Abstract
Pyrimidine derivative 3 was afforded through the reaction of compound (1) with 5-ureidohydantion (2). Product 3 underwent a cyclization to produce fused pyrimidine derivative 7, although the latter product 7 was synthesized through one step via the reaction of compound (1) with 5-ureidohydantion (2) using another catalyst. Compound 3 was oriented to react with cyclic ketones 8a,b in the presence of elemental sulfur, salicylaldehyde (10), aryldiazonium chlorides 12a,b and ω-bromo-4-methoxy- acetophenone (14), which afforded, fused thiophene derivatives 9a,b, coumarin derivative 11, arylhdrazono derivatives 13a,b and 4-methoxyphenyl butenyl derivative 15, respectively. The latter product 15 was reacted with either potassium cyanide (16a) or potassium thiocyanide (16b) to form cyano and thiocyano derivatives 17a,b, respectively. Compound 17a underwent further cyclization to afford pyridopyrimidine derivative 19. Compound 15 was reacted with either hydrazine (20a) or phenylhydrazine (20b) to produce hydrazo derivatives 21a,b and these products were cyclize to produce pyrrole derivatives 23a,b. Finally, 5-ureidohydantion (2) was reacted with compounds 24a,b,c to afford pyrimidine derivatives 25a,b,c. The structures of the synthesized compounds were confirmed using IR, 1H NMR, 13C NMR and mass spectrometry techniques. Compounds 11 and 19 have promising as analgesic and antipyretic activities.
Keywords:
Pyrimidine derivative; Thiophene; Coumarin; Pyridine; Pyrrole; Analgesic; Antipyretic and anti-inflammatory agents
Introduction
A series of studies was introduced to discover that hydantoin derivatives, important heterocyclic compounds, act as antioxidant agents (Gus’kov et al., 20045. Gus'Kov EP, Prokofev VN, Kletskii ME, Kornienko IV, Ogapurenko A, Olekhnovich LP, Chistyakov VA, Shestopalov AV, Sazykina MA. Allantoin as a vitamin. Dokl Biochem Biophys. 2004;398:320-324.). Moreover, Imidazolidine-2,4 dione derivatives are specific biologically active compounds and act as anti-proliferative agents (Reddy et al., 201013. Reddy YT, Reddy PN, Kodru S, Damodran C, Crooks PA. Aplysinopsin analogs: Synthesis and anti-proliferative activity of substituted (Z)-5-(N-benzylindol-3-ylmethylene) imidazolidine-2,4-diones. Bioorg Med Chem. 2010;18(10):3570-3574.), hypoglycemic, aldose reductase inhibitor agents (Iqbal et al., 20157. Iqbal Z, Hameed S, Ali S, Tehseen Y, Shahid M, Iqbal J. Synthesis, characterization, hypoglycemic and aldose reductase inhibition activity of arylsulfonylspiro[fluorene-9,5'-imidazolidine]-2',4'-diones. Eur J Med Chem. 2015;98:127-138.) and Bcl-2 inhibitors (Wang et al., 201516. Wang G, Wang Y, Wang L, Han L, Hou X, Fu H, Fang H. Design, synthesis and preliminary bioactivity studies of imidazolidine-2,4-dione derivatives as Bcl-2 inhibitors. Bioorg Med Chem. 2015;23(23):7359-7365.).
Pyridopyrimidine derivatives have a wide variety of biological properties, including antileishmanial (Agarwal et al., 20051. Agarwal A, Ashutosh R, Goyal N, Chawhan PMS, Gupta S. Dihydropyrido [2,3-d]pyrimidines as a new class of antileishmanial agents. Bioorg Med Chem. 2005;13(24):6678-6684.) and antitubercular activities (Horvati et al., 20156. Horvati K, Bacasa B, Szabo N, Fodor K, Balaka G, Rusavai M, Kiss É, Mezo G, Grolmusz V, Vertessy B, Hudecz F, Bosze S. Antimycobacterial activity of peptide conjugate of pyridopyrimidine derivative against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Tuberculosis. 2015;95(Suppl 1):S207-S211.; Rajesh et al., 201112. Rajesh SM, Kumar RS, Libertsen LA, Perumal S, Yogeeswari P, Sriram D. A green expedient synthesis of pyridopyrimidine-2-thiones and their antitubercular activity. Bioorg Med Chem Lett. 2011;21(10):3012-3016.). Additionally fused thiophene derivatives have antitumor activity (Dallemagne et al., 20032. Dallemagne P, Khanh LP, Alsaidi A, Varlet I, Collot V, Paillet M, Bureau R, Rault S. Synthesis and biological evaluation of five-Membered heterocycles fused to cyclopenta[c]thiophene as new antitumor agents. Bioorg Med Chem. 2003;11(7):1161-1167.) and pyrimidine derivatives containing the coumarin moiety have analgesic and anti-pyretic effects (Keri et al., 20108. Keri RS, Hosamani KM, Shingalapure RV, Hugar MH. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur J Med Chem. 2010;45(6):2597-2605.). Hydrazono derivatives have shown anticancer activity (Sztanke, Rzymowska, Sztanke, 201314. Sztanke M, Rzymowska J, Sztanke K. Synthesis, structure elucidation and in vitro anticancer activities of novel derivatives of diethyl (2E)-2-[(2E)-(1-aryl- imidazolidin-2-ylidene) hydrazono]succinate and ethyl (4-oxo-8-aryl-4,6,7,8-tetra-hydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetate. Bioorg Med Chem. 2013;21(23):7465-7480.) and pyrrole derivatives have antibacterial activity (Padmavathi et al., 201111. Padmavathi V, Prema Kumari C, Venkatesh BC, Padmaja A. Synthesis and antimicrobial activity of amido linked pyrrolyl and pyrazolyl-oxazoles, thiazoles and imidazoles. Eur J Med Chem. 2011;46(11):5317-5326.). In this article we aimed to improve and discover the analgesic, antipyretic and anti-inflammatory activities of synthesized compounds.
Material and Methods
General procedures
The melting points of the synthesized compounds were determined in open capillaries and are uncorrected. Elemental analyses were performed on a Yanaco CHNS Corder elemental analyzer (Japan). IR spectra were measured using KBr discs on a Pye Unicam SP-1000 spectrophotometer. 1H NMR and 13C NMR spectra were measured on a Varian EM 390-200 MHz instrument with CD3SOCD3 as the solvent using TMS as an internal standard material, the chemical shifts were expressed as δ ppm. Mass spectra were recorded on Kratos (75 eV) MS equipment (Germany).
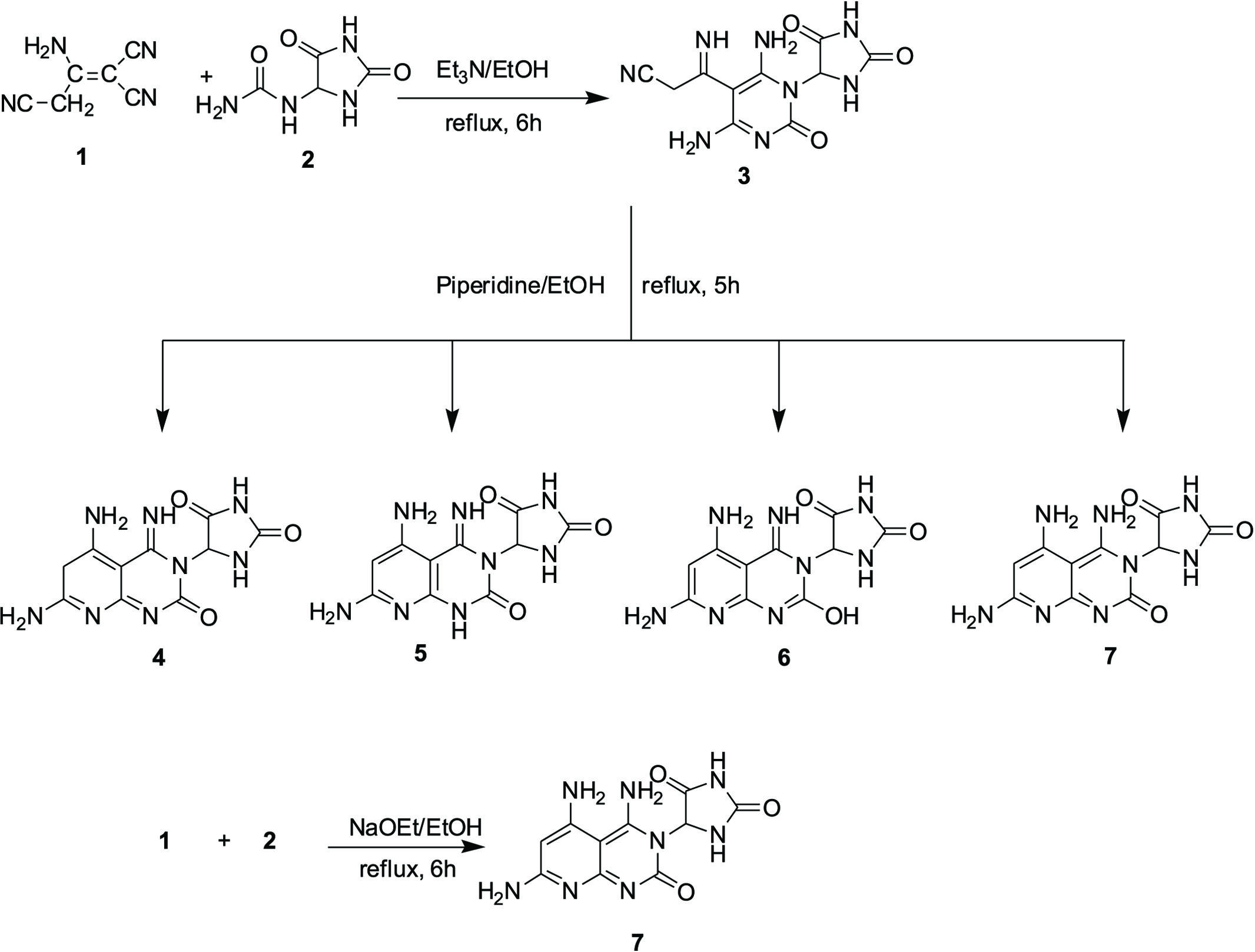
General procedures for the synthesis of compound: 3-(4,6-diamino-1-(2,5-dioxoimidazolidin-4-yl)-2-oxo-1,2-dihydropyrimidin-5-yl)-3-iminopropanenitrile (3)
β-amino-α,γ-dicyanocrotononitrile (1) (3.96 g, 0.03 mol) was added to a 5-ureidohydantoin solution (2) (4.743 g, 0.03 mol) in 100 mL of ethanol containing dimethylformamide (5mL) and triethylamine (1.0 mL) as a catalyst. The reaction mixture was heated under reflux for 6 h, cooled and poured onto ice containing a few drops of HCl. Then, the formed solid product was filtered out.
Compound 3: Faint yellow crystals from ethanol, yield 54%, 4.701 g, m.p. 168-170 ºC. IR (KBr): υ/cm-1 = 3438-3355 (2NH2, 3NH), 2883 (CH2), 2765 (CH), 2223 (CN), 1673, 1668, 1661 (3CO), 1648 (C=C). 1H NMR (DMSO-d6) δ = 3.05-3.13 (s, 2H, CH2), 4.87, 5.12 (2s, 4H, D2O-exchangeable, 2NH2), 5.74 (s, 1H, imidazolidindione ring), 8.35, 9.22, 9.79 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 40.9 (CH2), 62.1 (CH), 106.4 (C=NH), 116.9 (CN), 120.1, 148.6, 152.7 (pyrimidine C), 164.4, 168.3, 171.7 (3C=O). MS (relative intensity) m/z: 290 (M+, 32.2%). Calcd for C10H10N8O3 (290.24): C, 41.38; H, 3.47; N, 38.61%. Found: C, 41.65; H, 3.24; N, 38.90%.
General procedure for the synthesis of compound: 5-(4,5,7-triamino-2-oxopyrido-[2,3-d]pyrimidin-3(2H)-yl)imidazolidine-2,4-dione (7)
Method (A): A solution of compound 3 (0.58 g, 0.002 mol) in ethanol (50 mL) containing a catalytic amount of piperidine (0.5 mL) was heated under reflux for 5 h, poured onto an ice/water mixture containing a few drops of hydrochloric acid. The formed solid product was collected by filtration.
Method (B): β-Amino-α, γ-dicyanocrotononitrile (1) (0.396 g, 0.003 mol) was added to a solution of compound 2 (0.474 g, 0.003 mol) in sodium ethoxide (0.003 mol) [prepared by dissolving sodium metal (0.069 g, 0.003 mol) in absolute ethanol (50 mL)]. The reaction mixture was heated under reflux for 6 h and then evaporated under vacuum. The product was triturated with ethanol and the formed solid product was collected by filtration.
Compound 7: Brown crystals from ethanol, yield 66%, 0.575 g, m.p. 115-117 ºC. IR (KBr): υ/cm-1 = 3488-3327 (3NH2, 2NH), 2783 (CH), 1695, 1684, 1662 (3CO), 1651 (C=N). 1H NMR (DMSO-d6) δ = 4.78, 4.93, 5.27 (3s, 6H, D2O-exchangeable, 3NH2), 5.67 (s, 1H, imidazolidindione ring), 6.87 (s, 1H, pyridine ring), 8.73, 9.95 (2s, 2H, D2O-exchangeable, 2NH). 13C NMR: δ = 55.7 (CH), 124.3, 133.4, 138.5, 144.7, 150.3, 152.4 (pyridine C, pyrimidine C), 166.1, 169.7, 173.2 (3C=O). Calcd for C10H10N8O3 (290.24): C, 41.38; H, 3.47; N, 38.61%. Found: C, 41.11; H, 3.73; N, 38.99%.
General procedure for the synthesis of compounds: 5-(4,6-diamino-5-((2-amino-5,6-dihydro-4H-cyclopenta[b]thiophen-3-yl)(imino)methyl)-2-oxo-pyrimidin-1(2H)-yl) imidazolidine-2,4-dione (9a) and 5-(4,6-diamino-5-((2-amino-4,5,6,7-tetrahydrobenzo [b]thiophen-3-yl(imino) methyl)-2-oxopyrimidin-1(2H) yl) imidazolidine-2,4-dione (9b)
To a solution of compound 3 (0.58 g, 0.002 mol) in ethanol (50 mL) containing trimethylamine (0.5 mL), either cyclopentanone (8a) (0.168 g, 0.002 mol) or cyclohexanone (8b) (0.196 g, 0.002 mol) together with elemental sulfur (0.064 g, 0.002 mol) were added. The reaction mixture was heated under reflux for 4 h, cooled and poured onto an ice/water mixture containing a few drops of HCl. The formed precipitate was collected by filtration.
Compound 9a: Brown crystals from 1,4-dioxane, yield 63%, 0.489 g, m.p. 202-204 ºC. IR (KBr): υ/cm-1 = 3458-3336 (3NH2, 3NH), 2880 (CH2), 2796 (CH), 1683, 1674, 1663 (3CO), 1653 (C=N), 1646 (C=C), 684 (C-S). 1H NMR (DMSO-d6) δ = 2.07-2.18 (m, 6H, 3CH2), 4.54, 4.65, 5.81 (3s, 6H, D2O-exchangeable, 3NH2), 5.98 (s, 1H, imidazolidindione ring), 8.52, 8.77, 9.12 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 22.4, 27.3, 29.9 (3 CH2), 51.3 (CH), 93.8 (C=NH), 126.2, 131.5, 136.3, 140.5, 142.7, 149.1, 151.6 (thiophene C, pyrimidine C), 165.4, 168.8, 172.1 (3C=O). Calcd for C15H16N8O3S (388.40): C, 46.38; H, 4.15; N, 28.85; S, 8.26%. Found: C, 46.17; H, 3.89; N, 28.66; S, 8.01%.
Compound 9b: Brown crystals from 1,4-dioxane, yield 58%, 0.477 g, m.p. 212-214 ºC. IR (KBr): υ/cm-1 = 3460-3349 (3NH2, 3NH), 2882 (CH2), 2768 (CH), 1677, 1671, 1664 (3CO), 1655 (C=N), 1649 (C=C), 676 (C-S). 1H NMR (DMSO-d6) δ = 2.18-2.39 (m, 8H, 4CH2), 4.48, 4.69, 5.45 (3s, 6H, D2O-exchangeable, 3NH2), 5.86 (s, 1H, imidazolidindione ring), 8.61, 8.86, 9.08 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 22.8, 25.4, 27.3, 29.6 (4 CH2), 54.7 (CH), 97.1 (C=NH), 128.1, 134.7, 138.6, 141.5, 146.6, 150.8, 153.3 (thiophene C, pyrimidine C), 162.2, 166.7, 170.6 (3C=O). Calcd for C16H18N8O3S (402.43): C, 47.75; H, 4.51; N, 27.84; S, 7.97%. Found: C, 47.56; H, 4.78; N, 28.09; S, 7.68%.
General procedure for the synthesis of compound:- 5-(4,6-diamino-5-(imino(2-oxo-2H-chromen-3-yl)methyl)-2-oxopyrimidin-1(2H)-yl)imidazolidine-2,4-dione (11)
Salicylaldehyde (10) (0.244 g, 0.002 mol) was added to a solution of compound 3 (0.58 g, 0.002 mol) in 1,4-dioxane (50 mL) containing piperidine (0.5 mL). The reaction mixture was heated under reflux for 5 h and then evaporated under vacuum. The solid product was triturated with ethanol and the formed solid product was collected by filtration.
Compound 11: Yellowish brown crystals from 1,4-dioxane, yield 70%, 0.553 g, m.p. 185-187 ºC. IR (KBr): υ/cm-1 = 3443-3311 (2NH2, 3NH), 3052 (CH-aromatic.), 2762 (CH), 1835, 1681, 1666, 1660 (4C=O), 1652 (C=N), 1112 (CO), 1649 (C=C). 1H NMR (DMSO-d6) δ = 4.76, 5.25 (2s, 4H, D2O-exchangeable, 2NH2), 5.53 (s, 1H, imidazolidindione ring), 6.73 (s, 1H, coumarin H-4), 7.51- 7.66 (m, 4H, C6H4), 8.88, 9.28, 9.55 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 59.5 (CH), 95.3 (C=NH), 125.5, 131.9, 136.8, 140.7, 144.5, 147.2, 149.1, 151.3, 153.5 (pyrimidine C, coumarin C), 161.5, 165.8, 170.3, 181.5 (4C=O). Calcd for C17H13N7O5 (395.33): C, 51.65; H, 3.31; N, 24.80%. Found: C, 51.93; H, 3.59; N, 24.55%.
General procedure for the synthesis of compounds:- 2-(4,6-diamino-1-(2,5-dioxo- imidazolidin-4-yl)-2-oxo1,2-dihydropyrimidin-5-yl)-2-imino-N’-phenyl-acetohydrazonoyl cyanide (13a) and N’-(4-chlorophenyl)-2-(4,6-diamino-1-(2,5-dioxo- imidazolidin-4-yl-2-oxo-1,2-dihydropyrimidin-5-yl)-2-iminoaceto-hydrazonoyl cyanide (13b)
To a cold solution (0-5 C) of pyrimidine derivative 3 (0.58 g, 0.002 mol) in ethanol (50 mL) containing sodium acetate (0.164 g, 0.002 mol) either benzenediazonium chloride (12a) or 4-chlorobenzenediazonium chloride (12b) (0.002 mol) [prepared by adding an aqueous sodium nitrite solution (0.138 g, 0.002 mol) to a cold solution of either aniline or 4-chloroaniline (0.002 mol) in the appropriate amount of glacial acetic acid at (0-5 C) with continuous stirring] was added with continuous stirring. The reaction mixture was stirred at room temperature for an additional 4 h and the solid product was collected by filtration.
Compound 13a: Pale brown crystals from ethanol, yield 67%, 0.528 g, m.p. 137- 139 ºC. IR (KBr): υ/cm-1 = 3476-3354 (2NH2, 4NH), 3053 (CH aromatic), 2761 (CH), 2223 (CN), 1678, 1667, 1663 (3CO), 1657 (C=N), 1646 (C=C). 1H NMR (DMSO-d6) δ = 4.54, 5.17 (2s, 4H, D2O-exchangeable, 2NH2), 5.61 (s, 1H, imidazolidindione ring), 7.31- 7.62 (m, 5H, C6H5), 8.76, 9.13, 9.38, 9.59 (4s, 4H, D2O-exchangeable, 4NH). 13C NMR: δ = 67.3 (CH), 92.4, 109.2 (2C=N), 120.4 (CN), 123.3, 125.9, 127.9, 130.4, 132.8, 134.6, 138.4, 142.5 (pyrimidine C, C6H5), 163.9, 166.8, 169.3 (3C=O). MS (relative intensity) m/z: 394 (M+, 17.9%). Calcd for C16H14N10O3 (394.35): C, 48.73; H, 3.58; N, 35.52%. Found: C, 48.48; H, 3.29; N, 35.80%.
Compound 13b: Dark brown crystals from ethanol, yield 61%, 0.523 g, m.p. 193-195 ºC. IR (KBr): υ/cm-1 = 3451-3290 (2NH2, 4NH), 3050 (CH aromatic), 2770 (CH), 2224 (CN), 1673, 1663, 1659 (3CO), 1652 (C=N), 1647 (C=C). 1H NMR (DMSO-d6) δ = 4.63, 5.08 (2s, 4H, D2O-exchangeable, 2NH2), 5.89 (s, 1H, imidazolidindione ring), 7.44- 7.58 (d.d, 4H, C6H4), 8.58, 9.24, 9.55, 9.67 (4s, 4H, D2O-exchangeable, 4NH). 13C NMR: δ = 62.5 (CH), 98.4, 106.4 (2C=N), 121.7 (CN), 122.5, 124.8, 125.8, 128.3, 131.6, 135.3, 137.7, 141.5 (pyrimidine C, C6H5), 162.8, 165.7, 168.8 (3C=O). MS (relative intensity) m/z: 428 (M+, 23.4%). Calcd for C16H13ClN10O3 (428.79): C, 44.82; H, 3.06; N, 32.67%. Found: C, 44.56; H, 3.33; N, 32.40%.
General procedure for synthesis of compound: 4-bromo-2-((4,6-diamino-1-(2,5-dioxo- imidazolidin-4-yl)-2-oxo-1,2-dihydropyrimidin-5-yl) (imino) methyl)-3-(4-methoxy- phenyl) but-2-enenitrile (15)
ω-Bromo-4-methoxyacetophenone (14) (0.524 g, 0.002 mol) was added to a solution of compound 3 (0.58 g, 0.002 mol) in 1,4-dioxane (40 mL). The reaction mixture was stirred at room temperature for 2 h and then poured on to a beaker containing ice/water mixture. The formed solid product was collected by filtration.
Compound 15: Brown crystals from ethanol, yield 72%, 0.712 g, m.p. 121-123 ºC. IR (KBr): υ/cm-1 = 3422-3286 (2NH2, 3NH), 3055 (CH aromatic), 2986 (CH3), 2867 (CH2), 2766 (CH), 2225 (CN), 1680, 1669, 1662 (3CO), 1658 (C=N), 1648 (C=C). 1H NMR (DMSO-d6) δ = 3.32 (s, 3H, OCH3), 3.77 (s, 2H, CH2), 4.81, 5.29 (2s, 4H, D2O-exchangeable, 2NH2), 5.55 (s, 1H, imidazolidindione ring), 7.48-7.71 (d.d, 4H, C6H4), 8.66, 9.22, 9.45 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 41,2 (CH3), 49.5 (CH2), 65.3 (CH), 81.3, 88.6 (C=C), 103.7 (C=N), 118.3 (CN), 124.6, 126.7, 129.3, 131.5, 133.9, 135.4, 137.8 (pyrimidine C, C6H4), 160.7, 165.7, 169.8 (3C=O). MS (relative intensity) m/z: 500 (M+, 13.8%), 502 (M+, 13.4%), Calcd for C19H17BrN8O4 (501.29): C, 45.52; H, 3.42; N, 22.35%. Found: C, 45.81; H, 3.19; N, 22.63%.
General procedure for the synthesis of compounds: 2-((4,6-diamino-1-(2,5-dioxo- imidazolidin-4-yl)-2-oxo-1,2-dihydropyrimidin-5-yl) (imino) methyl)-3- (4- methoxy-phenyl-pent-2-enedinitrile (17a) and 2-((4,6-diamino-1-(2,5-dioxo-imidazolidin-4-yl)-2-oxo-1,2-dihydropyrimidin-5-yl)(imino)methyl)-3-(4-methoxyphenyl)-4-thio-cyanatobut-2-enenitrile (17b)
Either potassium cyanide (16a) (0.122 g, 0.002 mol) or potassium thiocyanate (16b) (0.189 g, 0.002 mol) was added to a solution of compound 15 (1.002 g, 0.002 mol) in ethanol (50 mL) in water bath at 60 oC, with continuous stirring. The reaction mixture was maintained in the water bath for 1 h at 60 ºC and then poured into a beaker containing an ice/water mixture and a few drops of HCl. The formed solid product was collected by filtration.
Compound 17a: Dark brown crystals from ethanol, yield 69%, 0.617 g, m.p. 157-159 ºC. IR (KBr): υ/cm-1 = 3445-3266 (2NH2, 3NH), 3051 (CH aromatic), 2978 (CH3), 2881 (CH2), 2754 (CH), 2225, 2223 (2CN), 1682, 1673, 1661 (3CO), 1657 (C=N), 1649 (C=C). 1H NMR (DMSO-d6) δ = 3.41 (s, 3H, OCH3), 3.76 (s, 2H, CH2), 4.55, 4.87 (2s, 4H, D2O-exchangeable, 2NH2), 5.63 (s, 1H, imidazolidindione ring), 7.33-7.54 (d.d, 4H, C6H4), 8.45, 8.76, 9.33 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 38.1 (CH3), 48.9 (CH2), 62.7 (CH), 77.7, 83.5 (C=C), 97.6 (C=N), 116.5, 119.2 (2CN), 122.8, 125.4, 128.6, 130.6, 134.4, 136.7, 138.9 (pyrimidine C, C6H4), 161.4, 164.5, 168.1 (3C=O). MS (relative intensity) m/z: 447 (M+, 28.4%). Calcd for C20H17N9O4 (447.41): C, 53.69; H, 3.83; N, 28.18%. Found: C, 53.96; H, 3.59; N, 28.43%.
Compound 17b: Brown crystals from ethanol, yield 64%, 0.613 g, m.p. 181-183 ºC. IR (KBr): υ/cm-1 = 3423-3233 (2NH2, 3NH), 3053 (CH aromatic), 2960 (CH3), 2884 (CH2), 2760 (CH), 2224, 2221 (2CN), 1680, 1672, 1662 (3CO), 1659 (C=N), 1651 (C=C). 1H NMR (DMSO-d6) δ = 3.25 (s, 3H, OCH3), 3.44 (s, 2H, CH2), 4.51, 4.73 (2s, 4H, D2O-exchangeable, 2NH2), 5.55 (s, 1H, imidazolidindione ring), 7.39- 7.61 (d.d, 4H, C6H4), 8.56, 8.74, 9.22 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 41.2 (CH3), 49.7 (CH2), 63.9 (CH), 76.7, 86.8 (C=C), 97.9 (C=N), 117.2, 119.8 (2CN), 123.9, 126.7, 129.9, 132.5, 136.2, 138.9, 140.7 (pyrimidine C, C6H4), 162.2, 164.8, 167.8 (3C=O). MS (relative intensity) m/z: 479 (M+, 23.3%). Calcd for C20H17N9O4S (479.47): C, 50.10; H, 3.57; N, 26.29; S, 6.69%. Found: C, 50.34; H, 3.28; N, 26.57; S, 6.41%.
General procedure for the synthesis of compound: 6-amino-2-(4,6-diamino-1-(2,5-dioxoimidazolidin-4-yl)-2-oxo-1,2-dihydropyrimidin-5-yl)-4-(4-methoxyphenyl)- nicotinonitrile (19)
The solution of compound 17a (0.447 g, 0.001 mol) in sodium ethoxide (0.001 mol) [prepared by dissolving sodium metal (0.023 g, 0.001 mol) in absolute ethanol (50 mL)]. The reaction was heated under reflux for 4 h and then evaporated under vacuum. The product was triturated with ethanol and the formed product was collected by filtration.
Compound 19: Yellow crystals from ethanol, yield 57%, 0.255 g, m.p. 207-209 ºC. IR (KBr): υ/cm-1 = 3462-3220 (3NH2, 2NH), 3054 (CH aromatic), 2985 (CH3), 2766 (CH), 2221 (CN), 1688, 1672, 1664 (3CO), 1655 (C=N), 1647 (C=C). 1H NMR (DMSO-d6) δ = 3.68 (s, 3H, OCH3), 4.38, 4.93, 5.33 (3s, 6H, D2O-exchangeable, 3NH2), 5.77 (s, 1H, imidazolidindione ring), 7.14 (s, 1H, pyridine), 7.28-7.49 (d.d, 4H, C6H4), 8.41, 8.82 (2s, 2H, D2O-exchangeable, 2NH). 13C NMR: δ = 40.4 (CH3), 63.3 (CH), 117.8 (CN), 120.6, 123.9, 125.2, 127.6, 131.1, 133.5, 136.2, 138.4, 140.7, 142.6, 144.5, 145.8 (Pyridine C, pyrimidine C, C6H4), 160.8, 163.3, 166.7 (3C=O). MS (relative intensity) m/z: 447 (M+, 30.5%). Calcd for C20H17N9O4 (447.41): C, 53.69; H, 3.83; N, 28.18%. Found: C, 53.44; H, 4.09; N, 28.37%.
General procedure for the synthesis of compounds: 2-((4,6-diamino -1-(2,5-dioxo- imidazolidin-4-yl) -2-oxo -1,2-dihydropyrimidin -5-yl) (imino) methyl) -4-hydrazinyl-3-(4-methoxyphenyl)but-2-enenitrile (21a) and 2-((4,6-diamino-1-(2,5-dioxo -imidazol- idin-4-yl)-2-oxo-1,2-dihydropyrimidin-5-yl) (imino) methyl)-3-(4-methoxy -phenyl)-4-(2-phenyl- hydrazinyl)but-2-enenitrile (21b)
Either hydrazine hydrate (20a) (0.1 g, 0.002 mol) or phenylhydrazine (20b) (0.22 g, 0.002) was added to a solution of compound 15 (1.002 g, 0.002 mol) in ethanol (50 mL). The reaction mixture was heated under reflux for 4 h and then poured onto an ice/water mixture containing a few drops of hydrochloric acid. The formed solid product was collected by filtration.
Compound 21a: Pale yellow crystals from ethanol, yield 74%, 0.67 g, m.p. 221-223 ºC. IR (KBr): υ/cm-1 = 3389-3212 (3NH2, 4NH), 3050 (CH aromatic), 2974 (CH3), 2881 (CH2), 2760 (CH), 2227 (CN), 1683, 1667, 1660 (3CO), 1655 (C=N), 1649 (C=C). 1H NMR (DMSO-d6) δ = 3.19 (s, 3H, OCH3), 3.28 (s, 2H, CH2), 4.58, 5.12, 5.28 (3s, 6H, D2O-exchangeable, 3NH2), 5.71 (s, 1H, imidazolidindione ring), 6.83-7.17 (d.d, 4H, C6H4), 8.43, 8.68, 8.77, 9.53 (4s, 4H, D2O-exchangeable, 4NH). 13C NMR: δ = 37.5 (CH3), 53.3 (CH2), 66.7 (CH), 79.4, 86.4 (C=C), 107.6 (C=N), 115.7 (CN), 120.5, 125.9, 128.2, 132.3, 134.7, 136.7, 138.9 (pyrimidine C, C6H4), 164.4, 166.9, 170.2 (3C=O). MS (relative intensity) m/z: 452 (M+, 27.4%). Calcd for C19H20N10O4 (452,43): C, 50.44; H, 4.46; N, 30.96%. Found: C, 50.71; H, 4.73; N, 30.68%.
Compound 21b: Pale yellow crystals from ethanol, yield 65%, 0.688 g, m.p. 240-242 ºC. IR (KBr): υ/cm-1= 3368-3188 (2NH2, 5NH), 3056 (CH aromatic), 2988 (CH3), 2879 (CH2), 2758 (CH), 2225 (CN), 1688, 1666, 1661 (3CO), 1657 (C=N), 1650 (C=C). 1H NMR (DMSO-d6) δ = 3.12 (s, 3H, OCH3), 3.22 (s, 2H, CH2), 5.17, 5.44 (2s, 4H, D2O-exchangeable, 2NH2), 5.55 (s, 1H, imidazolidindione ring), 6.91-7.12 (d.d, 4H, C6H4), 7.38-7.53 (m, 5H, C6H5), 8.22, 8.51, 8.79, 9.11, 9.58 (5s, 5H, D2O-exchangeable, 5NH). 13C NMR: δ = 39.4 (CH3), 51.7 (CH2), 69.5 (CH), 78.8, 83.2 (C=C), 110.4 (C=N), 117.6 (CN), 120.3, 122.5, 124.7, 127.1, 130.8, 132.6, 134.8, 136.5, 139.6, 141.2, 143.4, 146.4 (pyrimidine C, C6H4, C6H5), 163.3, 165.8, 167.8 (3C=O). MS (relative intensity) m/z: 528 (M+, 23.6%). Calcd for C25H24N10O4 (528.52): C, 56.81; H, 4.58; N, 26.50%. Found: C, 56.55; H, 4.33; N, 26.21%.
General procedure for the synthesis of compounds: 5-(4,6-diamino-5-((1,2-diamino-4-(4-methoxyphenyl)-1H- pyrrol- 3- yl) (imino)methyl)-2-oxopyrimidin-1(2H)-yl) imidazolidine-2,4-dione (23a) and 5-(4,6-diamino-5-((2-amino-4-(4-methoxyphenyl)-1-(phenylamino)-1H-pyrrol-3-yl)(imino)methyl)-2-oxopyrimidin-1(2H)-yl)imidazolidine-2,4-dione (23b)
The reactions began either with solutions of compound 21a (0.452 g, 0.001 mol) or compound 21b (0.528 g, 0.001 mol) in sodium ethoxide (0.001 mol) in absolute ethanol (50 mL). The reaction was heated under reflux for 3 h and then evaporated under vacuum. The product was triturated with ethanol and the formed product was collected by filtration.
Compound 23a: Creamy white crystals from ethanol, yield 57%, 0.258 g, m.p. 178-180 ºC. IR (KBr): υ/cm-1 = 3411-3264 (4NH2, 3NH), 3055 (CH aromatic), 2981 (CH3), 2768 (CH), 1689, 1668, 1663 (3CO), 1651 (C=N), 1645 (C=C). 1H NMR (DMSO-d6) δ = 3.27 (s, 3H, OCH3), 4.38, 4.59, 4.95, 5.23 (4s, 8H, D2O-exchangeable, 4NH2), 5.63 (s, 1H, imidazolidindione ring), 6.95-7.28 (m, 4H, C6H4, 1H, pyrrole), 8.51, 8.77, 9.38 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 39.2 (CH3), 64.4 (CH), 103.8 (C=N), 121.7, 123.6, 126.7, 128.9, 131.4, 133.6, 136.5, 138.5, 140.2, 143.4, 145.1 (pyrrole C, pyrimidine C, C6H4), 167.1, 169.8, 173.4 (3C=O). MS (relative intensity) m/z: 452 (M+, 21.5%). Calcd for C19H20N10O4 (452,43): C, 50.44; H, 4.46; N, 30.96%. Found: C, 50.18; H, 4.69; N, 30.72%.
Compound 23b: Pale yellow crystals from ethanol, yield 55%, 0.29 g, m.p. 150-152 ºC. IR (KBr): υ/cm-1= 3386-3187 (3NH2, 4NH), 3053 (CH aromatic), 2975 (CH3), 2782 (CH), 1685, 1671, 1665 (3CO), 1656 (C=N), 1650 (C=C). 1H NMR (DMSO-d6) δ = 3.41 (s, 3H, OCH3), 4.44, 4.67, 5.21 (3s, 6H, D2O-exchangeable, 3NH2), 5.54 (s, 1H, imidazolidindione ring), 6.86-7.37 (m, 4H, C6H4, 5H, C6H5, 1H, pyrrole), 8.43, 8.68, 9.17, 9.56 (4s, 4H, D2O-exchangeable, 4NH). 13C NMR: δ = 35.7 (CH3), 57.4 (CH), 101.4 (C=N), 120.2, 121.9, 123.3, 125.8, 127.5, 130.1, 132.7, 134.6, 136.7, 139.1, 141.5, 144.1, 146.3, 147.5 (pyrrole C, pyrimidine C, C6H4, C6H5), 163.3, 169.4, 171.8 (3C=O). MS (relative intensity) m/z: 528 (M+, 28.3%). Calcd for C25H24N10O4 (528.52): C, 56.81; H, 4.58; N, 26.50%. Found: C, 56.55; H, 4.86; N, 26.33%.
General procedure for the synthesis of compounds: 5-(4-amino-6-imino-2-oxo-5-(1-phenylethylidene)-5,6-dihydropyrimidin-1(2H)-yl)imidazolidine-2,4-diones (25a), 5-(4-amino-6-imino-2-oxo-5-(2-phenylhydrazono-5,6-dihydropyrimidin-1(2H)-yl) imidazol- idine-2,4-dione (25b) and 5-(4-amino-5-(2-(4-chlorophenyl)hydrazono)-6-imino-2-oxo-5,6-dihydro- pyrimidin-1(2H)-yl)imidazolidine-2,4-dione (25c)
Either compound 24a (0.505 g, 0.003 mol), 24b (0.614 g, 0.003 mol) or 24c (0.474 g, 0.003 mol) was added to a solution of 5-ureidohydantion (2) (0.474 g, 0.003 mol) in 50 mL of ethanol containing dimethylformamide (5.0 mL) and triethylamine (1.0 mL) as a catalyst. The reaction mixture was heated under reflux for 5 h, cooled and poured onto ice containing a few drops of HCl. The formed solid product was filtered out.
Compound 25a: Pale brown crystals from ethanol, yield 61%, 0.597 g, m.p. 251-253 ºC. IR (KBr): υ/cm-1= 3407-3326 (NH2, 3NH), 3051 (CH aromatic), 2978 (CH3), 2734 (CH), 1688, 1671, 1662 (3CO), 1657 (C=N), 1647 (C=C). 1HNMR (DMSO) δ = 1.87 (s, 3H, CH3), 4.63 (s, 2H, D2O-exchangeable, NH2), 5.65 (s, 1H, imidazolidindione ring), 7.27-7.44 (m, 5H, C6H5), 8.22, 8.46, 9.37 (3s, 3H, D2O-exchangeable, 3NH). 13C NMR: δ = 23.3 (CH3), 61.4 (CH), 86.4 (C=C), 118.7, 123.5, 126.7, 128.9, 130.2, 133.6, 137.4, 139.3 (pyrimidine C, C6H5), 160.2, 162.7, 165.6 (3C=O). MS (relative intensity) m/z: 326 (M+, 19.8%). Calcd for C15H14N6O3 (326.31): C, 55.21; H, 4.32; N, 25.75%. Found: C, 55.48; H, 4.05; N, 25.49%.
Compound 25b: Brown crystals from ethanol, yield 53%, 0.522 g, m.p. 197-199 ºC. IR (KBr): υ/cm-1= 3428-3335 (NH2, 4NH), 3053 (CH aromatic), 2766 (CH), 1684, 1672, 1664 (3CO), 1656 (C=N), 1649 (C=C). 1HNMR (DMSO) δ = 4.55 (s, 2H, D2O-exchangeable, NH2), 5.43 (s, 1H, imidazolidindione ring), 7.18-7.37 (m, 5H, C6H5), 8.33, 8.49, 8.74, 9.37 (4s, 4H, D2O-exchangeable, 4NH). 13C NMR: δ = 65.5 (CH), 120.4, 122.7, 125.9, 127.4, 130.4, 132.8, 135.8, 138.7 (pyrimidine C, C6H5), 161.7, 163.9, 165.5 (3C=O). MS (relative intensity) m/z: 328 (M+, 15.7%). Calcd for C13H12N8O3 (328.29): C, 47.56; H, 3.68; N, 34.13%. Found: C, 47.31; H, 3.94; N, 34.37%.
Compound 25c: Brown crystals from ethanol, yield 57%, 0.62 g, m.p. 218-220 ºC. IR (KBr): υ/cm-1= 3444-3352 (NH2, 4NH), 3057 (CH aromatic), 2761 (CH), 1682, 1670, 1663 (3CO), 1653 (C=N), 1647 (C=C). 1HNMR (DMSO) δ = 4.72 (s, 2H, D2O-exchangeable, NH2), 5.66 (s, 1H, imidazolidindione ring), 7.39-7.54 (d.d, 4H, C6H4), 8.38, 8.62, 8.88, 9.28 (4s, 4H, D2O-exchangeable, 4NH). 13C NMR: δ = 54.8 (CH), 121.4, 123.6, 125.7, 128.5, 131.5, 133.9, 135.7, 139.5 (pyrimidine C, C6H4), 162.8, 164.7, 167.3 (3C=O). MS (relative intensity) m/z: 362 (M+, 15.7%). Calcd for C13H11ClN8O3 (362.73): C, 43.05; H, 3.06; N, 30.89%. Found: C, 43.33; H, 3.34; N, 30.62%.
Pharmacology
Analgesic activity
Analgesic activity was introduced by the tail flick method (Fadeyi et al., 20044. Fadeyi OO, Obafemi CA, Adewunmi CO, Iwalewa EO. Antipyretic, analgesic, anti-inflammatory and cytotoxic effects of four derivatives of salicylic acid and anthranilic acid in mice and rats. Afr J Biotech. 2004;3(8):426-431.; Vogel, 200215. Vogel HG. Drug discovery and evaluation. Pharmacological assay. 2 ed. Berlin, Heidelberg: Springer-Verlag; 2002. p. 695-696; 759-762; 772-773.). Healthy albino mice weighing 20.0 g to 30.0 g were divided into different groups with six animals in each group. The control group received a 0.5% w/v carboxymethylcellulose (CMC) solution and the treated groups were given a 132 µmol/kg orally dose of compounds 3, 7, 9a ,b, 11, 13a ,b, 15, 17a, b, 19, 21a, b, 23a, b and 25a, b, c. The reaction times were noted at 2 h and 4 h intervals after drug administration. The percentage analgesic activity was calculated by the following formula:-
where:- T1 is the normal reaction time; T2 is the reaction time after treatment.
Antipyretic activity
Healthy Wistar rats were given s.c. 10mL/kg of a 20% aqueous suspension of sterilized brewer’s yeast powder (Fadeyi et al., 20044. Fadeyi OO, Obafemi CA, Adewunmi CO, Iwalewa EO. Antipyretic, analgesic, anti-inflammatory and cytotoxic effects of four derivatives of salicylic acid and anthranilic acid in mice and rats. Afr J Biotech. 2004;3(8):426-431.; Vogel, 200215. Vogel HG. Drug discovery and evaluation. Pharmacological assay. 2 ed. Berlin, Heidelberg: Springer-Verlag; 2002. p. 695-696; 759-762; 772-773.) weighting between 150 g and 200 g. Eighteen hours later, the animals showing an increase in rectal temperature greater than 0.5 ºC were selected. The control group received a 0.5% w/v carboxymethylcellulose solution and the treated groups received a of 132 µmol/kg dose of compounds 3, 7, 9a, b, 11, 13a, b, 15, 17a, b, 19, 21a, b, 23a, b and 25a, b, c. Rectal temperatures were noted using digital thermometer 30 minute before (pretreated) and at 1 h, 2 h and 4 h after administration of the dose.
Anti-inflammatory activity
The anti-inflammatory activity was examined using a hind paw edema method on albino rats of either six (Fadeyi et al., 20044. Fadeyi OO, Obafemi CA, Adewunmi CO, Iwalewa EO. Antipyretic, analgesic, anti-inflammatory and cytotoxic effects of four derivatives of salicylic acid and anthranilic acid in mice and rats. Afr J Biotech. 2004;3(8):426-431.; Vogel, 200215. Vogel HG. Drug discovery and evaluation. Pharmacological assay. 2 ed. Berlin, Heidelberg: Springer-Verlag; 2002. p. 695-696; 759-762; 772-773.). A freshly prepared of carrageenan solution (0.1mL, 1%w/v) was injected into the sub-plantar surface of the right hind limb of each animal. The control group received a 0.5% w/v CMC solution and the treated groups were orally given a 132 µmol/kg dose of compounds 3, 7, 9a ,b, 11, 13a ,b, 15, 17a, b, 19, 21a, b, 23a, b and 25a, b, c 30 minute before carrageenan. The volume of each paw was measured with a plethysmometer at 2 h and 4 h intervals after carrageenan injection. The percentage inhibition of edema was calculated by the following formula:
where: VC is the paw volume of control animal; VT is the paw volume of treated animals (standard /test compound).
Results and Discussion
This study was a continuation of our efforts aimed at the synthesis of new heterocyclic compounds with significant biological potential (El-Sharkawy et al., 20123. El-Sharkawy KA, Nasser NE, Zaki MY. Uses of 2-Amino-5, 6-dihydro-4H-cyclopenta [b] thiophene-3-carbonitrile in the synthesis of novel heterocyclic compounds with anticonvulsant, behavioral and CNS antidepressant activities. Int Res J Pure App Chem. 2012;2(1):91-104.; Mohareb, El-Sharkawy, Sherif, 200810. Mohareb R, El-Sharkawy KA, Sherif SM. The reaction of ß-amino-a,?-dicyano -crotononitrile with acetophenone: Synthesis of pyridine, pyridazine and thiophene derivatives with antimicrobial activities. Acta Pharm. 2008;58:429-444.). The goals of this work were to study the possibility of using compounds 2 and 3 in heterocyclic synthesis to produce the pyridopyrimidine derivative 7; thiophene derivatives 9a,b; coumarin derivative 11; pyrimidine derivatives 13,15,17a,b,21a,b; pyridine derivative 19; pyrazole derivatives 23a,b and iminopyrimidine derivatives 25a,b,c, as well as biologically evaluate these compounds for analgesic, antipyretic and anti-inflammatory activities. The reaction of β-amino-α, γ-dicyanocrotono- nitrile (1) with 5-ureidohydantion (2) using triethylamine as catalyst produced compound 3. The latter product underwent cyclization in the presence of piperidine. Four isomeric structures were considered, including 4,5,6 and 7. The 1HNMR spectral data showed that the final product contained three singlets at δ = 4.78, 4.93, 5.27 ppm and two singlets at δ = 8.73, 9.95 ppm which represented the presence of 3NH2 and 2NH groups, respectively; thus, the structures of compounds 4,5 and 6 were ruled out, as those latter structures only containing 2NH2 groups. Additionally, structure 6 contained an OH group which it was absent in the analytical and spectral data. In contrast, compound 7 was produced by another pathway, through the reaction of β-amino-α, γ-dicyanocrotononitrile (1) with 5-ureidohydantion (2) in the presence of sodium ethoxide directly. Compound 3 reacted with either cyclopentanone (8a) or cyclohexanone (8b) in the presence of elemental sulfur and trimethylamine afforded compounds 9a,b respectively. The structures of compounds 9a,b were verified by elemental analysis and spectral data. In compound 9a, the 1HNMR spectrum indicated the presence of a multiplet at δ = 2.07-2.18 ppm which could be assigned to the 3CH2 groups; three singlets at δ = 4.54, 4.65, 5.81 ppm, which indicate the presence of 3NH2 groups; a singlet at δ = 5.98 ppm, which indicate the presence of 1H of an imidazolidindione ring and three singlets at δ = 8.52, 8.77, 9.12 ppm corresponding to 3NH groups. Coumarin derivative 11 was formed via the reaction of compound 3 with salicylaldehyde (10) and the structure of the compound was confirmed. The 1HNMR spectrum indicated the presence of two singlets at δ = 4.76, 5.25 ppm, which indicate the presence of 2NH2 groups; a singlet at δ = 5.53 ppm, which indicates the presence of an 1H of imidazolidindione ring; a singlet at δ = 6.73 ppm, which indicate the presence of a coumarin 1H; a multiplet at δ = 7.51-7.66 ppm corresponding to 4H of benzene ring; and three singlets at δ = 8.88, 9.28, 9.55 ppm, which indicate the presence of 3NH groups. Compound 3 was also reacted with aryldiazonium salts 12a,b to afford arylhydrazono derivatives 13a,b respectively. The elucidation of the structure for these compounds was then confirmed. The 1HNMR spectrum for compound 13a showed the presence of two singlets at δ = 4.54, 5.17 ppm, which indicate the presence of 2NH2 groups; a singlet at δ = 5.61 ppm, which indicates the presence of 1H of an imidazolidindione ring; a multiplet at δ = 7.31-7.62 ppm corresponding to 5H of benzene ring; and four singlets at δ = 8.76, 9.13, 9.38, 959 ppm which indicate the presence of 4NH groups.
The last reaction of compound 3, was performed with ω-bromo-4-methoxyacetophenone (14), and the 4-methoxyphenylbutenyl derivative 15 was afforded. The elucidation of this structure was based on analytical and spectral data. Compound 15 was reacted with either potassium cyanide (16a) or potassium thiocyanate (16b) to form either the 4-methoxyphenylbutenyl cyanide derivative 17a or 4-methoxyphenylbutenyl thiocyanide derivative 17b, respectively. The structures of compounds 17a,b were verified by analytical and spectral data. Compound 17a underwent a cyclization in presence of sodium ethoxide to afford pyridine derivative 19 via formation of intermediate 18. The structure of compound 19 was then confirmed. The 1HNMR spectrum of compound 19 detected the presence of singlet at δ = 3.68 ppm, which indicates the presence of 3H of CH3 group; three singlets at δ = 4.38, 4.93 5.33 ppm, which indicate the presence of 3NH2 groups; a singlet at δ = 5.77 ppm, which indicates the presence of 1H of imidazolidindione ring; a singlet at δ = 7.14 ppm which indicates the presence of 1H of pyridine ring; a doublet of doublets at δ = 7.28-7.49 ppm corresponding to 4H of benzene ring and two singlets at δ = 8.41, 8.82 ppm, which indicate the presence of 2NH groups. Compound 15 was reacted with either hydrazine hydrate (20a) or phenyl hydrazine (20b) to produce hydrazono derivatives 21a,b, respectively. The structures of these compounds were confirmed by analytical and spectral data. The latter products underwent a cyclization to form pyrrole derivatives 23a,b through the intermediate formation of 22a,b, respectively. The structures of compounds 23a,b were confirmed using analytical and spectral data. The 1HNMR spectrum of compound 23a detected the presence of a singlet at δ = 3.27 ppm, which indicates the presence of 3H from a CH3 group; four singlets at δ = 4.38, 4.59, 4.95, 5.23 ppm, which indicate the presence of 4NH2 groups; a singlet at δ = 5.63 ppm, which indicates the presence of 1H of imidazolidindione ring; a multiplet at δ = 6.95-7.28 ppm corresponding to 4H of benzene ring and 1H of pyrrole ring and three singlets at δ = 8.51, 8.77, 9.38 ppm, which indicate the presence of 3NH groups. Finally 5-ureidohydantion (2) was reacted with compounds 24a,b,c to produce iminopyrimidine derivatives 25a,b,c, respectively and the structure of these compounds were confirmed by analytical and spectral data.
All the synthesized compounds were evaluated for their in vitro analgesic, antipyretic and anti-inflammatory activities. Acetaminophen was used as a reference standard drug. Based on the results from (Tables I and II), it is clear that compounds 11 and 19 showed promising actions as analgesic and antipyretic agents. This may be due their containing a coumarin moiety and 4-methoxyphenylpyridine moiety, respectively. In contrast, compounds 7, 9a, b, 13a, b showed moderate analgesic and antipyretic effects. The remaining compounds 3, 15, 17a, b, 21a, b, 23a, b, 25a, b, c exhibited poor analgesic and antipyretic effects. Compound 11 exhibited high significance as an anti-inflammatory agent, which may be due to the presence of the coumarin moiety. Compounds 7, 9b, 13b, 19 were considered as moderate anti-inflammatory effects. The remaining compounds 3, 9a, 13a, 15, 17a, b, 21a, b, 23a, b, 25a, b, c exhibited poor biological significance as anti-inflammatory agents (Table III).
Conclusions
In this article, the synthesized pyrimidines 3, 7, 13a,b, 15, 17a,b, 21a,b, 25a, b, c; thiophenes 9a, b; coumarin 11, pyridine 19 and pyrroles 23a, b were evaluated as analgesic, antipyretic and anti-inflammatory agents compared to the reference standard drug acetaminophen. Among the newly synthesized compounds, compounds 11 and 19 showed promising significant analgesic and antipyretic activities compared to the other compounds. Additionally compounds 7, 9a,b and 13a,b had moderately significant analgesic and antipyretic activities. Moreover, compound 11 had clear anti-inflammatory properties compared to the remaining compounds.
Acknowledgements
The authors would like to thank the research group working in the Pharamacology Depatment, Faculty of Pharmacy, October University for Modern Sciences and Arts. We would also like to thank the Poison Control and Medical Forensic Chemistry Center team from Jazan Health, Jazan City, Kingdom of Saudi Arabia for recording the analytical and spectral data for the newly synthesized compounds.
References
-
1Agarwal A, Ashutosh R, Goyal N, Chawhan PMS, Gupta S. Dihydropyrido [2,3-d]pyrimidines as a new class of antileishmanial agents. Bioorg Med Chem. 2005;13(24):6678-6684.
-
2Dallemagne P, Khanh LP, Alsaidi A, Varlet I, Collot V, Paillet M, Bureau R, Rault S. Synthesis and biological evaluation of five-Membered heterocycles fused to cyclopenta[c]thiophene as new antitumor agents. Bioorg Med Chem. 2003;11(7):1161-1167.
-
3El-Sharkawy KA, Nasser NE, Zaki MY. Uses of 2-Amino-5, 6-dihydro-4H-cyclopenta [b] thiophene-3-carbonitrile in the synthesis of novel heterocyclic compounds with anticonvulsant, behavioral and CNS antidepressant activities. Int Res J Pure App Chem. 2012;2(1):91-104.
-
4Fadeyi OO, Obafemi CA, Adewunmi CO, Iwalewa EO. Antipyretic, analgesic, anti-inflammatory and cytotoxic effects of four derivatives of salicylic acid and anthranilic acid in mice and rats. Afr J Biotech. 2004;3(8):426-431.
-
5Gus'Kov EP, Prokofev VN, Kletskii ME, Kornienko IV, Ogapurenko A, Olekhnovich LP, Chistyakov VA, Shestopalov AV, Sazykina MA. Allantoin as a vitamin. Dokl Biochem Biophys. 2004;398:320-324.
-
6Horvati K, Bacasa B, Szabo N, Fodor K, Balaka G, Rusavai M, Kiss É, Mezo G, Grolmusz V, Vertessy B, Hudecz F, Bosze S. Antimycobacterial activity of peptide conjugate of pyridopyrimidine derivative against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Tuberculosis. 2015;95(Suppl 1):S207-S211.
-
7Iqbal Z, Hameed S, Ali S, Tehseen Y, Shahid M, Iqbal J. Synthesis, characterization, hypoglycemic and aldose reductase inhibition activity of arylsulfonylspiro[fluorene-9,5'-imidazolidine]-2',4'-diones. Eur J Med Chem. 2015;98:127-138.
-
8Keri RS, Hosamani KM, Shingalapure RV, Hugar MH. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur J Med Chem. 2010;45(6):2597-2605.
-
9Kulkarni SK. Handbook of Experimental Pharmacology. 3 ed. Delhi: Vallabh Prakashan; 2003. P. 123-125; 128-131; 172-186.
-
10Mohareb R, El-Sharkawy KA, Sherif SM. The reaction of ß-amino-a,?-dicyano -crotononitrile with acetophenone: Synthesis of pyridine, pyridazine and thiophene derivatives with antimicrobial activities. Acta Pharm. 2008;58:429-444.
-
11Padmavathi V, Prema Kumari C, Venkatesh BC, Padmaja A. Synthesis and antimicrobial activity of amido linked pyrrolyl and pyrazolyl-oxazoles, thiazoles and imidazoles. Eur J Med Chem. 2011;46(11):5317-5326.
-
12Rajesh SM, Kumar RS, Libertsen LA, Perumal S, Yogeeswari P, Sriram D. A green expedient synthesis of pyridopyrimidine-2-thiones and their antitubercular activity. Bioorg Med Chem Lett. 2011;21(10):3012-3016.
-
13Reddy YT, Reddy PN, Kodru S, Damodran C, Crooks PA. Aplysinopsin analogs: Synthesis and anti-proliferative activity of substituted (Z)-5-(N-benzylindol-3-ylmethylene) imidazolidine-2,4-diones. Bioorg Med Chem. 2010;18(10):3570-3574.
-
14Sztanke M, Rzymowska J, Sztanke K. Synthesis, structure elucidation and in vitro anticancer activities of novel derivatives of diethyl (2E)-2-[(2E)-(1-aryl- imidazolidin-2-ylidene) hydrazono]succinate and ethyl (4-oxo-8-aryl-4,6,7,8-tetra-hydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetate. Bioorg Med Chem. 2013;21(23):7465-7480.
-
15Vogel HG. Drug discovery and evaluation. Pharmacological assay. 2 ed. Berlin, Heidelberg: Springer-Verlag; 2002. p. 695-696; 759-762; 772-773.
-
16Wang G, Wang Y, Wang L, Han L, Hou X, Fu H, Fang H. Design, synthesis and preliminary bioactivity studies of imidazolidine-2,4-dione derivatives as Bcl-2 inhibitors. Bioorg Med Chem. 2015;23(23):7359-7365.
Publication Dates
-
Publication in this collection
08 Apr 2019 -
Date of issue
2018
History
-
Received
16 Oct 2016 -
Accepted
09 Jan 2018