Abstract
In forest nurseries, one of the factors that change the growth and quality of seedlings is water supply, which has a direct influence on metabolic processes such as stomatal conductance and photochemical efficiency of photosystem II. This study aims to identify the influence of different watering regimes as well as the use of a water-retaining polymer on the initial growth and metabolic processes of Enterolobium contortisiliquum seedlings. The experimental design was in random blocks with a factorial scheme. Morphological attributes, as well as physiological and biochemical attributes were investigated. We verified that the performance of E. contortisiliquum seedlings depends on the watering regime provided to the plants; furthermore, up to 60 days after the application of the treatments, both the height and diameter of the collection and the leaf water potential were similar in the water regimes of 8 mm day-1 and 12 mm day-1.
Keywords:
abiotic stress; water potential; oxidative stress; antioxidant enzymes
1.1. INTRODUCTION
Enterolobium contortisiliquum (Vell.) Morong belongs to the Fabaceae family. It is a tree native to South America found in Brazil, Argentina, Paraguay, and Uruguay (Burkart, 1967Burkart A. Leguminosae. Buenos Aires: Instituto de Botanica Darwinion; 1967. (La flora de la provincia de Buenos Aires; vol. 4, pt. 3).). This species can occur in several forest formations; however, it is more commonly found in subtropical forests (Moreira et al., 2015Moreira PDA, Brandão MM, Araujo NH, Oliveira DA, Fernandes GW. Genetic diversity and structure of the tree Enterolobium contortisiliquum (Fabaceae) associated with remnants of a seasonally dry tropical forest. Flora: Morphology, Distribution, Functional Ecology of Plants 2015; 210: 40-46. 10.1016/j.flora.2014.10.005
https://doi.org/10.1016/j.flora.2014.10....
).
This species is important for different sectors of the economy, such as timber, shipbuilding, and civil construction (Zuchiwschi et al., 2010Zuchiwschi E, Fantini AC, Alves AC, Peroni N. Limitações ao uso de espécies florestais nativas pode contribuir com a erosão do conhecimento ecológico tradicional e local de agricultores familiares. Acta Botanica Brasilica 2010; 24(1): 270-282. 10.1590/S0102-33062010000100029
https://doi.org/10.1590/S0102-3306201000...
). It also has medicinal value in view of the anti-inflammatory and antibiotic properties of substances present in its bark and fruit, respectively (Nakahata et al., 2011Nakahata M, Mayer B, Ries C, De Paula CAA, Karow M, Neth P et al. The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines. Biological Chemistry 2011; 392(4): 327-336. 10.1515/bc.2011.031
https://doi.org/10.1515/bc.2011.031...
). In addition, E. contortisiliquum is recommended for reforestation of degraded areas, permanent preservation, and mixed plantings, mainly due to its rapid initial growth (Araújo & Paiva Sobrinho, 2011Araújo APD, Paiva Sobrinho SD. Germinação e produção de mudas de tamboril (Enterolobium contortisiliquum (Vell.) Morong) em diferentes substratos. Revista Árvore 2011; 35(3): 581-588. 10.1590/S0100-67622011000400001
https://doi.org/10.1590/S0100-6762201100...
).
In forest nurseries, one of the factors that influence the development and quality of seedlings is the management of irrigation (Silva et al., 2012Silva RBGD, Simões D, Silva MRD. Qualidade de mudas clonais de Eucalyptus urophylla × E. grandis em função do substrato. Revista Brasileira de Engenharia Agrícola e Ambiental 2012; 16(3): 297-302. http://dx.doi.org/10.1590/S1415-43662012000300010
http://dx.doi.org/10.1590/S1415-43662012...
). This aspect of forestry, together with the worldwide concern for sustainable use of water, requires studies that optimize irrigation in several productive sectors, such as forestry.
Water is an indispensable component for the maintenance of metabolic homeostasis and structural integrity of cell macromolecules. Leaf watering regime has a direct influence on metabolic processes, such as stomatal conductance, cell elongation and expansion, ψ, and photochemical efficiency of photosystem II (Silva et al., 2007Silva MDA, Jifon JL, Silva JAGD, Sharma V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology 2007; 19(3): 193-201. 10.1590/S1677-04202007000300003
https://doi.org/10.1590/S1677-0420200700...
). In addition, water acts as a solvent for electrolytes and is involved in the assembly and reactivity of native biomolecules (Vyumvuhore et al., 2015Vyumvuhore R, Tfayli A, Biniek K, Duplan H, Delalleau A, Manfait M et al. The relationship between water loss, mechanical stress, and molecular structure of human stratum corneum ex vivo. Journal of Biophotonics 2015; 8(3): 217-225. 10.1002/jbio.201300169
https://doi.org/10.1002/jbio.201300169...
).
The effects of environmental stresses, such as water restriction, can be measured by the increase in production of reactive oxygen species (ROS) (Møller et al., 2007Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 2007; 58(1): 459-481. 10.1146/annurev.arplant.58.032806.103946
https://doi.org/10.1146/annurev.arplant....
). ROS, such as singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide (O2•-), and hydroxyl radical (HO•) are capable of causing oxidative damage to lipids, proteins, and DNA within the plant (Møller et al., 2007Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 2007; 58(1): 459-481. 10.1146/annurev.arplant.58.032806.103946
https://doi.org/10.1146/annurev.arplant....
; Sharma et al., 2012Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012; 2012: 217037. 10.1155/2012/217037
https://doi.org/10.1155/2012/217037...
), which ultimately causes reduced growth and death. Thus, to minimize the cytotoxic effects of ROS, plants have a complex antioxidative system, where specific enzymes act to neutralize the action of these radicals, starting with superoxide dismutase (SOD) that dismutes the superoxide anion (O2•-) to H2O2 (Pandey et al., 2016Pandey P, Srivastava RK, Rajpoot R, Rani A, Pandey AK, Dubey RS. Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance. Environmental Science and Pollution Research 2016; 23(2): 1516-1528. 10.1007/s11356-015-5392-8
https://doi.org/10.1007/s11356-015-5392-...
). In sequence, hydrogen peroxide is detoxified by peroxidases such as ascorbate peroxidases (APXs), glutathione peroxidase (GPX) and catalase (Asada, 2006Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 2006; 141: 391-396. 10.1104/pp.106.082040
https://doi.org/10.1104/pp.106.082040...
; Choudhury et al., 2016Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signaling & Behavior 2013; 8(4): e23681. 10.4161/psb.23681
https://doi.org/10.4161/psb.23681...
; Waszczak et al., 2018Waszczak C, Carmody M, Kangasjärvi J. Reactive oxygen species in plant signaling. Annual Review of Plant Biology 2018; 69: 209-236. 10.1146/annurev-arplant-042817-040322
https://doi.org/10.1146/annurev-arplant-...
).
In addition, the use of synthetic conditioners is reported to contribute toward an increase in the water retention capacity of the substrate, which reduces the frequency of irrigation, and thus, allows more effective use of resources from the culture medium and water, which ultimately optimizes crop yield (Navroski et al., 2015Navroski MC, Araujo MM, Fior CS, Cunha FDS, Berghetti ÁLP, Pereira MDO. Uso de hidrogel possibilita redução da irrigação e melhora o crescimento inicial de mudas de Eucalyptus dunnii Maiden. Scientia Forestalis 2015; 43(106): 467-476.). Moreover, the use of water-retaining polymers improves the chemical and physical characteristics of the substrate, allowing better utilization of the nutrients incorporated into the culture medium (Felippe et al., 2016Felippe D, Navroski MC, Sampietro JA, Frigotto T, Albuquerque JA, Mota CS, Pereira MO. Efeito do hidrogel no crescimento de mudas de Eucalyptus benthamii submetidas a diferentes frequências de irrigação. Floresta 2016; 46(2): 215-225. 10.5380/rf.v46i2.43920
https://doi.org/10.5380/rf.v46i2.43920...
; Navroski et al., 2016Navroski MC, Araújo MM, Cunha FDS, Berghetti ÁLP, Pereira MDO. Redução da adubação e melhoria das características do substrato com o uso do hidrogel na produção de mudas de Eucalyptus dunnii Maiden. Ciência Florestal 2016; 26(4): 1155-1165. 10.5902/1980509825106
https://doi.org/10.5902/1980509825106...
)
This investigation sought to identify the influence of different watering regimes and the addition of water-retaining polymer to the substrate on the initial growth and metabolic processes of E. contortisiliquum seedlings. The main questions that we aimed to answer are: a) Is the growth of E. contortisiliquum seedlings dependent on the watering regime adopted?; b) Does the use of water-retaining polymer allow a reduction in the amount of water available to the plants?; c) Under conditions of low water availability, does E. contortisiliquum keep the metabolic processes unchanged?
2. MATERIAL AND METHODS
2.1. Plant material and experimental design
The experiment was conducted in the forest nursery of the Universidade de Santa Maria (29°43’S and 53°43’W), located in the central region of the Rio Grande do Sul State, Brazil. The approximate altitude here is 90 meters. The local climate, according to the Köppen classification, is subtropical, type Cfa, with annual average precipitation between 1,900 and 2,200 mm. The average temperature is 19.1 °C, with maximum and minimum values of 32 and 9 °C, respectively (Alvares et al., 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLDM, Sparovek G. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 2013; 22(1): 711-728. 10.1127/0941-2948/2013/0507
https://doi.org/10.1127/0941-2948/2013/0...
), which sets four distinct seasons in the area under study. The research was conducted from February to June 2016, corresponding to the period between summer and fall.
Mature fruits of E. contortisiliquum were collected from eight trees located in a fragment of Deciduous Seasonal Forest (29°38’S and 53°40’W), in Santa Maria, Rio Grande do Sul State, Brazil. After the collection, seeds were extracted by pruning shears and manual processing. Only completely formed seeds were selected for further analysis. Following seed collection, the tegument was manually scarified with sandpaper in the region opposite to the micropyle without accessing the cotyledons, as recommended by Brasil (2013Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instruções para análise de espécies florestais. Brasília, DF; 2013. ).
Seedlings were germinated with the use of cylindrical-conical polypropylene tubes with a volume of 110 cm³ that were placed in plastic trays with a capacity for 96 tubes. They were suspended at a depth of 16 cm from the soil surface. We used a commercial substrate composed of sphagnum peat and expanded vermiculite with charcoal rice husk in a 4:1 (v:v) ratio. For base fertilization, 8 g per liter substrate of the controlled-release fertilizer with an NPK formulation of 18-05-09 were employed. Substrates were prepared in a concrete mixer by adding the fertilizer and the water-retaining polymer for the treatment that required its presence.
Two seeds were sowed per container. After about 15 days, thinning was performed to leave only one seedling per container, which was central and the strongest one. The seedlings were kept for 30 days in a greenhouse, where they received micro-sprinkler irrigation (5 mm day-1). After this period, the trays were placed in an open and sunny environment, where the seedlings were subjected to different watering regimes. In this environment, there were low tunnels and clear polyethylene tarpaulins that were manually operated to cover the seedlings whenever necessary in order to avoid contact with precipitation.
The experimental design was random blocks in a factorial scheme. Factor A was constituted by three randomized irrigation regimes (4, 8, and 12 mm day˗1). Factor D was prepared on the basis of the absence (P0 - 0 g L-1) or presence (P4 - 4 g L-1) of the water-retaining polymer. The assay was performed using four replicates per treatment, totaling 24 experimental units, each unit composed of 24 seedlings.
Before installing the experiment, physical and chemical analyses of the tested substrates with or without the water-retaining polymer tested (Table 1) were carried out in the Laboratory of Analysis of Substrates for Plants from the Departamento de Diagnóstico e Pesquisa Agropecuária. The analyses were conducted according to Brasil (2008Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instrução normativa nº 31, de 24 de outubro de 2008. Diário Oficial da União, Brasília, DF (2008 Oct. 24); Sec. 1.) and Verdonck et al. (1984Verdonck O, Penninck R, De Boodt M. The physical properties of different horticultural substrates. Acta Horticulturae 1984; 150: 155-160. 10.17660/ActaHortic.1984.150.16
https://doi.org/10.17660/ActaHortic.1984...
).
Previously, the uniformity of the irrigation system was determined using the Christiansen Uniformity Coefficient (CUC) as described by Bernardo et al. (2006Bernardo S, Soares AA, Mantovani EC. Manual de irrigação. Viçosa: UFV; 2006.). The CUC obtained was 83.2%, which was considered adequate by these authors. Irrigation regimes (RR) were programmed at different frequencies and schedules by means of timers linked to the irrigation system: RR4 - 4 mm day-1 (2 mm at 8 a.m. and 1 p.m.); RR8 - 8 mm day-1 (2 mm at 8 a.m. and 1 p.m. and 4 mm at 3 p.m.); and RR12 - 12 mm day-1 (4 mm at 8 a.m., 1 p.m. and 3 p.m.).
2.2. Morphological attributes
The morphological attributes were determined at four-time intervals (30, 60, 90, and 120 days after sowing), corresponding to 0, 30, 60, and 90 days after applying the irrigation regimes, in 8 seedlings per experimental unit. Morphological attributes included height, and stem diameter. The relationship between height and stem diameter of the collected samples from the eight central plants of each replicate was also evaluated. The height was measured with a millimeter ruler from the apical bud of the plant to the surface of the substrate. The diameter of each specimen was measured with a digital caliper (accuracy of 0.01 mm). Shoot dry mass, root dry mass, and total dry mass were obtained from four individuals of each experimental unit. Dry mass was measured by weighing samples after drying them for a period of approximately 72 hours in a forced circulation oven at 70 °C.
2.3. Water potential
The water potential (ψ) was measured at four-time intervals (30, 60, 90, and 120 days after sowing). The measurements were performed with the aid of a Scholander Pressure Chamber (model 600) in four plants per replicate and the evaluations carried out between 1 p.m. and 2 p.m. To perform this evaluation, about 8 cm of plant were sectioned from the apex of the molt and inserted into the chamber, followed by the application of pressure until the first droplets of exudation were observed. At this time the reading was performed in MPa.
2.4. Biochemical attributes
At the end of the experiment (90 days after application of irrigation regimes), the photosynthetic pigments (chlorophyll a, b, and carotenoids content) and attributes of oxidative stress (lipid peroxidation, hydrogen peroxide concentration, and activity of the enzyme superoxide dismutase) were measured. Samples of expanded leaves were collected and immediately frozen in liquid N2 and then stored in an ultrafreezer (−80 °C) until the moment of determination. The analyses were carried out at the Plant Biotechnology Laboratory, Department of Biology (Universidade Federal de Santa Maria).
In the quantification of the photosynthetic pigments, the concentrations of chlorophyll a, chlorophyll b, and carotenoids were determined according to the methodology described by Hiscox & Israelstam (1979Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 1979; 57(12): 1332-1334. https://doi.org/10.1139/b79-163
https://doi.org/10.1139/b79-163...
) and were then estimated using the Lichtenthaler formula (Lichtenthaler, 1987Lichtenthaler HK. [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 1987; 148: 350-382. 10.1016/0076-6879(87)48036-1
https://doi.org/10.1016/0076-6879(87)480...
). Fresh leaf samples (0.05 g) were incubated at 65 °C with dimethyl sulfoxide for 1.5 hours. The absorbances of the solutions were measured in a spectrophotometer (Celm E-205D) at 663, 645, and 470 nm for chlorophyll a, chlorophyll b, and carotenoids, respectively.
Lipid peroxidation was estimated following the method of El-moshaty et al. (1993El-Moshaty FIB, Pike SM, Novacky AJ, Sehgal OP. Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ringspot virus or southern bean mosaic virus. Physiological and Molecular Plant Pathology 1993; 43(2): 109-119. 10.1006/pmpp.1993.1044
https://doi.org/10.1006/pmpp.1993.1044...
). Samples of leaves (0.5 g) previously macerated in liquid N2 were homogenized and then centrifuged, with 1 ml of the supernatant added to 1 ml of 20% (w/v) of trichloroacetic acid containing 0.5% (w/v) of thiobarbituric acid. The mixture was heated at 95 °C for 40 min, cooled in an ice bath for 15 min, and then centrifuged at 10,000 × g for 15 min. The absorbance of the supernatant was read in a spectrophotometer at 532 and 600 nm (to correct non-specific turbidity). Lipid peroxidation was expressed as nmol MDA mg-1 protein.
The concentration of H2O2 was determined according to the method described by Loreto & Velikova (2001Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology 2001; 127(4): 1781-1787. 10.1104/pp.010497
https://doi.org/10.1104/pp.010497...
). Approximately 0.1 g of leaf samples were homogenized in 3 mL of 0.1% (w/v) TCA. The homogenate was centrifuged at 12,000 × g for 10 min at 4 °C. Following this, 0.5 mL of the supernatant were added to 0.5 mL of 10 mM K-phosphate buffer (pH 7.0) and 1 mL of 1M KI. H2O2 concentration of the supernatant was evaluated by comparing the absorbance obtained in a spectrophotometer at 390 nm with a standard calibration curve. The concentration of hydrogen peroxide was expressed as μmol g-1 of fresh mass.
SOD activity was determined according to the spectrophotometric method described by Giannopolitis & Ries (1977Giannopolitis CN, Ries SK. Superoxide dismutases: II: purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiology 1977; 59(2): 315-318. 10.1104/pp.59.2.315
https://doi.org/10.1104/pp.59.2.315...
). Samples (0.5 g) were homogenized in 3 mL of 0.05 M sodium phosphate buffer (pH 7.8) containing 1 mM EDTA and 2% (w/v) polyvinylpyrrolidone (PVP). Afterwards, the homogenate was centrifuged at 13,000 × g for 15 min at 4 °C (Zhu et al., 2004Zhu Z, Wei G, Li J, Qian Q, Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Science 2004; 167(3): 527-533. 10.1016/j.plantsci.2004.04.020
https://doi.org/10.1016/j.plantsci.2004....
). To determine the activity, 50 µl of sample was mixed with 50 mM potassium phosphate buffer (pH 7.8), 13 mM methionine, 2 µM riboflavin, 75 µM Nitroblue tetrazolium (NBT), and 0.1 mM EDTA. The tubes containing the solution were packed in 15-watt bulbs for 15 min followed by reading the absorbances at 560 nm (Beauchamp & Fridovich, 1971Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry 1971; 44(1): 276-287. 10.1016/0003-2697(71)90370-8
https://doi.org/10.1016/0003-2697(71)903...
). The activity of the enzyme was expressed as U mg-1 protein. Total proteins were determined following the method of Bradford (1976Bradford MM. A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 1976; 72(1-2): 248-254. 10.1016/0003-2697(76)90527-3
https://doi.org/10.1016/0003-2697(76)905...
) using bovine serum albumin as standard.
2.5. Statistical analysis
The data were subjected to analysis of normality and homogeneity assumptions, followed by analysis of variance (ANOVA), Tukey HSD test (p < 0.05), and/or linear regression to observe the significance coefficient of determination (R²). For statistical analysis, we used the software SISVAR v. 5.3 (Ferreira, 2014Ferreira DF. Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia 2014; 38(2): 109-112. 10.1590/S1413-70542014000200001
https://doi.org/10.1590/S1413-7054201400...
). When considering the quantitative factor, time intervals of 0, 30, 60, and 90 days after the application of irrigation regimes were performed.
3. RESULTS
The analysis of variance indicated an interaction between irrigation regimes (IR) and the time of measurements, for height, stem diameter, height/stem diameter ratio, root dry mass, and water potential (p < 0.05) variables. Individually, the factors affected the other morphological and physiological variables (Table 2).
We verified the highest averages of height, stem diameter, and height/stem diameter ratio, when the E. contortisiliquum seedlings were grown under irrigation regime RR12 (12 mm day-1). In addition, after 90 days in the nursery, seedlings under the RR12 regime showed an increase in height and stem diameter, which was about 50% higher than of those grown under RR4 (4 mm day-1) (Figure 1).
Height (a), stem diameter (b), height/stem diameter ratio (c) and root dry mass (d) of Enterolobium contortisiliquum, according to the irrigation regimes of 4 (RR4), 8 (RR8) and 12 (RR12) mm day-1 at 0, 30, 60 and 90 days after irrigation.
On the other hand, we found that under the irrigation regime RR12 (12 mm day-1), root dry mass had the lowest mean. For this attribute, the regimes that provided the lowest water supply (RR4 and RR8) showed the highest values of dry mass of roots (Figure 1).
The attributes of height, stem diameter, and shoot dry mass presented higher values (p < 0.05), when the E. contortisiliquum seedlings were raised in the presence of the water-retaining polymer (Figure 2).
Height (a), stem diameter (b) shoot dry mass (c) of Enterolobium contortisiliquum seedlings, conducted in the absence (P0 - 0 g L-1) or in the presence (P4 - 4 g L-1) of the water-retaining polymer in the substrate formulation. Data are mean ± SE.
We observed that the ψ of the plants was influenced by the watering regimes used. Water supply restriction (RR4 - 4 mm day-1) reduced the hydration state of the cells (ψ −2.04 MPa) 90 days after the application of the treatments (Figure 3). With other regimens, RR8 and RR12, 60 days after the application of the treatments, similar results of −0.56 and −0.69 MPa, respectively, were obtained.
Water potential (ψ) of Enterolobium contortisiliquum seedlings, according to irrigation regimes of 4 (RR4), 8 (RR8) and 12 (RR12) mm day-1 at 0, 30, 60 and 90 days after application of irrigation.
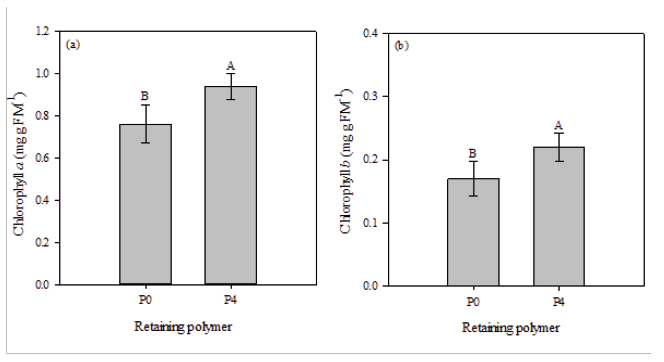
The analysis of the photosynthetic pigments revealed that E. contortisiliquum seedlings had the same behavior regarding the use of water-retaining polymer. We observed that seedlings grown in the presence of the polymer (P4 - 4 g L-1) had the highest levels of chlorophyll a (0.94 mg g MF-1) and b (0.22 mg g MF-1) (Figure 4).
Chlorophyll a (a) and chlorophyll b (b) of Enterolobium contortisiliquum seedlings, conducted in the absence (P0 - 0 g L-1) or in the presence (P4 - 4 g L-1) of water-retaining polymer in the substrate formulation. Data are mean ± SE
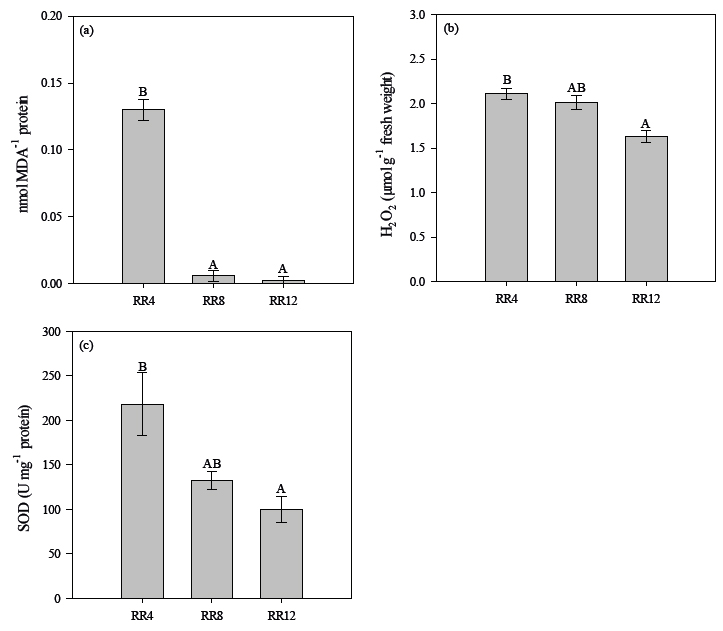
For other biochemical attributes, we observed a significant effect of the irrigation regimes, when analyzing lipid peroxidation, hydrogen peroxide concentration, and the activity of the enzyme superoxide dismutase (Figure 5). The level of lipid peroxidation that was measured by the accumulation of malondialdehyde (MDA) and H2O2 content increased as the water availability decreased. The highest values of 0.13 μmol MDA mg-1 protein and 2.11 μmol g-1 of fresh mass, respectively, were observed in the seedlings subjected to the RR4 irrigation regime (4 mm day-1) as compared to the RR12 irrigation regime (12 mm day-1) (Figure 5).
Malondialdehyde (MDA) content of shoot (a), hydrogen peroxide (H2O2) concentration (b) and superoxide dismutase (SOD) enzyme activity (c) of Enterolobium contortisiliquum seedlings, according to the irrigation regimens of 4 (RR4), 8 (RR8) and 12 (RR12) mm day-1 at 90 days after the application of irrigation. Data are mean ± SE.
The plant’s defense system investigated in this study by the activity of the SOD increased significantly with the reduction of their water supply (Figure 5). In general, plants subjected to RR4 irrigation regimen (4 mm day-1) showed a 46% increase in SOD activity as compared to RR12 (12 mm day-1) regimen.
4. DISCUSSION
Morphologically, E. contortisiliquum seedlings presented positive results when the RR12 irrigation regime (12 mm day-1) was applied. Silva et al. (2016Silva P, Campoe O, De Paula R, Lee D. Seedling growth and physiological responses of sixteen eucalypt taxa under controlled water regime. Forests 2016; 7(6): 110. 10.3390/f7060110
https://doi.org/10.3390/f7060110...
) studied the responses of water availability in sixteen species of the Eucalyptus genus to verify the negative effect of water restriction during seedling production. However, Dutra et al. (2016Dutra AF, Araujo MM, Turchetto F, Rorato DG, Aimi SC, Gomes DR, Nishijima T. Substrate and irrigation scheme on the growth of Parapiptadenia rigida (angico-vermelho) seedlings. Ciência Rural 2016; 46(6): 1007-1013. 10.1590/0103-8478cr20141732
https://doi.org/10.1590/0103-8478cr20141...
) evaluated the effect of different substrates and irrigation regimes to report that the use of 4 mm day-1 was enough to produce healthy seedlings of Parapiptadenia rigida (Benth.) Brenan. Similarly, we also verified it is fundamental to characterize the specificities of each species in relation to the factor of watering regimes. This will allow the optimization of the productive activity and the rational use of water in nurseries because even species from the same family, such as E. contortisiliquum and P. rigida, have different water requirements.
Enterolobium contortisiliquum expressed the opposite behavior under limited water conditions in the substrate concerning root dry mass. The results show that water deficiency in the substrate has a significant influence on root growth, where this deficit increases the root biomass under stress. The contrasting response in the production of root biomass reflects a strategy used by different forest species (Dutra et al., 2016Dutra AF, Araujo MM, Turchetto F, Rorato DG, Aimi SC, Gomes DR, Nishijima T. Substrate and irrigation scheme on the growth of Parapiptadenia rigida (angico-vermelho) seedlings. Ciência Rural 2016; 46(6): 1007-1013. 10.1590/0103-8478cr20141732
https://doi.org/10.1590/0103-8478cr20141...
; Silva et al., 2016Silva P, Campoe O, De Paula R, Lee D. Seedling growth and physiological responses of sixteen eucalypt taxa under controlled water regime. Forests 2016; 7(6): 110. 10.3390/f7060110
https://doi.org/10.3390/f7060110...
) in relation to carbon allocation. In general, water restriction induces carbon partitioning for root synthesis in order to increase the surface of fine roots, and thus, to increase the area of contact with the soil, which consequently enhances the capacity of water and nutrients absorption (Litton et al., 2007Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Global Change Biology 2007; 13(10): 2089-2109. 10.1111/j.1365-2486.2007.01420.x
https://doi.org/10.1111/j.1365-2486.2007...
; Ryan et al., 2010Ryan MG, Stape JL, Binkley D, Fonseca S, Loos RA, Takahashi EN et al. Factors controlling Eucalyptus productivity: how water availability and stand structure alter production and carbon allocation. Forest Ecology and Management 2010; 259(9): 1695-1703. 10.1016/j.foreco.2010.01.013
https://doi.org/10.1016/j.foreco.2010.01...
).
One of the main results obtained in our study refers to the fact that the watering regimes had a significant interaction (p < 0.05) with the time of permanence of the seedlings in the nursery. Up to 60 days after the application of irrigation regimes, there was no difference observed between seedlings grown at RR12 and RR8. Therefore, both irrigation regimes can be applied during the growth phase. However, this should be done in combination, where in the first 60 days, 8 mm day-1 may be used and then changed to 12 mm-1 after this period. In this manner, 77.8% of water was saved during the production period of the E. contortisiliquum seedlings.
The positive effects of the combined use of irrigation regimes, RR8 and RR12, are explicitly observable, when analyzing data on water potential in plants. We observed here that up to 60 days after the application of the treatments, water potential presented values close to RR8 and RR12: −0.56 MPa and −0.69 MPa, respectively. However, when analyzing the irrigation regime, RR4 (4 mm day-1), we identified severe water stress (−1.56 MPa). According to Taiz et al. (2017Taiz L, Zeiger E, Møller IM, Murphy A. Fisiologia e desenvolvimento vegetal. 6th ed. Porto Alegre: Artmed; 2017.), plants with water potential below −1.00 MPa are under severe water stress, and adverse symptoms can be seen in the processes of expansion and cell expansion.
Additionally, according to Bergonci et al. (2000Bergonci JI, Bergamaschi H, Berlato MA, Santos AO. Potencial da água na folha como um indicador de déficit hídrico em milho. Pesquisa Agropecuária Brasileira 2000; 35(8): 1531-1540. 10.1590/S0100-204X2000000800005
https://doi.org/10.1590/S0100-204X200000...
), the potential of water in a plant indicates the state of turgidity of its cells. In a situation of low availability of water in the soil, plants reduce the loss of water by restricting stomatal conductance. Water stress substantially changes the metabolism and gas exchange in plants (Taiz et al., 2017Taiz L, Zeiger E, Møller IM, Murphy A. Fisiologia e desenvolvimento vegetal. 6th ed. Porto Alegre: Artmed; 2017.), which can, in turn, directly hinder the growth if water stress inhibits photosynthesis by stalling the dehydration of mesophyll cells (Silva et al., 2007Silva MDA, Jifon JL, Silva JAGD, Sharma V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology 2007; 19(3): 193-201. 10.1590/S1677-04202007000300003
https://doi.org/10.1590/S1677-0420200700...
). Thus, the lower water potential confirms the lower growth rates and aerial biomass allocation in plants grown under the 4 mm day-1 regime (RR4).
We observed that the addition of water-retaining polymer to the substrate contributes to the growth and biomass increase in the E. contortisiliquum seedlings, regardless of the watering regime adopted. These results corroborate those obtained by Navroski et al. (2015Navroski MC, Araujo MM, Fior CS, Cunha FDS, Berghetti ÁLP, Pereira MDO. Uso de hidrogel possibilita redução da irrigação e melhora o crescimento inicial de mudas de Eucalyptus dunnii Maiden. Scientia Forestalis 2015; 43(106): 467-476.) and Felippe et al. (2016Felippe D, Navroski MC, Sampietro JA, Frigotto T, Albuquerque JA, Mota CS, Pereira MO. Efeito do hidrogel no crescimento de mudas de Eucalyptus benthamii submetidas a diferentes frequências de irrigação. Floresta 2016; 46(2): 215-225. 10.5380/rf.v46i2.43920
https://doi.org/10.5380/rf.v46i2.43920...
) on the production of the genus Eucalyptus seedlings. The increase in biomass with the addition of water-retaining polymer occurs due to the reduction of percolation of irrigation water and improvement in the aeration and drainage in soil (Bernardi et al., 2012Bernardi MR, Sperotto M Jr, Daniel O, Vitorino ACT. Crescimento de mudas de Corymbia citriodora em função do uso de hidrogel e adubação. Cerne 2012; 18(1): 67-74. 10.1590/S0104-77602012000100009
https://doi.org/10.1590/S0104-7760201200...
) as well as the reduction in nutrient leaching (Navroski et al., 2015Navroski MC, Araujo MM, Fior CS, Cunha FDS, Berghetti ÁLP, Pereira MDO. Uso de hidrogel possibilita redução da irrigação e melhora o crescimento inicial de mudas de Eucalyptus dunnii Maiden. Scientia Forestalis 2015; 43(106): 467-476.), which contributes directly to the improvement of the nutritional status of the plant, reducing the consumption of fertilizers from 25% to 50% (Navroski et al., 2016Navroski MC, Araújo MM, Cunha FDS, Berghetti ÁLP, Pereira MDO. Redução da adubação e melhoria das características do substrato com o uso do hidrogel na produção de mudas de Eucalyptus dunnii Maiden. Ciência Florestal 2016; 26(4): 1155-1165. 10.5902/1980509825106
https://doi.org/10.5902/1980509825106...
).
The results obtained for the photosynthetic pigment content demonstrate the beneficial effect of the use of water-retaining polymers in reducing the leaching of nutrients from the substrate, especially nitrogen (N). Chlorophyll a and b contents were higher in plants grown with the addition of the polymer to the substrate. Photosynthetic pigments are used to estimate the nutritional status of N in plants because the amount of these pigments correlates positively with N content in the plant (Smeal & Zhang, 1994Smeal D, Zhang H. Chlorophyll meter evaluation for nitrogen management in corn. Communications in Soil Science and Plant Analysis 1994; 25(9-10): 1495-1503. 10.1080/00103629409369130
https://doi.org/10.1080/0010362940936913...
). This relationship is attributed mainly to the fact that, in general, 50% to 70% of the total N of leaves is incorporated in enzymes that are associated to the chloroplasts (Chapman & Barreto, 1997Chapman SC, Barreto HJ. Using a chlorophyll meter to estimate specific leaf nitrogen of tropical maize during vegetative growth. Agronomy Journal 1997; 89(4): 557-562. 10.2134/agronj1997.00021962008900040004x
https://doi.org/10.2134/agronj1997.00021...
), other than being a part of the chlorophyll molecule (Taiz et al., 2017Taiz L, Zeiger E, Møller IM, Murphy A. Fisiologia e desenvolvimento vegetal. 6th ed. Porto Alegre: Artmed; 2017.).
However, stressful environmental conditions, such as water restriction, provoke a common response involving the overproduction of reactive oxygen species (ROS), including 1O2, H2O2, and O2•- in plant cells (Pandey et al., 2016Pandey P, Srivastava RK, Rajpoot R, Rani A, Pandey AK, Dubey RS. Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance. Environmental Science and Pollution Research 2016; 23(2): 1516-1528. 10.1007/s11356-015-5392-8
https://doi.org/10.1007/s11356-015-5392-...
). These ROS are unstable and highly reactive molecules that cause serious damage to vital organelles, such as chloroplasts, mitochondria, and DNA on reaction with other molecules, thus causing peroxidation of membrane lipids, protein oxidation, and nucleic acid fragmentation (Gill & Tuteja, 2010Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 2010; 48(12): 909-930. 10.1016/j.plaphy.2010.08.016
https://doi.org/10.1016/j.plaphy.2010.08...
). We demonstrated higher lipid peroxidation in plants cultivated under water restriction (4 mm day-1) as compared to plants grown in irrigation regimes with higher water supply (8 and 12 mm day-1). This indicates that the low water supply to E. contortisiliquum seedlings causes oxidative stress, causing damage to membrane lipids. In addition, there was a higher content of ROS represented by H2O2 in plants under water restriction (4 mm day-1), as compared to the plants in the irrigation regime of 12 mm day-1.
The aforementioned responses obtained are attributable to the fact that under conditions of water stress the photosynthetic activity is inhibited in plant tissues due to an imbalance between light capture and its use (Foyer & Noctor, 2000Foyer CH, Noctor G. Tansley review no. 112. New Phytologist 2000; 146(3): 359-388. 10.1046/j.1469-8137.2000.00667.x
https://doi.org/10.1046/j.1469-8137.2000...
). The dissipation of excess light energy in the PSII core and the antenna complex leads to the generation of ROS that are potentially hazardous under water restriction conditions (Sharma et al., 2012Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012; 2012: 217037. 10.1155/2012/217037
https://doi.org/10.1155/2012/217037...
).
In an attempt to neutralize the ROS and achieve a balance between the synthesis and degradation of these molecules, the plant defense system, which is represented by the antioxidant enzyme superoxide dismutase (SOD), showed 105% higher activity in plants under water restriction (4 mm day-1) as compared to the watering regime that provided 12 mm day-1. Activation of antioxidative enzyme activity has been reported in several plant species under water stress (Sgherri et al., 2004Sgherri C, Stevanovic B, Navari-Izzo F. Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiologia Plantarum 2004; 122(4): 478-485. 10.1111/j.1399-3054.2004.00428.x
https://doi.org/10.1111/j.1399-3054.2004...
; Sharma & Dubey, 2005Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regulation 2005; 46(3): 209-221. 10.1007/s10725-005-0002-2
https://doi.org/10.1007/s10725-005-0002-...
). This higher activity of the SOD enzyme under water restriction observed in our study coincides with the higher content of H2O2. Overproduction of ROS is opposed enzymatically by means of a complex coordinate system of antioxidant enzymes (Shapiguzov et al., 2012Shapiguzov A, Vainonen J, Wrzaczek M, Kangasjärvi J. ROS-talk: how the apoplast, the chloroplast, and the nucleus get the message through. Frontiers in Plant Science 2012; 3: 292. 10.3389/fpls.2012.00292
https://doi.org/10.3389/fpls.2012.00292...
). Among these enzymes, SOD is considered the first line of defense of the plant against O2
•- (Favaretto et al., 2011Favaretto VF, Martinez CA, Soriani HH, Furriel RPM. Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environmental and Experimental Botany 2011; 70(1): 20-28. 10.1016/j.envexpbot.2010.06.003
https://doi.org/10.1016/j.envexpbot.2010...
); it plays a primordial role in the protection of cells against oxidative damage by dismutation of O2
•- to H2O2 and O2 (Wang et al., 2005Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. Journal of Plant Physiology 2005; 162(4): 465-472. 10.1016/j.jplph.2004.09.009
https://doi.org/10.1016/j.jplph.2004.09....
). The H2O2 has a deleterious action because it participates, together with the O2
•-, in the formation reaction of OH•, the most reactive oxidant in the ROS family (Sharma et al., 2012Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012; 2012: 217037. 10.1155/2012/217037
https://doi.org/10.1155/2012/217037...
). Thus, after the dismutation of the O2
•-, the produced H2O2 requires the action of other enzymes such as catalase (CAT), ascorbate peroxidase (APX) and guaiacol peroxidases (POX), which catalyzes the dismutation of H2O2 into water and oxygen (Barbosa et al., 2014Barbosa MR, Silva MMDA, Willadino L, Ulisses C, Camara TR. Geração e desintoxicação enzimática de espécies reativas de oxigênio em plantas. Ciência Rural 2014; 44(3): 453-460. 10.1590/S0103-84782014000300011
https://doi.org/10.1590/S0103-8478201400...
).
According to results of MDA, H2O2 and SOD activity, the irrigation condition of 4 mm day-1 presented oxidative damages due to the high levels of peroxidation of lipids and H2O2. The accumulation of H2O2 favors the peroxidation of lipids, causing oxidative damages (Sharma et al., 2012Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012; 2012: 217037. 10.1155/2012/217037
https://doi.org/10.1155/2012/217037...
). As there was a high production of H2O2, SOD probably performed the dismutation of the superoxide anion in H2O2. However, the enzymes responsible for H2O2 elimination, such as catalase and peroxidases, were not efficient in the control of the production of H2O2. This probably favored oxidative stress. Thus, in the 4 mm day-1 condition, the SOD activity was elevated, but, together with the other antioxidant enzymes, they were not able to neutralize the oxidative damages to the more severe condition of water restriction, observed by the high levels of MDA and H2O2, resulting in seedlings with lower morphological quality. Thus, we noticed that water restriction in the 4 mm day-1 irrigation regime triggered an abiotic stress situation.
For conditions of 8 and 12 mm day-1 even with high levels of H2O2 production, there was no oxidative damage, indicated by the reduced levels of lipid peroxidation. ROS under low levels participate in various biological processes in plants such as cell signaling to regulate development, cellular proliferation and differentiation, redox levels, stress signaling, immune response, interactions with other organisms, systemic responses, circadian rhythms, and cell death regulation (Mittler, 2017Mittler, R. ROS are good. Trends in Plant Science 2017; 22(1): 11-19. 10.1016/j.tplants.2016.08.002
https://doi.org/10.1016/j.tplants.2016.0...
). That is, ROS participate in various biochemical and physiological processes even under normal growth conditions.
We emphasize the importance of research involving concomitant morphophysiological analysis to allow technical recommendations based on morphological and metabolic analyses.
5. CONCLUSIONS
The development of E. contortisiliquum seedlings is dependent on the water regime adopted by the nursery. In this sense, it can be used 8 mm day-1 (RR8) in the first 60 days and for the remainder of the period the daily 12 mm day-1 (RR12).
The use of water-retaining polymer does not reduce the need for irrigation in E. contortisiliquum seedlings. However, it provides greater growth and biomass allocation, regardless of the daily water regime.
E. contortisiliquum seedlings tend to reduce their metabolism when submitted under conditions of low water availability, independent of the addition of the water-retaining polymer. Under these conditions, the seedlings present a smaller increase in biomass due to the overproduction of ROS and oxidative damages, not effectively neutralized by the action of the antioxidant system.
ACKNOWLEDGEMENTS
We would like to thank the Universidade Federal de Santa Maria for the necessary infrastructure for this study.
REFERENCES
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLDM, Sparovek G. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 2013; 22(1): 711-728. 10.1127/0941-2948/2013/0507
» https://doi.org/10.1127/0941-2948/2013/0507 - Araújo APD, Paiva Sobrinho SD. Germinação e produção de mudas de tamboril (Enterolobium contortisiliquum (Vell.) Morong) em diferentes substratos. Revista Árvore 2011; 35(3): 581-588. 10.1590/S0100-67622011000400001
» https://doi.org/10.1590/S0100-67622011000400001 - Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 2006; 141: 391-396. 10.1104/pp.106.082040
» https://doi.org/10.1104/pp.106.082040 - Barbosa MR, Silva MMDA, Willadino L, Ulisses C, Camara TR. Geração e desintoxicação enzimática de espécies reativas de oxigênio em plantas. Ciência Rural 2014; 44(3): 453-460. 10.1590/S0103-84782014000300011
» https://doi.org/10.1590/S0103-84782014000300011 - Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry 1971; 44(1): 276-287. 10.1016/0003-2697(71)90370-8
» https://doi.org/10.1016/0003-2697(71)90370-8 - Bergonci JI, Bergamaschi H, Berlato MA, Santos AO. Potencial da água na folha como um indicador de déficit hídrico em milho. Pesquisa Agropecuária Brasileira 2000; 35(8): 1531-1540. 10.1590/S0100-204X2000000800005
» https://doi.org/10.1590/S0100-204X2000000800005 - Bernardi MR, Sperotto M Jr, Daniel O, Vitorino ACT. Crescimento de mudas de Corymbia citriodora em função do uso de hidrogel e adubação. Cerne 2012; 18(1): 67-74. 10.1590/S0104-77602012000100009
» https://doi.org/10.1590/S0104-77602012000100009 - Bernardo S, Soares AA, Mantovani EC. Manual de irrigação. Viçosa: UFV; 2006.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 1976; 72(1-2): 248-254. 10.1016/0003-2697(76)90527-3
» https://doi.org/10.1016/0003-2697(76)90527-3 - Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instrução normativa nº 31, de 24 de outubro de 2008. Diário Oficial da União, Brasília, DF (2008 Oct. 24); Sec. 1.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instruções para análise de espécies florestais. Brasília, DF; 2013.
- Burkart A. Leguminosae. Buenos Aires: Instituto de Botanica Darwinion; 1967. (La flora de la provincia de Buenos Aires; vol. 4, pt. 3).
- Chapman SC, Barreto HJ. Using a chlorophyll meter to estimate specific leaf nitrogen of tropical maize during vegetative growth. Agronomy Journal 1997; 89(4): 557-562. 10.2134/agronj1997.00021962008900040004x
» https://doi.org/10.2134/agronj1997.00021962008900040004x - Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signaling & Behavior 2013; 8(4): e23681. 10.4161/psb.23681
» https://doi.org/10.4161/psb.23681 - Dutra AF, Araujo MM, Turchetto F, Rorato DG, Aimi SC, Gomes DR, Nishijima T. Substrate and irrigation scheme on the growth of Parapiptadenia rigida (angico-vermelho) seedlings. Ciência Rural 2016; 46(6): 1007-1013. 10.1590/0103-8478cr20141732
» https://doi.org/10.1590/0103-8478cr20141732 - El-Moshaty FIB, Pike SM, Novacky AJ, Sehgal OP. Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ringspot virus or southern bean mosaic virus. Physiological and Molecular Plant Pathology 1993; 43(2): 109-119. 10.1006/pmpp.1993.1044
» https://doi.org/10.1006/pmpp.1993.1044 - Favaretto VF, Martinez CA, Soriani HH, Furriel RPM. Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environmental and Experimental Botany 2011; 70(1): 20-28. 10.1016/j.envexpbot.2010.06.003
» https://doi.org/10.1016/j.envexpbot.2010.06.003 - Felippe D, Navroski MC, Sampietro JA, Frigotto T, Albuquerque JA, Mota CS, Pereira MO. Efeito do hidrogel no crescimento de mudas de Eucalyptus benthamii submetidas a diferentes frequências de irrigação. Floresta 2016; 46(2): 215-225. 10.5380/rf.v46i2.43920
» https://doi.org/10.5380/rf.v46i2.43920 - Ferreira DF. Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia 2014; 38(2): 109-112. 10.1590/S1413-70542014000200001
» https://doi.org/10.1590/S1413-70542014000200001 - Foyer CH, Noctor G. Tansley review no. 112. New Phytologist 2000; 146(3): 359-388. 10.1046/j.1469-8137.2000.00667.x
» https://doi.org/10.1046/j.1469-8137.2000.00667.x - Giannopolitis CN, Ries SK. Superoxide dismutases: II: purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiology 1977; 59(2): 315-318. 10.1104/pp.59.2.315
» https://doi.org/10.1104/pp.59.2.315 - Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 2010; 48(12): 909-930. 10.1016/j.plaphy.2010.08.016
» https://doi.org/10.1016/j.plaphy.2010.08.016 - Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 1979; 57(12): 1332-1334. https://doi.org/10.1139/b79-163
» https://doi.org/10.1139/b79-163 - Lichtenthaler HK. [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 1987; 148: 350-382. 10.1016/0076-6879(87)48036-1
» https://doi.org/10.1016/0076-6879(87)48036-1 - Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Global Change Biology 2007; 13(10): 2089-2109. 10.1111/j.1365-2486.2007.01420.x
» https://doi.org/10.1111/j.1365-2486.2007.01420.x - Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology 2001; 127(4): 1781-1787. 10.1104/pp.010497
» https://doi.org/10.1104/pp.010497 - Mittler, R. ROS are good. Trends in Plant Science 2017; 22(1): 11-19. 10.1016/j.tplants.2016.08.002
» https://doi.org/10.1016/j.tplants.2016.08.002 - Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 2007; 58(1): 459-481. 10.1146/annurev.arplant.58.032806.103946
» https://doi.org/10.1146/annurev.arplant.58.032806.103946 - Moreira PDA, Brandão MM, Araujo NH, Oliveira DA, Fernandes GW. Genetic diversity and structure of the tree Enterolobium contortisiliquum (Fabaceae) associated with remnants of a seasonally dry tropical forest. Flora: Morphology, Distribution, Functional Ecology of Plants 2015; 210: 40-46. 10.1016/j.flora.2014.10.005
» https://doi.org/10.1016/j.flora.2014.10.005 - Nakahata M, Mayer B, Ries C, De Paula CAA, Karow M, Neth P et al. The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines. Biological Chemistry 2011; 392(4): 327-336. 10.1515/bc.2011.031
» https://doi.org/10.1515/bc.2011.031 - Navroski MC, Araújo MM, Cunha FDS, Berghetti ÁLP, Pereira MDO. Redução da adubação e melhoria das características do substrato com o uso do hidrogel na produção de mudas de Eucalyptus dunnii Maiden. Ciência Florestal 2016; 26(4): 1155-1165. 10.5902/1980509825106
» https://doi.org/10.5902/1980509825106 - Navroski MC, Araujo MM, Fior CS, Cunha FDS, Berghetti ÁLP, Pereira MDO. Uso de hidrogel possibilita redução da irrigação e melhora o crescimento inicial de mudas de Eucalyptus dunnii Maiden. Scientia Forestalis 2015; 43(106): 467-476.
- Pandey P, Srivastava RK, Rajpoot R, Rani A, Pandey AK, Dubey RS. Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance. Environmental Science and Pollution Research 2016; 23(2): 1516-1528. 10.1007/s11356-015-5392-8
» https://doi.org/10.1007/s11356-015-5392-8 - Ryan MG, Stape JL, Binkley D, Fonseca S, Loos RA, Takahashi EN et al. Factors controlling Eucalyptus productivity: how water availability and stand structure alter production and carbon allocation. Forest Ecology and Management 2010; 259(9): 1695-1703. 10.1016/j.foreco.2010.01.013
» https://doi.org/10.1016/j.foreco.2010.01.013 - Sgherri C, Stevanovic B, Navari-Izzo F. Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiologia Plantarum 2004; 122(4): 478-485. 10.1111/j.1399-3054.2004.00428.x
» https://doi.org/10.1111/j.1399-3054.2004.00428.x - Shapiguzov A, Vainonen J, Wrzaczek M, Kangasjärvi J. ROS-talk: how the apoplast, the chloroplast, and the nucleus get the message through. Frontiers in Plant Science 2012; 3: 292. 10.3389/fpls.2012.00292
» https://doi.org/10.3389/fpls.2012.00292 - Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regulation 2005; 46(3): 209-221. 10.1007/s10725-005-0002-2
» https://doi.org/10.1007/s10725-005-0002-2 - Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012; 2012: 217037. 10.1155/2012/217037
» https://doi.org/10.1155/2012/217037 - Silva MDA, Jifon JL, Silva JAGD, Sharma V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology 2007; 19(3): 193-201. 10.1590/S1677-04202007000300003
» https://doi.org/10.1590/S1677-04202007000300003 - Silva P, Campoe O, De Paula R, Lee D. Seedling growth and physiological responses of sixteen eucalypt taxa under controlled water regime. Forests 2016; 7(6): 110. 10.3390/f7060110
» https://doi.org/10.3390/f7060110 - Silva RBGD, Simões D, Silva MRD. Qualidade de mudas clonais de Eucalyptus urophylla × E. grandis em função do substrato. Revista Brasileira de Engenharia Agrícola e Ambiental 2012; 16(3): 297-302. http://dx.doi.org/10.1590/S1415-43662012000300010
» http://dx.doi.org/10.1590/S1415-43662012000300010 - Smeal D, Zhang H. Chlorophyll meter evaluation for nitrogen management in corn. Communications in Soil Science and Plant Analysis 1994; 25(9-10): 1495-1503. 10.1080/00103629409369130
» https://doi.org/10.1080/00103629409369130 - Taiz L, Zeiger E, Møller IM, Murphy A. Fisiologia e desenvolvimento vegetal. 6th ed. Porto Alegre: Artmed; 2017.
- Verdonck O, Penninck R, De Boodt M. The physical properties of different horticultural substrates. Acta Horticulturae 1984; 150: 155-160. 10.17660/ActaHortic.1984.150.16
» https://doi.org/10.17660/ActaHortic.1984.150.16 - Vyumvuhore R, Tfayli A, Biniek K, Duplan H, Delalleau A, Manfait M et al. The relationship between water loss, mechanical stress, and molecular structure of human stratum corneum ex vivo. Journal of Biophotonics 2015; 8(3): 217-225. 10.1002/jbio.201300169
» https://doi.org/10.1002/jbio.201300169 - Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. Journal of Plant Physiology 2005; 162(4): 465-472. 10.1016/j.jplph.2004.09.009
» https://doi.org/10.1016/j.jplph.2004.09.009 - Waszczak C, Carmody M, Kangasjärvi J. Reactive oxygen species in plant signaling. Annual Review of Plant Biology 2018; 69: 209-236. 10.1146/annurev-arplant-042817-040322
» https://doi.org/10.1146/annurev-arplant-042817-040322 - Zhu Z, Wei G, Li J, Qian Q, Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Science 2004; 167(3): 527-533. 10.1016/j.plantsci.2004.04.020
» https://doi.org/10.1016/j.plantsci.2004.04.020 - Zuchiwschi E, Fantini AC, Alves AC, Peroni N. Limitações ao uso de espécies florestais nativas pode contribuir com a erosão do conhecimento ecológico tradicional e local de agricultores familiares. Acta Botanica Brasilica 2010; 24(1): 270-282. 10.1590/S0102-33062010000100029
» https://doi.org/10.1590/S0102-33062010000100029
-
1
Associate editor: Marcel Carvalho Abreu https://orcid.org/0000-0002-6457-421X
Publication Dates
-
Publication in this collection
17 Apr 2020 -
Date of issue
2020
History
-
Received
10 June 2018 -
Accepted
07 July 2019