Abstract
This study aimed to carry out a phytosociological survey in a riparian area of an intermittent stream in the semi-arid region of Paraíba, Brazil. Fifty-one contiguous plots of 10 × 20 m (1.02 ha) were distributed along the watercourse. Living and dead shrub-tree individuals, still standing, with stem diameter at ground level (DGL) ≥ 3 cm and total height ≥ 1 m were sampled. Fifty-one species distributed in 22 families were sampled. Fabaceae, Euphorbiaceae, and Anacardiaceae had the highest species richness. Aspidosperma pyrifolium Mart. & Zucc., Combretum monetaria Mart., and Cenostigma pyramidale (Tul.) E. Gagnon & G.P. Lewis had the highest importance values. Shannon index was 2.61 nats.ind.−1 and the total basal area was 25.4 m2. Height and diameter mean values were 5.4 m and 12.4 cm, respectively. Phytosociological parameters recorded for the studied riparian vegetation have higher values in comparison with those obtained in other areas of the caatinga.
Keywords:
phytosociology; caatinga; riparian forest
1. INTRODUCTION AND OBJECTIVES
The course of human evolution was defined by its proximity to watercourses, of which banks were comprised of forests and were used by humans to supply their immediate needs (Lacerda, 2016Lacerda AV. Os cílios das águas: espaços plurais no contexto do semiárido brasileiro. Campina Grande: EDUFCG; 2016.). According to this author, all these aspects denoted values that have been expanded over time, and currently the riparian vegetation has immense potentialities in the pharmacological, food and artisanal fields. These potentials can be revealed through sustainable practices in opportunities for the development of urban and rural communities.
Riparian forests contribute to the water table supply, protect fountainheads and prevent soil erosion, reduce impacts on aquatic biota, and are closely related to water quality for human and animal consumption, in addition to being related to energy generation and irrigation (Lima & Zakia, 2009Lima WP, Zakia MJB. Hidrologia de matas ciliares. In: Rodrigues RR, Leitão-Filho HF, editors. Matas ciliares: conservação e recuperação. São Paulo: Edusp; 2009. p. 33-44.). These forest formations have high biodiversity and are important ecological corridors. Attanasio et al. (2006Attanasio CM, Rodrigues RR, Gandolfi S, Nave AG. Adequação ambiental de propriedades rurais: recuperação de áreas degradadas: restauração de matas ciliares. Piracicaba: Universidade de São Paulo; 2006.) point out that these forests also provide organic matter, such as trunks and branches, for river food webs, create microhabitats in watercourses, and protect flora and fauna species.
The relevance of the riparian areas has led many countries to develop laws to conserve their vegetation. In Brazil, riparian forests are located in Permanent Preservation Areas (PPAs) protected by the Brazilian Forest Code - Law 12,651/2012 (Brasil, 2012Brasil. Lei nº 12.651, de 25 de maio de 2012. Diário Oficial da União, Brasília, DF (2012 May 28); Sec. 1: 1.).
However, there has been a reduction in the components of their biodiversity due to negative impacts. These negative impact factors are related to forest fires, cutting and falling of several trees, frequent trampling by cattle, intense traffic of agricultural machinery, waste disposal, occupation of areas unfit for cultivation, indiscriminate use of pesticides, among others (EMBRAPA, 2012Empresa Brasileira de Pesquisa Agropecuária - EMBRAPA. Cerrado: restauração de matas de galeria e ciliares. Brasília: Embrapa Cerrados; 2012.). Castro et al. (2012Castro D, Mello RSP, Poester GC. Práticas para restauração da mata ciliar. Porto Alegre: Catarse; 2012.) explain that the degradation of riparian forests occurs due to the expansion of agricultural areas precisely because these areas are close to water bodies, which facilitates the installation of irrigation systems. According to these authors, the urban expansion, often occurring with disorderly growth of cities, is another negative impact factor on riparian ecosystems.
Therefore, it is important to emphasize studies aimed at the definition of the vegetation structure in riparian areas in the semi-arid region of Brazil. Lacerda (2016Lacerda AV. Os cílios das águas: espaços plurais no contexto do semiárido brasileiro. Campina Grande: EDUFCG; 2016.) explains that these areas in the semi-arid are characterized by their biological richness, which associated with the physical variability of the natural systems results in a diversity with great potentials. The peculiarities that define the floristic composition and the structure of the riparian communities are remarkable and their laws are imposed by interactions within the connections established, making these systems complex in bands of drylands. Thus, this study aimed to carry out a phytosociological survey of the riparian vegetation in a conserved area of an intermittent stream in the semi-arid region of Paraíba.
2. MATERIALS AND METHODS
2.1. Study area
This study was conducted in the hydrographic basin of the Taperoá River in the semi-arid region of Paraíba, Brazil. This basin drains an area of approximately 5,667.49 km2 (Souza et al., 2004Souza BI, Silans AMBP, Santos JB. Contribuição ao estudo da desertificação na bacia do Taperoá. Revista Brasileira de Engenharia Agrícola Ambiental 2004; 8(2-3): 292-298. 10.1590/S1415-43662004000200019
https://doi.org/10.1590/S1415-4366200400...
) and is located in the central part of Paraíba State, between 6° 51’ 31” and 7° 34’ 21” S latitude and 36° 0’ 55” and 37° 13’ 9” W longitude (Lacerda et al., 2010Lacerda AV, Barbosa FM, Soares JJ, Barbosa MRV. Flora arbustiva-arbórea de três áreas no semiárido paraibano, Brasil. Biota Neotropica 2010; 10(4): 275-284. 10.1590/S1676-06032010000400032
https://doi.org/10.1590/S1676-0603201000...
).
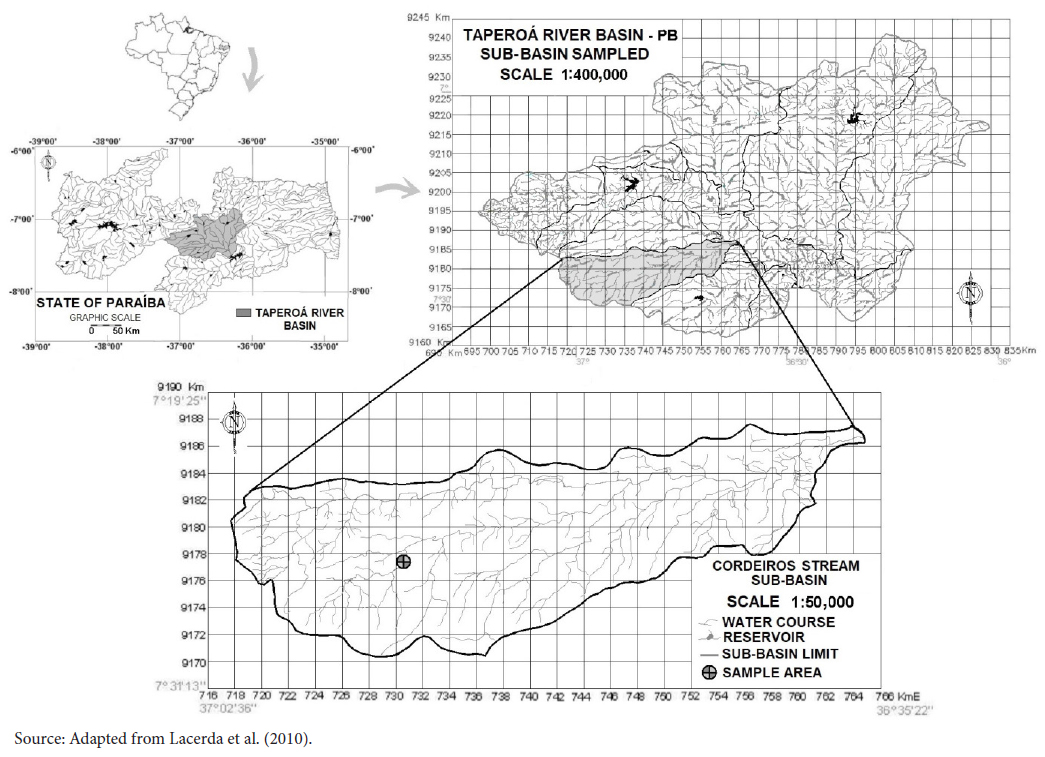
The survey was carried out in the riparian forest of the Cazuzinha stream located in the sub-basin of the Cordeiros stream, which is part of the Taperoá basin (Figure 1).
Cazuzinha stream located in the sub-basin of the Cordeiros stream - hydrographic basin of the Taperoá River, semi-arid region of Paraíba, Brazil.
The Cazuzinha stream is intermittent, runs northeast and has an extension of 15 km and a drainage basin of 59 km2, at an altitude ranging from 564 to 579 m, with a channel of about 12 m of mean width. The riparian area sampled in this stream is located in the Almas Farm, a Natural Heritage Private Reserve, in the municipality of São José dos Cordeiros, between 7° 26’ 13” and 7° 25’ 46” S latitude and 36° 54’ 30” and 36° 54’ 35” W longitude. This reserve has 3,505 ha and was created by the IBAMA Decree No. 1,343/90 and Decree No. 98,914 of January 31, 1990.
The information on rainfall, temperature, relative air humidity and evaporation was systematized from the data of the historical series (from January 1996 to December 2005), provided by the Water Resources Laboratory of the Department of Civil Engineering of the Universidade Federal de Campina Grande. In this period, the annual average rainfall was 486.9 mm and the average annual air temperature ranged from 23.6 °C to 27.4 °C. The lowest temperatures were recorded in July and August, and the highest in November and December. Average monthly relative air humidity reached a maximum of 75% in June and July and a minimum of 64% in the dry season in November and December.
The potential evaporation in the region was quite high, reaching 2,697 mm per year. The riparian vegetation is predominantly arboreal, with the occurrence of shrub species quite branched from the base, in addition to the presence of an herbaceous stratum abundant in the rainy season.
2.2. Data collection and analysis
For the definition of the community structure, fifty-one contiguous plots of 10 × 20 m (1.02 ha) were established, distributed along the watercourse. The following inclusion criteria were considered for sampling: shrub-tree, living and dead, and standing specimens with stem diameter at ground level (DGL) ≥ 3 cm and total height ≥ 1 m. Specimens were tagged with small plaques, numbered, and identified by scientific name. In the case of unidentified species, collections were made for later identification. The perimeter was measured at ground level using a tape measure and subsequently converted into diameter. For the trees and shrubs with multiple trunks, all the branches with DGL ≥ 3 cm were measured. Plant height was determined using a 5 m rod. For higher specimens, estimates were made by comparing with this rod.
The data recorded in the field were organized in an electronic spreadsheet (Microsoft® Excel, version 2010) and the phytosociological parameters were calculated using the MATA NATIVA 2 software (Cientec, 2006Cientec. Mata nativa 2: sistema para análise fitossociológica e elaboração de planos de manejo de florestas nativas. São Paulo; 2006.). The following data were analyzed: number of species and individuals per species, basal area per species and total basal area, absolute and relative densities (AD and RD), absolute and relative frequencies (AF and RF), and absolute and relative dominance (ADo and RDo) (Mueller-Dombois & Ellenberg, 1974Mueller-Dombois D, Ellenberg H. Aims and methods of vegetation ecology. New York: John Wiley & Sons; 1974.). From the relative parameters, the importance value (IV) and the cover value (CV) were calculated for each species. The Shannon specific diversity index (H’) and the equability index (J’) were used for the floristic heterogeneity analysis, according to Magurran (1988Magurran AE. Ecological diversity and its measurement. London: Chapman and Hall; 1988.) and Pielou (1975Pielou EC. Ecological diversity. New York: Jonh Wiley & Sons; 1975.), respectively, based on the proportional abundance of species.
The evaluation of the distribution of the sampled individuals into the height classes was performed through the elaboration of frequency histograms with an interval of 1 m. Frequency distribution histograms of diameter classes at 3 cm intervals were also performed for all individuals surveyed. For species with more than 10% of the total number of individuals, graphs of distribution by diameter and height classes were made, considering the previously mentioned intervals. The species were classified into families according to the APG III (2009Angiosperm Phylogeny Group - APG III. An update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG III. Botanical Journal of Linnean Society 2009; 161: 105-121. 10.1111/boj.12385
https://doi.org/10.1111/boj.12385...
) system and the taxonomic update of the species and their authors followed the List of Brazilian Flora Species (Reflora, 2016Reflora. Flora do Brasil 2020 em construção [Internet]. 2016 [cited 2016 May 18]. Available from: Available from: https://bit.ly/2ITnPXi
https://bit.ly/2ITnPXi...
).
3. RESULTS AND DISCUSSION
In the 51 surveyed plots, 1,929 living and 209 standing dead individuals were sampled. Living individuals were distributed into 51 species, 43 genera, and 22 families. Considering all trees and shrubs recorded, we obtained a total density of 2,096 individuals.ha−1 and a total basal area of 25.4 m2.
Comparing the data presented here with those from studies on different deciduous communities of the semi-arid region (Alcoforado-Filho et al., 2003Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
https://doi.org/10.1590/S0102-3306200300...
; Fabricante & Andrade, 2007Fabricante JR, Andrade LA. Análise estrutural de um remanescente de caatinga no Seridó paraibano. Oecologia Brasiliensis 2007; 11(3): 341-349.; Ferraz et al., 2013Ferraz RC, Mello AA, Ferreira RA, Prata APN. Levantamento fitossociológico em área de caatinga no Monumento Natural Grota do Angico, Sergipe, Brasil. Revista Caatinga 2013; 26(3): 89-98.; Parente et al., 2010Parente HN, Araújo KD, Silva EE, Andrade AP, Dantas RT, Silva DS, Ramalho CI. Parâmetros fitossociológicos do estrato arbóreo-arbustivo em áreas contíguas de caatinga no Cariri Paraibano. Revista Científica de Produção Animal 2010; 12(2): 138-141. 10.15528/2176-4158/rcpa.v12n2p138-141
https://doi.org/10.15528/2176-4158/rcpa....
; Pinto et al., 2012Pinto MSC, Sampaio EVSB, Nascimento LM. Florística e estrutura da vegetação de um brejo de altitude em Pesqueira, PE, Brasil. Revista Nordestina de Biologia 2012; 21(1): 47-79.; Sabino et al., 2016Sabino FGS, Cunha MCL, Santana GM. Estrutura da vegetação em dois fragmentos de caatinga antropizada na Paraíba. Floresta e Ambiente 2016; 23(4): 487-497. 10.1590/2179-8087.017315
https://doi.org/10.1590/2179-8087.017315...
; Santana et al., 2016Santana JAS, Santana JAS Jr, Barreto WS, Ferreira ATS. Estrutura e distribuição espacial da vegetação da caatinga na Estação Ecológica do Seridó, RN. Pesquisa Florestal Brasileira 2016; 36(88): 355-361. 10.4336/2016.pfb.36.88.1002
https://doi.org/10.4336/2016.pfb.36.88.1...
; Santana & Souto, 2006Santana JAS, Souto JS. Diversidade e estrutura fitossociológica da caatinga na Estação Ecológica do Seridó - RN. Revista de Biologia e Ciências da Terra 2006; 6(2): 232-242.; Silva et al., 2014Silva N, Lucena RFP, Lima JRF, Lima GDS, Carvalho TKN, Sousa SP Jr, Alves CAB. Conhecimento e uso da vegetação nativa da caatinga em uma comunidade rural da Paraíba, Nordeste do Brasil. Boletim do Museu de Biologia Mello Leitão 2014; (34): 5-37.), we observed that the number of species found in the riparian environment was higher than those recorded in the majority of the phytosociological surveys carried out in solid ground areas of the caatinga. For Alcoforado-Filho et al. (2003Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
https://doi.org/10.1590/S0102-3306200300...
), the density variation in areas of caatinga is due to water availability. However, these authors recognize the absence of estimates of this parameter in areas of native vegetation in the semi-arid region of Brazil. In addition, for these authors, water availability involves other variables, such as the distribution of rainfall throughout the year and the soil water retention. Thus, the total annual rainfall does not explain the density variations in the surveys conducted in the caatinga, which sometimes occur in very close areas.
Among the 22 families identified, Euphorbiaceae had the highest number of individuals, followed by Combretaceae, Apocynaceae, Fabaceae, and Boraginaceae. Together, these five families comprised 90.6% of the living shrub-tree individuals recorded. Of the 627 Euphorbiaceae family individuals, 62.2% belong to a single species: Croton echioides Baill. Combretaceae family, which accounted for 25.4% of the individuals, was represented by two species: Combretum leprosum Mart. and Combretum monetaria Mart.. Fabaceae species have been found in riparian forest areas in the caatinga (Farias et al., 2017Farias RC, Lacerda AV, Gomes AC, Barbosa FM, Dornelas CSM. Riqueza florística em uma área ciliar de caatinga no Cariri Ocidental da Paraíba, Brasil. Revista Brasileira de Gestão Ambiental e Sustentabilidade 2017; 4(7): 109-118. 10.21438/rbgas.040711
https://doi.org/10.21438/rbgas.040711...
; Ferraz et al., 2006Ferraz JSF, Albuquerque UP, Meunier IMJ. Valor de uso e estrutura da vegetação lenhosa às margens do riacho do Navio, Floresta, PE, Brasil. Acta Botânica Brasílica 2006; 20(1): 125-134. 10.1590/S0102-33062006000100012
https://doi.org/10.1590/S0102-3306200600...
; Holanda et al., 2005Holanda FSR, Santos LGC, Santos CM, Casado APB, Pedrotti A, Ribeiro GTl. Riparian vegetation affected by bank erosion in the lower São Francisco river, Northeastern Brazil. Revista Árvore 2005; 29(2): 327-336. 10.1590/S0100-67622005000200016
https://doi.org/10.1590/S0100-6762200500...
; Lacerda et al., 2007Lacerda AV, Barbosa FM, Barbosa MRV. Estudo do componente arbustivo-arbóreo de matas ciliares na bacia do rio Taperoá, semi-árido paraibano: uma perspectiva para sustentabilidade dos recursos naturais. Oecologia Brasiliensis 2007; 11(3): 331-340. 10.4257/oeco.2007.1103.03
https://doi.org/10.4257/oeco.2007.1103.0...
, 2010Lacerda AV, Barbosa FM, Soares JJ, Barbosa MRV. Flora arbustiva-arbórea de três áreas no semiárido paraibano, Brasil. Biota Neotropica 2010; 10(4): 275-284. 10.1590/S1676-06032010000400032
https://doi.org/10.1590/S1676-0603201000...
; Santos & Vieira, 2006Santos RM, Vieira FA. Florística e estrutura da comunidade arbórea de fragmentos de matas ciliares dos rios São Francisco, Cochá e Carinhanha, norte de Minas Gerais, Brasil. Revista Científica Eletrônica de Engenharia Florestal 2006; 4(8): 1-21.; Silva FG et al., 2015Silva FG, Silva RH, Araújo RM, Lucena MFA, Sousa JM. Levantamento florístico de um trecho de mata ciliar na mesorregião do Sertão Paraibano. Revista Brasileira Biociência 2015; 13(4): 250-258.; Souza & Rodal, 2010Souza JAS, Rodal MJN. Levantamento florístico em trecho de vegetação ripária de caatinga no rio Pajeú, Floresta/Pernambuco-Brasil. Revista Caatinga 2010; 23(4): 54-62.; Trovão et al., 2010Trovão DMBM, Freire AM, Melo IJMM. Florística e fitossociologia do componente lenhoso da mata Ciliar do Riacho de Bodocongó, semiárido paraibano. Revista Caatinga 2010; 23(2): 78-86.).
Euphorbiaceae, Fabaceae, Combretaceae, Apocynaceae, and Sapotaceae were the most prominent families in the study community, considering the importance value. The high IV of Euphorbiaceae was basically due to the high number of individuals of C. echioides and to the uniform distribution of its species in the area. Fabaceae stood out due to the higher relative dominance of its species, especially Cenostigma pyramidale (Tul.) E. Gagnon & G.P. Lewis. Combretaceae had the third most significant IV because of its higher density and relative frequency of its species. Likewise, Apocynaceae had the fourth highest IV due to its higher number of individuals (347), wide distribution, and high relative density of Aspidosperma pyrifolium Mart. & Zucc. Sapotaceae was represented only by Sideroxylon obtusifolium (Roemer & Schultes) T.D. Penn. and had the fifth highest IV due to the higher relative dominance of its individuals.
The phytosociological parameters of the species identified are shown, in decreasing order of IV, in Table 1.
The AD and RD values show that C. echioides, A. pyrifolium, and C. monetaria were the most representative species in the community structure. Together, these three species accounted for 50.2% of the total relative density. A. pyrifolium and C. monetaria had the highest AF and RF (Table 1), occurring in 47 of the 51 plots, followed by dead individuals (44 plots), C. pyramidale (42), C. echioides (36), C. leprosum (35), C. blanchetianus (32), S. macrocarpa (31), A. colubrina and C. trichotoma (18 plots each), C. foliolosum and S. obtusifolium (16 plots each). These 11 species together with the dead individuals accounted for 68.2% of the total relative frequency. Regarding the ADo and RDo, C. pyramidale and S. obtusifolium stood out with 4.40 m2 and 4.19 m2 of basal area, respectively.
The first ten species together with the dead individuals comprised 74.9% of the total IV. Regarding the cover value (CV), with the exception of C. trichotoma, which was replaced by S. brasiliensis, the same species that had the highest importance values (IVs) also had the highest cover values (CVs) (Table 1). Thus, analyzing the species and their respective IVs, we noticed few of them have high values, whereas many others, with few individuals, have low IVs. Considering the most important species in terms of IV, some authors have also evidenced the importance of A. pyrifolium and C. pyramidale covering areas of the caatinga (Alcoforado-Filho et al., 2003Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
https://doi.org/10.1590/S0102-3306200300...
; Fabricante & Andrade, 2007Fabricante JR, Andrade LA. Análise estrutural de um remanescente de caatinga no Seridó paraibano. Oecologia Brasiliensis 2007; 11(3): 341-349.; Ferraz et al., 2013Ferraz RC, Mello AA, Ferreira RA, Prata APN. Levantamento fitossociológico em área de caatinga no Monumento Natural Grota do Angico, Sergipe, Brasil. Revista Caatinga 2013; 26(3): 89-98.; Parente et al., 2010Parente HN, Araújo KD, Silva EE, Andrade AP, Dantas RT, Silva DS, Ramalho CI. Parâmetros fitossociológicos do estrato arbóreo-arbustivo em áreas contíguas de caatinga no Cariri Paraibano. Revista Científica de Produção Animal 2010; 12(2): 138-141. 10.15528/2176-4158/rcpa.v12n2p138-141
https://doi.org/10.15528/2176-4158/rcpa....
; Sabino et al., 2016Sabino FGS, Cunha MCL, Santana GM. Estrutura da vegetação em dois fragmentos de caatinga antropizada na Paraíba. Floresta e Ambiente 2016; 23(4): 487-497. 10.1590/2179-8087.017315
https://doi.org/10.1590/2179-8087.017315...
; Santana et al., 2016Santana JAS, Santana JAS Jr, Barreto WS, Ferreira ATS. Estrutura e distribuição espacial da vegetação da caatinga na Estação Ecológica do Seridó, RN. Pesquisa Florestal Brasileira 2016; 36(88): 355-361. 10.4336/2016.pfb.36.88.1002
https://doi.org/10.4336/2016.pfb.36.88.1...
; Santana & Souto, 2006Santana JAS, Souto JS. Diversidade e estrutura fitossociológica da caatinga na Estação Ecológica do Seridó - RN. Revista de Biologia e Ciências da Terra 2006; 6(2): 232-242.; Silva et al., 2014Silva N, Lucena RFP, Lima JRF, Lima GDS, Carvalho TKN, Sousa SP Jr, Alves CAB. Conhecimento e uso da vegetação nativa da caatinga em uma comunidade rural da Paraíba, Nordeste do Brasil. Boletim do Museu de Biologia Mello Leitão 2014; (34): 5-37.), evidencing thus the wide distribution of these species in different environment typologies. Rodrigues et al. (2003Rodrigues LA, Carvalho DA, Oliveira-Filho AT, Botrel RT, Silva EA. Florística e estrutura da comunidade arbórea de um fragmento florestal em Luminárias, MG. Acta Botanica Brasilica 2003; 17(1): 71-87. 10.1590/S0102-33062003000100006
https://doi.org/10.1590/S0102-3306200300...
) argue many species found in riparian vegetation are also found in forest formations of the caatinga, demonstrating their wide adaptation to different ecological systems.
The dead category had the fifth highest IV and also accounted for 9.8% of the total individuals recorded. Felfili et al. (2004Felfili, JM, Silva MC Jr, Sevilha AC, Fagg CW, Walter BMT, Nogueira PE, Rezende AV. Diversity, floristic and structural patterns of cerrado vegetation in Central Brazil. Plant Ecology 2004; 175: 37-46. 10.1023/B:VEGE.0000048090.07022.02
https://doi.org/10.1023/B:VEGE.000004809...
) state that, for dead individuals in gallery forest areas, it is generally found a percentage around 3% to 9%. Associated with this, we observed the frequency data indicate there is no disturbance in the area, since the dead individuals had a high frequency, occurring in 86% of the plots.
Individuals had a mean diameter of 12.4 cm. This value was higher than those recorded in studies carried out in areas of the caatinga (Amorim et al., 2005Amorim IL, Sampaio EVSB, Araújo EL. Flora e estrutura da vegetação arbustivo-arbórea de uma área de caatinga do Seridó, RN, Brasil. Acta Botanica Brasilica 2005; 19(3): 615-623. 10.1590/S0102-33062005000300023
https://doi.org/10.1590/S0102-3306200500...
; Guedes et al., 2012Guedes RS, Zanella FCV, Costa JEV Jr, Santana GM, Silva JA. Caracterização florístico-fitossociológica do componente lenhoso de um trecho de caatinga no semiárido paraibano. Revista Caatinga 2012; 25(2): 99-108.; Santana & Souto, 2006Santana JAS, Souto JS. Diversidade e estrutura fitossociológica da caatinga na Estação Ecológica do Seridó - RN. Revista de Biologia e Ciências da Terra 2006; 6(2): 232-242.). Maximum diameter was 86.1 cm (Z. joazeiro). Analysis of frequency of the diameter classes indicates a decrease in the number of individuals as the diameter increases (Figure 2).
Distribution of shrub-tree individuals according to diameter classes in the riparian area of the Cazuzinha stream, semi-arid region of Paraíba, Brazil.
Thus, when evaluating the diameter classes, we observed an inverted letter J. Of the 2,138 individuals sampled in the Cazuzinha stream, 1,802 (84.28%) were concentrated in the first three diameter classes, with 1,095 (51.22%) in the 3-6 cm class, whereas the last diameter class accounted only for two individuals. There were interruptions in the highest diameter classes. According to Silva (2004Silva MC Jr. Fitossociologia e estrutura diamétrica da mata de galeria do Taquara, na reserva ecológica do IBGE, DF. Revista Árvore 2004; 28(3): 419-428. 10.1590/S0100-67622004000300013
https://doi.org/10.1590/S0100-6762200400...
), the J-inverted curve pattern indicates a positive balance between recruitment and mortality and characterizes the vegetation as self-regenerating. For Assmann (1970Assmann E. The principles of forest yield: studies in the organic production, structure, increment and yield of forest stands. Braunschweig: Pergamon Press; 1970.), the highest concentration of individuals in the smaller diameter classes is considered a typical pattern of uneven-aged natural forests.
Analyzing a riparian forest ecosystem in a transition area between the cerrado and caatinga, Silva LS et al. (2015Silva LS, Alves AR, Nunes AKA, Macedo WS, Martins AR. Florística e fitossociologia em um remanescente de mata ciliar na bacia do Rio Gurguéia - PI. Nativa 2015; 3(03): 156-164. 10.14583/2318-7670.v03n03a02
https://doi.org/10.14583/2318-7670.v03n0...
) also recorded the highest number of individuals in the first diameter classes. This was also observed in areas of caatinga (Alcoforado-Filho et al., 2003Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
https://doi.org/10.1590/S0102-3306200300...
; Fabricante & Andrade, 2007Fabricante JR, Andrade LA. Análise estrutural de um remanescente de caatinga no Seridó paraibano. Oecologia Brasiliensis 2007; 11(3): 341-349.; Guedes et al., 2012Guedes RS, Zanella FCV, Costa JEV Jr, Santana GM, Silva JA. Caracterização florístico-fitossociológica do componente lenhoso de um trecho de caatinga no semiárido paraibano. Revista Caatinga 2012; 25(2): 99-108.; Pinto et al., 2012Pinto MSC, Sampaio EVSB, Nascimento LM. Florística e estrutura da vegetação de um brejo de altitude em Pesqueira, PE, Brasil. Revista Nordestina de Biologia 2012; 21(1): 47-79.; Sabino et al., 2016Sabino FGS, Cunha MCL, Santana GM. Estrutura da vegetação em dois fragmentos de caatinga antropizada na Paraíba. Floresta e Ambiente 2016; 23(4): 487-497. 10.1590/2179-8087.017315
https://doi.org/10.1590/2179-8087.017315...
).
In this study, the density of individuals with a diameter larger than 42 cm was higher than those found in studies conducted in other areas of caatinga (Alcoforado-Filho et al., 2003Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
https://doi.org/10.1590/S0102-3306200300...
; Pinto et al., 2012Pinto MSC, Sampaio EVSB, Nascimento LM. Florística e estrutura da vegetação de um brejo de altitude em Pesqueira, PE, Brasil. Revista Nordestina de Biologia 2012; 21(1): 47-79.).
For species with more than 10% of the total number of individuals, diametric distribution graphs were established (Figure 3).
Distribution of shrub-tree individuals according to diameter classes of species with more than 10% of representative in the riparian area of the Cazuzinha stream, semi-arid region of Paraíba, Brazil.
The configuration resembling an inverted letter J shows the occurrence of a continuous supply of seedlings for the larger diameter classes, i.e., these species have had a complete life cycle and the population can be considered in equilibrium with the environment. A. pyrifolium and C. monetaria have had interruptions in the larger diameter classes. This fact indicates a non-continuous growth process, i.e., it must have been interrupted due to some factors, such as intense and prolonged droughts, diseases, senility, or selective cutting of large-sized individuals for the use of wood. Santana & Souto (2006Santana JAS, Souto JS. Diversidade e estrutura fitossociológica da caatinga na Estação Ecológica do Seridó - RN. Revista de Biologia e Ciências da Terra 2006; 6(2): 232-242.) confirm this fact, stating it may be due to diseases, senility, or exploitation. However, according to these authors, the lack of knowledge of the growth dynamics of plants from the caatinga makes it difficult to draw meaningful conclusions on this subject.
Individuals had a mean height of 5.4 m, higher than those from some studies carried out in the caatinga (Guedes et al., 2012Guedes RS, Zanella FCV, Costa JEV Jr, Santana GM, Silva JA. Caracterização florístico-fitossociológica do componente lenhoso de um trecho de caatinga no semiárido paraibano. Revista Caatinga 2012; 25(2): 99-108.; Santana & Souto, 2006Santana JAS, Souto JS. Diversidade e estrutura fitossociológica da caatinga na Estação Ecológica do Seridó - RN. Revista de Biologia e Ciências da Terra 2006; 6(2): 232-242.). The maximum height (17 m) was recorded for S. brasiliensis and the minimum (1.3 m) for J. molissima and A. pyrifolium. The highest frequency classes were composed of individuals with heights between 3.1 m and 5 m (Figure 4).
Distribution of shrub-tree individuals according to height classes in the riparian area of the Cazuzinha stream, semi-arid region of Paraíba, Brazil.
An impressive number of individuals higher than 8 m were recorded in the riparian vegetation, in comparison with the studies of Alcoforado-Filho et al. (2003Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
https://doi.org/10.1590/S0102-3306200300...
) and Pinto et al. (2012Pinto MSC, Sampaio EVSB, Nascimento LM. Florística e estrutura da vegetação de um brejo de altitude em Pesqueira, PE, Brasil. Revista Nordestina de Biologia 2012; 21(1): 47-79.). Araújo et al. (2005Araújo EL, Silva KA, Ferraz EMN, Sampaio EVSB, Silva SI. Diversidade de herbáceas em microhabitats rochoso, plano e ciliar em uma área de caatinga, Caruaru, PE, Brasil. Acta Botanica Brasilica 2005; 19(2): 285-294. 10.1590/S0102-33062005000200011
https://doi.org/10.1590/S0102-3306200500...
) consider the higher soil moisture content in riparian environments also favors the occurrence of larger woody plants.
The distribution, according to height classes, of the number of individuals of the species with more than 10% of the total number of individuals recorded is shown in Figure 5. For C. echioides, the distribution curves showed the highest frequency class was defined by heights between 3.1 m and 4.0 m. Regarding the tree species A. pyrifolium and C. monetaria, the highest frequency class consisted of heights ranging from 4.1 m and 5.0 m.
Distribution of shrub-tree individuals according to height classes of species with more than 10% of representatives in the riparian area of the Cazuzinha stream, semi-arid region of Paraíba, Brazil.
For the riparian vegetation studied, values of diversity and equability were 2.61 nats.ind.-1 and 0.66, respectively. The values presented here are higher than those recorded by Trovão et al. (2010Trovão DMBM, Freire AM, Melo IJMM. Florística e fitossociologia do componente lenhoso da mata Ciliar do Riacho de Bodocongó, semiárido paraibano. Revista Caatinga 2010; 23(2): 78-86.) and Santos & Vieira (2006Santos RM, Vieira FA. Florística e estrutura da comunidade arbórea de fragmentos de matas ciliares dos rios São Francisco, Cochá e Carinhanha, norte de Minas Gerais, Brasil. Revista Científica Eletrônica de Engenharia Florestal 2006; 4(8): 1-21.) in riparian forest areas of the caatinga.
4. CONCLUSIONS
The riparian vegetation structure of the Cazuzinha stream in the Cariri Paraibano is characterized by many typical species of solid ground areas of the caatinga and by species that require more favorable environmental conditions, resulting in higher species richness in these riparian ecosystems. The values of diameter and mean height of the individuals were higher than those from studies carried out in the caatinga solid ground areas. For the diameter classes, we obtained a J-inverted curve pattern, characterizing the vegetation as self-regenerating. Diversity and equability indexes had values higher than those observed in other riparian forest environments in the caatinga. Therefore, the phytosociological parameters recorded for the riparian vegetation were higher than those obtained in studies conducted in areas of caatinga and in riparian ecosystems in this biome.
ACKNOWLEDGEMENTS
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the RPPN Fazenda Almas owners.
REFERENCES
- Alcoforado-Filho FG, Sampaio EVSB, Rodal MJN. Florística e fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru, Pernambuco. Acta Botanica Brasilica 2003; 17(2): 287-303. 10.1590/S0102-33062003000200011
» https://doi.org/10.1590/S0102-33062003000200011 - Amorim IL, Sampaio EVSB, Araújo EL. Flora e estrutura da vegetação arbustivo-arbórea de uma área de caatinga do Seridó, RN, Brasil. Acta Botanica Brasilica 2005; 19(3): 615-623. 10.1590/S0102-33062005000300023
» https://doi.org/10.1590/S0102-33062005000300023 - Angiosperm Phylogeny Group - APG III. An update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG III. Botanical Journal of Linnean Society 2009; 161: 105-121. 10.1111/boj.12385
» https://doi.org/10.1111/boj.12385 - Araújo EL, Silva KA, Ferraz EMN, Sampaio EVSB, Silva SI. Diversidade de herbáceas em microhabitats rochoso, plano e ciliar em uma área de caatinga, Caruaru, PE, Brasil. Acta Botanica Brasilica 2005; 19(2): 285-294. 10.1590/S0102-33062005000200011
» https://doi.org/10.1590/S0102-33062005000200011 - Assmann E. The principles of forest yield: studies in the organic production, structure, increment and yield of forest stands. Braunschweig: Pergamon Press; 1970.
- Attanasio CM, Rodrigues RR, Gandolfi S, Nave AG. Adequação ambiental de propriedades rurais: recuperação de áreas degradadas: restauração de matas ciliares. Piracicaba: Universidade de São Paulo; 2006.
- Brasil. Lei nº 12.651, de 25 de maio de 2012. Diário Oficial da União, Brasília, DF (2012 May 28); Sec. 1: 1.
- Castro D, Mello RSP, Poester GC. Práticas para restauração da mata ciliar. Porto Alegre: Catarse; 2012.
- Cientec. Mata nativa 2: sistema para análise fitossociológica e elaboração de planos de manejo de florestas nativas. São Paulo; 2006.
- Empresa Brasileira de Pesquisa Agropecuária - EMBRAPA. Cerrado: restauração de matas de galeria e ciliares. Brasília: Embrapa Cerrados; 2012.
- Fabricante JR, Andrade LA. Análise estrutural de um remanescente de caatinga no Seridó paraibano. Oecologia Brasiliensis 2007; 11(3): 341-349.
- Farias RC, Lacerda AV, Gomes AC, Barbosa FM, Dornelas CSM. Riqueza florística em uma área ciliar de caatinga no Cariri Ocidental da Paraíba, Brasil. Revista Brasileira de Gestão Ambiental e Sustentabilidade 2017; 4(7): 109-118. 10.21438/rbgas.040711
» https://doi.org/10.21438/rbgas.040711 - Felfili, JM, Silva MC Jr, Sevilha AC, Fagg CW, Walter BMT, Nogueira PE, Rezende AV. Diversity, floristic and structural patterns of cerrado vegetation in Central Brazil. Plant Ecology 2004; 175: 37-46. 10.1023/B:VEGE.0000048090.07022.02
» https://doi.org/10.1023/B:VEGE.0000048090.07022.02 - Ferraz JSF, Albuquerque UP, Meunier IMJ. Valor de uso e estrutura da vegetação lenhosa às margens do riacho do Navio, Floresta, PE, Brasil. Acta Botânica Brasílica 2006; 20(1): 125-134. 10.1590/S0102-33062006000100012
» https://doi.org/10.1590/S0102-33062006000100012 - Ferraz RC, Mello AA, Ferreira RA, Prata APN. Levantamento fitossociológico em área de caatinga no Monumento Natural Grota do Angico, Sergipe, Brasil. Revista Caatinga 2013; 26(3): 89-98.
- Guedes RS, Zanella FCV, Costa JEV Jr, Santana GM, Silva JA. Caracterização florístico-fitossociológica do componente lenhoso de um trecho de caatinga no semiárido paraibano. Revista Caatinga 2012; 25(2): 99-108.
- Holanda FSR, Santos LGC, Santos CM, Casado APB, Pedrotti A, Ribeiro GTl. Riparian vegetation affected by bank erosion in the lower São Francisco river, Northeastern Brazil. Revista Árvore 2005; 29(2): 327-336. 10.1590/S0100-67622005000200016
» https://doi.org/10.1590/S0100-67622005000200016 - Lacerda AV. Os cílios das águas: espaços plurais no contexto do semiárido brasileiro. Campina Grande: EDUFCG; 2016.
- Lacerda AV, Barbosa FM, Barbosa MRV. Estudo do componente arbustivo-arbóreo de matas ciliares na bacia do rio Taperoá, semi-árido paraibano: uma perspectiva para sustentabilidade dos recursos naturais. Oecologia Brasiliensis 2007; 11(3): 331-340. 10.4257/oeco.2007.1103.03
» https://doi.org/10.4257/oeco.2007.1103.03 - Lacerda AV, Barbosa FM, Soares JJ, Barbosa MRV. Flora arbustiva-arbórea de três áreas no semiárido paraibano, Brasil. Biota Neotropica 2010; 10(4): 275-284. 10.1590/S1676-06032010000400032
» https://doi.org/10.1590/S1676-06032010000400032 - Lima WP, Zakia MJB. Hidrologia de matas ciliares. In: Rodrigues RR, Leitão-Filho HF, editors. Matas ciliares: conservação e recuperação. São Paulo: Edusp; 2009. p. 33-44.
- Magurran AE. Ecological diversity and its measurement. London: Chapman and Hall; 1988.
- Mueller-Dombois D, Ellenberg H. Aims and methods of vegetation ecology. New York: John Wiley & Sons; 1974.
- Parente HN, Araújo KD, Silva EE, Andrade AP, Dantas RT, Silva DS, Ramalho CI. Parâmetros fitossociológicos do estrato arbóreo-arbustivo em áreas contíguas de caatinga no Cariri Paraibano. Revista Científica de Produção Animal 2010; 12(2): 138-141. 10.15528/2176-4158/rcpa.v12n2p138-141
» https://doi.org/10.15528/2176-4158/rcpa.v12n2p138-141 - Pielou EC. Ecological diversity. New York: Jonh Wiley & Sons; 1975.
- Pinto MSC, Sampaio EVSB, Nascimento LM. Florística e estrutura da vegetação de um brejo de altitude em Pesqueira, PE, Brasil. Revista Nordestina de Biologia 2012; 21(1): 47-79.
- Reflora. Flora do Brasil 2020 em construção [Internet]. 2016 [cited 2016 May 18]. Available from: Available from: https://bit.ly/2ITnPXi
» https://bit.ly/2ITnPXi - Rodrigues LA, Carvalho DA, Oliveira-Filho AT, Botrel RT, Silva EA. Florística e estrutura da comunidade arbórea de um fragmento florestal em Luminárias, MG. Acta Botanica Brasilica 2003; 17(1): 71-87. 10.1590/S0102-33062003000100006
» https://doi.org/10.1590/S0102-33062003000100006 - Sabino FGS, Cunha MCL, Santana GM. Estrutura da vegetação em dois fragmentos de caatinga antropizada na Paraíba. Floresta e Ambiente 2016; 23(4): 487-497. 10.1590/2179-8087.017315
» https://doi.org/10.1590/2179-8087.017315 - Santana JAS, Santana JAS Jr, Barreto WS, Ferreira ATS. Estrutura e distribuição espacial da vegetação da caatinga na Estação Ecológica do Seridó, RN. Pesquisa Florestal Brasileira 2016; 36(88): 355-361. 10.4336/2016.pfb.36.88.1002
» https://doi.org/10.4336/2016.pfb.36.88.1002 - Santana JAS, Souto JS. Diversidade e estrutura fitossociológica da caatinga na Estação Ecológica do Seridó - RN. Revista de Biologia e Ciências da Terra 2006; 6(2): 232-242.
- Santos RM, Vieira FA. Florística e estrutura da comunidade arbórea de fragmentos de matas ciliares dos rios São Francisco, Cochá e Carinhanha, norte de Minas Gerais, Brasil. Revista Científica Eletrônica de Engenharia Florestal 2006; 4(8): 1-21.
- Silva FG, Silva RH, Araújo RM, Lucena MFA, Sousa JM. Levantamento florístico de um trecho de mata ciliar na mesorregião do Sertão Paraibano. Revista Brasileira Biociência 2015; 13(4): 250-258.
- Silva LS, Alves AR, Nunes AKA, Macedo WS, Martins AR. Florística e fitossociologia em um remanescente de mata ciliar na bacia do Rio Gurguéia - PI. Nativa 2015; 3(03): 156-164. 10.14583/2318-7670.v03n03a02
» https://doi.org/10.14583/2318-7670.v03n03a02 - Silva MC Jr. Fitossociologia e estrutura diamétrica da mata de galeria do Taquara, na reserva ecológica do IBGE, DF. Revista Árvore 2004; 28(3): 419-428. 10.1590/S0100-67622004000300013
» https://doi.org/10.1590/S0100-67622004000300013 - Silva N, Lucena RFP, Lima JRF, Lima GDS, Carvalho TKN, Sousa SP Jr, Alves CAB. Conhecimento e uso da vegetação nativa da caatinga em uma comunidade rural da Paraíba, Nordeste do Brasil. Boletim do Museu de Biologia Mello Leitão 2014; (34): 5-37.
- Souza BI, Silans AMBP, Santos JB. Contribuição ao estudo da desertificação na bacia do Taperoá. Revista Brasileira de Engenharia Agrícola Ambiental 2004; 8(2-3): 292-298. 10.1590/S1415-43662004000200019
» https://doi.org/10.1590/S1415-43662004000200019 - Souza JAS, Rodal MJN. Levantamento florístico em trecho de vegetação ripária de caatinga no rio Pajeú, Floresta/Pernambuco-Brasil. Revista Caatinga 2010; 23(4): 54-62.
- Trovão DMBM, Freire AM, Melo IJMM. Florística e fitossociologia do componente lenhoso da mata Ciliar do Riacho de Bodocongó, semiárido paraibano. Revista Caatinga 2010; 23(2): 78-86.
Publication Dates
-
Publication in this collection
08 May 2020 -
Date of issue
2020
History
-
Received
26 May 2018 -
Accepted
24 Nov 2018


 Source: Adapted from
Source: Adapted from 


