologia 67: 213-217. doi: 10.1007/BF00384286
» https://doi.org/10.1007/BF00384286 - Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic Forest. Annals of Botany 90: 517-524. doi: 10.1093/aob/mcf189
» https://doi.org/10.1093/aob/mcf189 - Scarano FR, Rios RI, Esteves FA (1998) Tree species richness, diversity and flooding regime: case studies of recuperation after anthropic impact in Brazilian flood-prone forests. International Journal of Ecology and Environmental Sciences 24: 223-235.
- Sick H (1997) Ornitologia Brasileira. Rio de Janeiro, Nova Fronteira, 912p.
- Stein A, Kreft H (2014) Terminology and quantification of environmental heterogeneity in species-richness research. Biological Reviews 90: 815-836. doi: 10.1111/brv.12135
» https://doi.org/10.1111/brv.12135 - Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters 17: 866-880. doi: 10.1111/ele.12277
» https://doi.org/10.1111/ele.12277 - Souza AF, Martins FR (2005) Spatial variation and dynamics of flooding, canopy openness, and structure in a Neotropical swamp forest. Plant Ecology 180: 161-173. doi: 10.1007/s11258-004-7811-7
» https://doi.org/10.1007/s11258-004-7811-7 - Sylvestre LS, Rosa MMT (2002) Manual metodológico para estudos botânicos na Mata Atlântica. Seropédica, Universidade Federal Rural do Rio de Janairo, 123p.
- Taulman JF, Smith KG (2002) Habitat mapping for bird conservation in North America. Bird Conservation International 12: 281-309. doi: 10.1017/S0959270902002186
» https://doi.org/10.1017/S0959270902002186 - Terborgh J (1985) Habitat selection in Amazon birds, p. 311-338. In: Cody ML (Ed.) Habitat selection in birds. New York, Academic Press, 558p.
- Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography 31: 79-92. doi: 10.1046/j.0305-0270.2003.00994.x
» https://doi.org/10.1046/j.0305-0270.2003.00994.x - Turner W, Spector S, Gardiner N, Fladeland M, Sterling E, Steininger M (2003) Remote sensing for biodiversity, science and conservation. Trends in Ecology & Evolution 18: 306-314. doi: 10.1016/S0169-5347(03)00070-3
» https://doi.org/10.1016/S0169-5347(03)00070-3 - Van Sluys M, Rocha CFD, Hatano FH, Boquimpani-Freitas L, Marra RV (2004) Anfíbios na Restinga de Jurubatiba: composição e história natural, p. 165-178. In: Rocha CFD, Esteves FA, Scarano FR (Eds.) Pesquisas ecológicas de longa duração na Restinga de Jurubatiba: Ecologia, História Natural e Conservação. São Carlos, RiMa, 374p.
- Wilkinson L (1990) SYSTAT: the system for statistics. Evanston, Systat Software.
- Ye X, Skidmore AK, Eang T (2013) Within-patch habitat quality determines the resilience of specialist species in fragmented landscapes. Landscape Ecology 28: 135-147. doi: 10.1007/s10980-012-9826-0
» https://doi.org/10.1007/s10980-012-9826-0
APPENDICES
Bird species occurrence in each study site in the Parque Nacional da Restinga de Jurubatiba (Taxonomy follows CBRO 2014CBRO (2014) Listas das aves do Brasil. Comitê Brasileiro de Registros Ornitológicos, 11th ed. Available online at: Available online at: http://www.cbro.org.br/CBRO/listabr.htm [Accessed 21/01/2014]
http://www.cbro.org.br/CBRO/listabr.htm... ). Source of record: n = nets, o = observations, v = voice recording. Feeding habit: S = seedeater, F = frugivorous, I = insectivorous, N = nectarivorous, C = carnivorous, based on Sick (1997Sick H (1997) Ornitologia Brasileira. Rio de Janeiro, Nova Fronteira, 912p.) and the authors experience - predominant items. FF = flooded forest, DF = dry forest, OS = open scrub, CS = closed scrub.
ONLINE SUPPLEMENTARY MATERIAL
About the Online Supplementary Material (available with the HTML version of the article at http://www.scielo.br/zool):
Figure S1. Snip of a satellite image of the Parque Nacional da Restinga de Jurubatiba region showing the four study sites (Original image: CBERS 2, CCD Sensor - Spectral Band 2 (green), Scale 1:35000. Acquisition on April 7th 2004, supplied by National Institute For Space Research - INPE, holder of Creative Commons License; http://www.dgi.inpe.br/CDSR). DMS shown are coordinates in the lower right edges of the figure.
Publication Dates
-
Publication in this collection
2016
History
-
Received
21 Dec 2015 -
Reviewed
19 Apr 2016 -
Accepted
21 June 2016