Abstract
The industrial development and the advance of the primary sector in Brazil generated an increase in the cases of structures damaged by sulfate attacks. A reduction in material lifetime is one of the most costly factors in the construction field. Therefore, it is necessary to understand the sulfate attack mechanism in order to provide repairs and prevent further attacks. This article aims to understand how the environmental condition and the material properties influence the attack’s severity. Hence, it combined an experimental program and analytical model to measure those parameter effects. Experiments show that cement with a higher amount of tricalcium aluminate (C3A), as the CP V ARI, presented a more pronounced deterioration. Visual changes such as cracking, crystallization of expansive products and a complete disintegration were also observed. In addition, loss of resistance occurred in the specimens with low slag content. Moreover, the model is useful to predict the delamination depth and to identify the most critical factors influencing the attack through sensitive analysis. Its results were compared with real cases based on literature and verifying the model reliability.
Keywords:
sulfate; sulfate-resistant cement; durability; expansion; ettringite
1. Introduction
There are several sources of sulfate, such as sewage, industrial waste, and fertilizers. The industrial development generated an increase in the cases of structures damaged by sulfate attacks. One of the most costly factors in the construction field is the reduction of material lifetime. Because this type of deterioration process is complex, it has raised many controversial theories in order to understand its mechanism (Santhanam et al, 2001SANTHANAM, M., COHEN, M. D., OLEK, J. Sulfate attack research - whither now? Cement and Concrete Research, n.31, p. 845-851, 2001.; Neville, 2004NEVILLE, A. M. The confused world of sulfate attack on concrete. Cement and Concrete Research, v. 34, p. 1275-1296, 2004.).
Most of the time, the sulfate salts in nature, such as Na2SO4 and MgSO4, cause external sulfate attacks, leading to the formation of expansive products, i.e. gypsum and ettringite (Skalny et al, 2002SKALNY, J., MARCHAND, J., ODLER, I. Sulfate attack on concrete. London, New York: Spon Press, 2002.). Whittaker and Black (2015)WHITTAKER, M., BLACK, L. Current knowledge of external sulfate attack. Advances in Cement Research, 2015. ISSN 0951-7197. defend that the presence of gypsum and ettringite is not enough to cause degradation. Although, after a certain period, a threshold concentration is reached and it initiates a pressure on the pore walls, which leads to expansion, cracking, spalling, loss of mechanical properties and the complete deterioration of the composite due to the concrete's low tensile strength (Monteiro and Kurtis, 2003MONTEIRO, P. J. M., KURTIS, K. E. Time to failure for concrete exposed to severe sulfate attack. Cement and Concrete Research, 33, p. 987-993, 2003.; Neville, 2016NEVILLE, A. M. Propriedades do concreto. ( 5. ed.). São Paulo: Bookman, 2016.; Idiart et al, 2011IDIART, A. E., LÓPEZ, C. M., CAROL, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: a meso-scale model. Cement and Concrete Composites, v. 33, p. 411-423, 2011.; Whittaker and Black, 2015WHITTAKER, M., BLACK, L. Current knowledge of external sulfate attack. Advances in Cement Research, 2015. ISSN 0951-7197.).
The attack mechanism is complex, since there are numerous factors that influence the severity of the process. These factors can be related to the material’s nature (w/c ratio, cure process, clinker constitution, additions and so on) and to the surrounding environmental circumstances (temperature, pH, external sulfate concentration, cyclic exposure and so on).
Concrete with a higher w/c ratio leads to high permeability that facilitates the ingress of hazardous chemical ions into the inner material. Moreover, it is known that Portlandite reacts with sulfate to form gypsum, causing a decalcification of the cement matrix and, consequently, loss of strength (Santhanam et al, 2002SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 1. Summary of experimental results. Cement and Concrete Research, n.32, p. 915-921, 2002.). Hence, the clicker composition plays an important role, since tricalcium silicate (C3S) hydration produces 2.2 times more CH (Calcium Hydroxide) than the dicalcium silicate (C2S) hydration, so the silica ratio is also significant for material durability (Irassar, 2000IRASSAR, E. F., GONZÁLEZ, M., RAHHAL, V. Sulfate resistance of type V cements with limestone filler and natural pozzolana. Cement and Concrete Composites, n.22, p.361-368, 2000.; Neville, 2016NEVILLE, A. M. Propriedades do concreto. ( 5. ed.). São Paulo: Bookman, 2016.). Furthermore, the tricalcium aluminate (C3A) reacts with sulfate to form ettringite (Basista and Weglewski, 2008BASISTA, M., WEGLEWSKI, W. Micromechanical modelling of sulfate corrosion in concrete: influence of ettringite forming reaction. Theoretical and Applied Mechanics, Belgrade, v. 35, p. 29-52, 2008.); thus, lowering its content is crucial to increase the concrete sulfate resistance.
Meanwhile, the environment is decisive when it comes to sulfate attack. The type of cation associated with SO42- modifies the mechanisms and the consequences of the attack (Neville, 2004NEVILLE, A. M. The confused world of sulfate attack on concrete. Cement and Concrete Research, v. 34, p. 1275-1296, 2004.). Additionally, a higher sulfate external concentration leads to a faster diffusion generating a more severe attack (Whittaker and Black, 2015WHITTAKER, M., BLACK, L. Current knowledge of external sulfate attack. Advances in Cement Research, 2015. ISSN 0951-7197.). Temperature and the pH of the solution are also determinant in the products formation (Navarro et al, 2000NAVARRO, C. R., DOEHNE, E., SEBASTIAN, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cement and Concrete Research, n.30, p.1527-1534, 2000.; Zhou et al, 2006ZHOU, Q., HILL, J., BYARS, E. A., CRIPPS, J. C., LYNSDALE, C. J., SHARP, J. H. The role of pH in thaumasite sulfate attack. Cement and Concrete Research, n.36, p.160-170, 2006.; Liu et al, 2013LIU, Z. et al. The effect of MgSO4 on thaumasite formation. Cement and Concrete Composites, n.35, p.102-108, 2013.; Hou et al 2015HOU, H. et al. Thaumasite sulfate attack: case studies and implications. In: INTERNATIONAL CONFERENCE OF CEMENT MICROSCOPY, 37. 2015.; Neville, 2016NEVILLE, A. M. Propriedades do concreto. ( 5. ed.). São Paulo: Bookman, 2016.).
This article aims to understand the influence of these factors. In order to have a wider view of the problem, it is divided into two complementary approaches: experimental and analytical models. The first one is dedicated to comprehending the attack in a short-term through experimental procedures. Lastly, it turns to analytical modeling to analyze the influences of those factors in a long-term period of attack. Then, the experimental and analytical approach are complementary visions.
2. Materials and methods
2.1 Experimental Approach
To understand how the cement type may affect the sulfate attack process, experiments of measuring dimensions and mechanical property variations took place. In Brazil there are four types of Portland cement: composite (CP II); blast furnace (CP III); pozolan (CP IV); high early strength (CP V). Thus, 4 x 4 x 16 cm prismatic mortar specimens were cast using the four different Brazilian Portland cement types, two of them are considered Sulfate Resistant (SR). Table 1 displays the regulatory standards of each Portland Cement and some specified data, as the content of mineral addition that could be ground granulated blast furnace slag (GGBS) and pozzolana. The research used each type to cast 10 specimens, configuring one series.
The NBR 5737:1992______________________. NBR 5737: Cimento Portland Resistente a Sulfatos. Rio de Janeiro, 1992 regulates the sulfate resistant cements commercialized in Brazil and stipulates that for a cement to be considered RS, it must have a content in mass of less than 8% of C3A in the clinker and less than 5% of carbonaceous material in the cement. For the specific cement types CP III and CP IV, the content of mineral addition should be 60-70% and 25-40%, respectively.
Only one brand of each cement type was tested, however there are national standards for all the types. Then, repeatability for those cements which are aligned to the standards is expected. Table 2 presents a characterization of cement types provided by the manufactures. Two different cement companies were used and are identified in the table by letters A and B.
The mortar mix proportion was 1:3.2 by mass and the w/c ratio was equal to 0.6, as defined in the Brazilian standard NBR 13583:2014______________________. NBR 13583: Determinação da variação dimensional de barras de argamassa de cimento Portland expostas à solução de sulfato de sódio. 2nd edition, Rio de Janeiro, 2014.. Limestone sand without organic compounds and with controlled gradation, passed through the No. 16 sieve and was retained on the No. 50 sieve, the grading curve is presented in Figure 1.
Specimens were cured during 14 days in a moist chamber, of which the last 12 days were in a saturated lime solution. Afterward, the researchers immersed eight specimens of each series in a sodium sulfate solution (0.704 mol/L SO42-) and the others remained in a saturated lime solution, all of them with controlled temperature of (40 ± 2) ºC and pH between 6.0 - 8.0. The use of a caliper and a precision scale measured, respectively, the length and mass of all the specimens with a precision of 0.5 mm and 0.1g at the scheduled ages of 0, 14, 28, 42, 56, 91 and 105 days after immersion.
The expansion experiment was performed in accordance with NBR 13583:2014. For the mass loss analysis, an analogous expansion procedure was followed. The sample was withdrawn from the oven and stabilized in environment with 23 +- 2ºC for 10 min. After that, each sample was weighted not exceeding a time of 5 min. Additional tests were performed to complement the analysis, such as compressive and flexural strength in different ages. Moreover, an ultrasonic pulse velocity test took place according to the Brazilian standard NBR 8802:2013______________________. NBR 8802: Concreto endurecido - Determinação da velocidade de propagação de onda ultrassônica. Rio de Janeiro, 2013.. The Table 3 summarizes the procedures realized and presents the standards used.
2.2 Analytical approach
Due to the expansive products, the sulfate attack consists of cyclic process where more cracks result in a further attack. Then, to predict this interactive process and simulate the deterioration mechanism, the researchers decided to use an analytical approach.
2.2.1 Initial considerations and simplifications
The model admits that the attack happens in one direction, which means that only one face of the concrete is exposed to the sulfate solution. This situation happens in sewer pipes and buried ducts. Santhanam and others admit similar models (Santhanam et al, 2003SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 2. Proposed mechanisms. Cement and Concrete Research, v. 33, p. 341-346, 2003. ).
The adoption of sodium sulfate occurred due to its large presence in nature. In addition, the choice is consistent with the experimental performance that used the same solution. The model admits that the sodium sulfate solution is infinite, coherent with the tiny model dimensions compared with the solution proportion. The concrete modeled has a fixed length, unitary width and is divided in packs with the same dimension.
The aggressive ions penetrate the concrete by diffusion, through its pores, and further react with hydration products forming ettringite and gypsum. We used the secondary Fick’s law adopting the Collepardi solution (Frederiksen et al, 2008FREDERIKSEN, J. M., MEJLBRO, L., NILSSON, L. Fick’s 2nd law - complete solutions for chloride ingress into concrete - with focus on time dependent diffusivity and boundary condition. Lund: Institute of Technology, 2008.), Equation 1, due to its compatibility with the model and simplicity. The diffusivity coefficient (D) was considered constant with the value of 6.69x10-9 m2/s (Sun et al, 2013SUN, C., CHEN, J., ZHU, J., ZHANG, M., YE, J. A new diffusion model of sulfate ions in concrete. Construction and Building Materials, v. 39, p. 39-45, 2013.). It is known that the coefficient changes with the advance of the cracking process; this parameter was simplified, assuming that no big losses in the results would occur. The concentration in a specific point and time (C(x,t)) is given in function of initial concentration of solute in concrete (Ci) and external solute concentration (Cs).
The sodium sulfate that penetrates the concrete is divided into mobile, bonded (ettringite) and about-to-bond (mobile). These divisions are necessary because there is a correct proportion between reagents to form products and the appropriated activation energy. The mobile sulfate is not yet bonded, hence it continues to penetrate inner concrete pores. The bonded sulfate has already reacted with concrete products forming ettringite and gypsum. The about-to-bond sulfate is the ion that is almost with the conditions to react. To model the attack mechanisms, just the mobile and bonded sulfate are necessary. Admitting that when the ions penetrate the concrete, the majority will react, and this proportion is given by the reaction ratio.
By combining the Chemical Kinect Laws of Instantaneous Velocity and Reaction Rate on the chemical reaction between sodium sulfate and monosulfate, Equation 2, it is possible to find the sulfate ion concentration available at a certain time, given by Equation 3. Then, the difference between the initial concentration [SO4]0 and the concentration available at a time [SO4](t) gives the amount of sulfate ions that has a destination, as bonded or mobile.
The C3A and Ca concentrations are adopted as constant. The rate constant (k) admits a value between 10-10 m3/mol.s a 10-6 m3/mol.s. given by Tixier (2000 apud Basista and Weglewski, 2008BASISTA, M., WEGLEWSKI, W. Micromechanical modelling of sulfate corrosion in concrete: influence of ettringite forming reaction. Theoretical and Applied Mechanics, Belgrade, v. 35, p. 29-52, 2008.) This way, the study leads to the fact that the relationship between the reacted sulfate (ettringite) by the total of sulfate (bonded + mobile) produces the reaction rate, this value stands around 97% and 99%. In an analogue way, by multiplying the reaction rate by the sulfate that had a destination ([SO4]0 - [SO4](t)), it is possible to achieve the ettringite concentration (bonded sulfate), Equation 4.
2.2.2 Mechanism of attack
The basic mechanism considered for sulfate attack is a cyclic process occurring in each element pack. First, the sulfate source is in contact with the cement matrix. In a further moment, the SO4 -2 penetrates the first element (pack) of the matrix by diffusion. Last, there is sulfate bonded (ettringite) around the matrix compounds and the mobile sulfate is penetrating to inner packs. Simultaneously, more sulfate ions are entering the first pack of the cement matrix, restarting the cycle. Summing up, each second, there is ettringite formation (Equation 3) while more sulfate penetrates the concrete (Equation 1).
The model algorithm is developed in three steps, as shown in Figure 2. The first step calculates the reaction rate using Equation 3 for a fixed time period (1 day) considering that all the sulfate is consumed and reposition does not occur. The second step simulates the mechanism of attack for the first division of time, which is the total period of attack divided by a selected number (nt), where more divisions of time result in a more accurate model. Therefore, in this step for each second, the bonded and mobile sulfate (by Equation 3 and 4) are calculated. During this process, sulfate is replaced (by diffusion, Equation 1). The third step verifies if the ettringite concentration accumulated inside concrete during the attack is larger than the threshold concentration. If so, the element pack is delaminated. In this step, the mechanism of attack for the other divisions of time is calculated, similar to the previous step, although simultaneously the occurrence of delamination is verified. The information about which pack delaminated and the time of occurrence is saved.
The three-step sulfate attack process considered in the Analytical Model. a) first b) second and c) third step.
The threshold concentration was arbitrated equal to the lower sulfate concentration found in natural environments. It is known that the delamination frequency depends on cement composition, tortuosity, environmental concentration and exposition period. Table 4 shows the model input and output data.
Moreover, the model is a simplification of diverse complex mechanisms of attack and the differences of expansion between the ettringite and gypsum, and the variation of pH or the formation of TSA (Thaumasite form of Sulfate Attack) were not considered.
2.2.3 Method for testing the model feasibility
Quantitative and qualitative analysis is necessary to verify the applicability of the model. For the quantitative analysis, researches were founded in literature that present study cases of sulfate attack on real structures and in the laboratory. Just one that attended the boundary conditions of the model was selected. Then, study cases where pH or temperature are relevant to the delamination depth were eliminated because in this situation, the formation of brucite or thaumasite probably happens, which are products with a different formation process and this mechanism is not considered in the model.
The qualitative analysis consists of evaluating the model constraint responses as expected, and they are: environmental concentration, slag content and water/cement ratio. The tortuosity is intrinsic of the other parameters, it varies in accordance with the factor that influences porosity. For example, a concrete with a higher water/cement ratio probably is more porous and it is easier for the sulfate ions to penetrate. Thus, a higher w/c ratio implies in a lower tortuosity.
3. Results
3.1 Experimental approach
The deterioration by a sulfate attack was analyzed considering the effects: visual, physical (expansion and mass loss), and mechanical.
3.1.1 Visual effects
The visual effects caused by a sulfate attack are usually perceived first. Due to the C-S-H decalcification, cracks and spalling may be observed (Neville, 2004NEVILLE, A. M. The confused world of sulfate attack on concrete. Cement and Concrete Research, v. 34, p. 1275-1296, 2004.; Santhanam et al, 2002SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 1. Summary of experimental results. Cement and Concrete Research, n.32, p. 915-921, 2002. and 2003SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 2. Proposed mechanisms. Cement and Concrete Research, v. 33, p. 341-346, 2003. ;Whittaker and Black, 2015WHITTAKER, M., BLACK, L. Current knowledge of external sulfate attack. Advances in Cement Research, 2015. ISSN 0951-7197.). However, it was only possible to note cracks and spalling in S3 after 91 days immersed, shown in Figure 3a. S3 and sulfate-resistant specimens (S4) are compared in Figure 3b. Samples from Series S3 led to complete disintegration before 105 days of immersion as shown in Figure 4.
Examples of visual effects on samples. a) S3 sample with 91 days exposure. b) Comparison between S3 and S4 samples on the immersion age of 105 days.
All the series portrayed efflorescence signs, as shown in Figure 5, probably caused by the deposition of mirabilite (Navarro et al, 2000NAVARRO, C. R., DOEHNE, E., SEBASTIAN, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cement and Concrete Research, n.30, p.1527-1534, 2000.; Neville, 2016NEVILLE, A. M. Propriedades do concreto. ( 5. ed.). São Paulo: Bookman, 2016.). To ensure the information, a mineralogical analysis is needed.
The samples only were analyzed at the scheduled ages, so the efflorescence could be observed only at this moment. Table 5 displays the age that efflorescence was first noted.
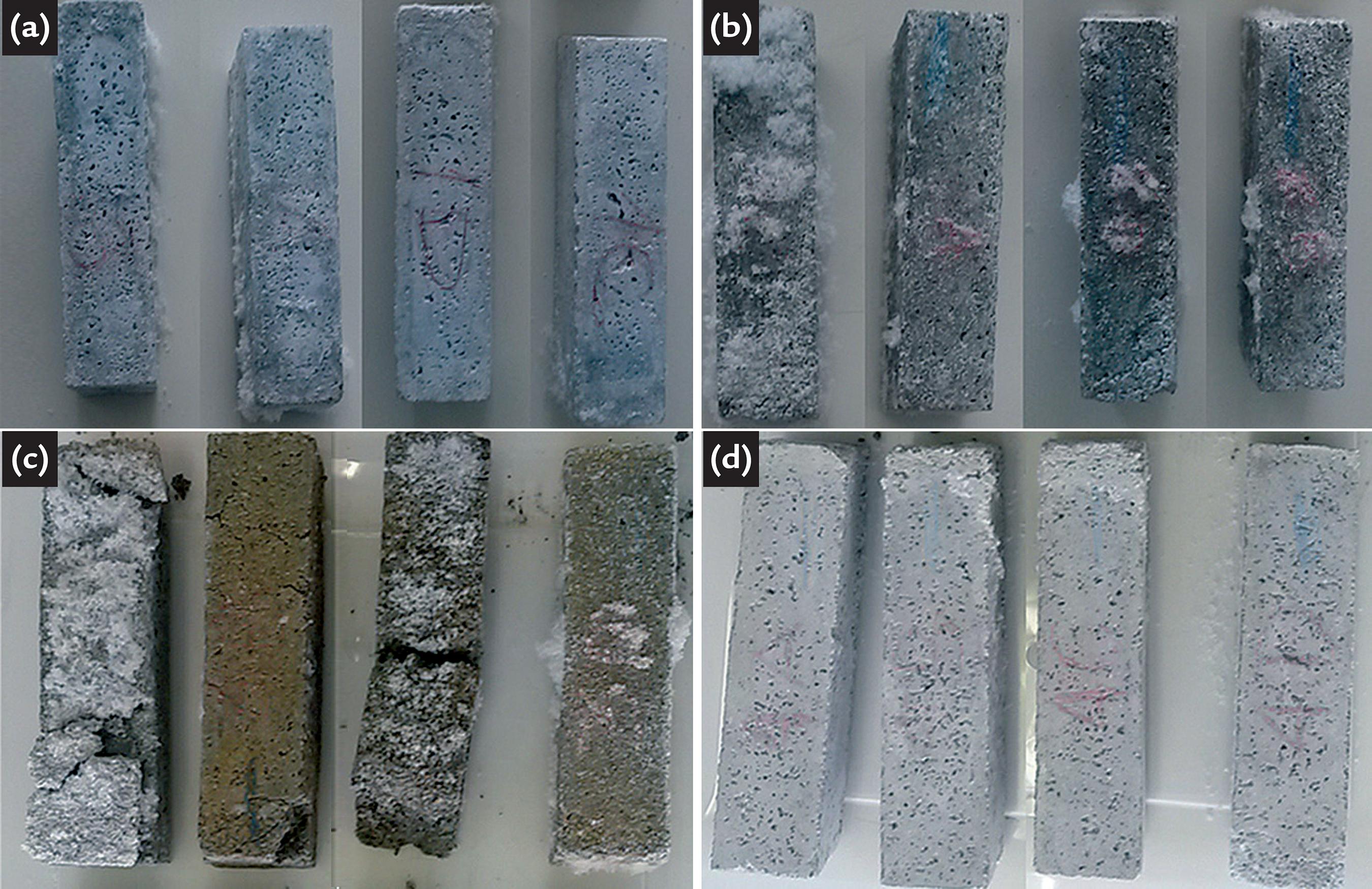
Figure 6 shows how the samples of the four series on 105 days. Note that Figure 6a and Figure 6d present samples less deteriorated with no cracks and few efflorescence signs, which are relative to the SR cement. Although, the mortar made with the non-resistant cements, in Figure 6b and Figurec6 had a large presence of efflorescence and the Series 3 has some samples already disintegrated.
Samples immersed for 105 days in sodium sulfate solution. a) Series S1 b) Series S2 c) Series S3 d) Series S4.
3.1.2 Expansion
The expansion obtained from the specimens immersed in sodium sulfate discounting the expansion measured in the specimens immersed in lime gives the resultant expansion (ER). Figure 7 shows the comparative expansion history of all the series. It indicates a better performance of sulfate resistant cement (S1 and S4). Even though S2 is non-sulfate resistant, it had a reasonable performance due to its GGBS (Ground-Granulated Blast-furnace Slag) content. The early-resistance cement series (S3) suffered a complete disintegration before 105 days of immersion. Since it had a high content of C3A and C3S, it was not possible to measure its expansion at this age.
Santhanam et al (2002)SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 1. Summary of experimental results. Cement and Concrete Research, n.32, p. 915-921, 2002. proposed that the mechanism of expansion of sulfate attack in mortars consists of two different stages. The first stage presents little or no expansion, and the second one an abrupt increase in the expansion ratio, observed in Figure 8. The experiments indicate that this is only true for sulfate non-resistant cement tested.
Two stages clearly defined in sulfate non-resistant cement behavior in S2 and S3, whereas in S1 and S4 the SR cements' resultant expansion profile does not behave in two clear stages as proposed by Santhanam.
As shown in Figure 8, S1 and S4 stages do not perform respecting this behavior. As observed in Figure 8, standard deviations for S2 and S3 Series were high after the sulfate attack. In order to assess these differences, the variation coefficient (VC) has been used to measure the variability before and after the sulfate attack. VC represents the ratio of the standard deviation to the mean, and it is a useful statistic for comparing the degree among datasets, even if the means are drastically different from one another. In S3 Series, for instance, the VC for 14, 28, 42 and 56 days were 0.637, 0.923, 0.484 and 0.472, respectively. After the sulfate attack, the VC for 91 days was 0.328 and for 105 days was 0.335. Although presenting higher mean expansion for 91 and 105 days, the variations after the attack were smaller than those previously observed the 14, 28, 42 and 56 days.
3.1.3 Mass loss
The calculation of resultant mass loss (dMR) is similar to the resultant expansion, by considering the effects from the specimens immersed in a saturated lime solution. Mass variation correlates with the expansive product recrystallization, which has a greater molarity (Whittaker and Black, 2015WHITTAKER, M., BLACK, L. Current knowledge of external sulfate attack. Advances in Cement Research, 2015. ISSN 0951-7197.). Figure 9 shows the comparative mass variation history of all the series. It indicates a connection with expansion, since the series with the higher expansion had the biggest mass increases.
3.1.4 Ultrasonic pulse velocity
Cracks may take place when sulfate attacks the concrete, so it is expected to have an increase in the material porosity. The more deteriorated the mortar specimens are, the lower is their ultrasonic pulse velocity. Hence, it is a relevant index to consider when analyzing the sulfate attack deterioration process. Comparative data between the mortars immersed in saturated lime and in the aggressive solution at 105 days are shown in Table 6. The specimen S3 presented total disintegration in 105 days, being destroyed during handling. Then, the result is not indicated.
Comparison between mortars immersed in sulfate and saturated lime solution at 105 day - Ultrasonic Pulse Velocity.
3.1.5 Flexural and compressive strength
Loss of strength is a direct consequence of the sulfate attack, since it causes loss of cohesion due to the C-S-H decalcification. It is reasonable to expect best performances for these series that demonstrated minor losses in the ultrasonic pulse velocity. Figure 10 shows the flexural and compressive strength development of all the series at 28, 56, and 105 days. Some of standard deviations were high, this is due to the sulfate attack that does not necessarily occur and is a linear way inside the samples, during the deterioration process.
In order to have a clear understanding of the solution influence, the data from the samples immersed in sulfate and in lime are compared in Table 7. It demonstrates that the sulfate resistant cement series (S1 and S4) did not suffer serious consequences in terms of strength. On the other hand, S1 and S4 performed better in the sulfate solution than in saturated lime. S3 had a complete disintegration which configures a 100% in strength loss and S2 showed big losses in both flexural and compressive strength. The comparison of S2 and S1 results may indicate that the addition of a small amount of GGBS is not as effective as the same amount of pozzolana. A high amount of GGBS revealed capable of significantly increasing the sulfate resistance as observed in S4 results.
Comparison between mortars immersed in sulfate and saturated lime solution at 105 day - Flexural and Compressive Strength.
3.2 Analytical approach
To prove the accuracy of the analytical approach, quantitative and sensitivity analyses were made.
3.2.1 Quantitative analysis
To verify the precision of the model, a comparison with cases reported in literature was needed, and the results are presented in Table 8, which specifies the input information used on the model. All the cases utilize Portland cement and adopted a threshold concentration of 0.001 mol/L, coherent with the smallest concentrations found in nature. The tortuosity values were arbitrated based on Ahmad et al. (2009)AHMAD, S., AZAD, A. K., LOUGHLIN, K. F. A study of permeability and tortuosity of concrete. In: CONFERENCE ON OUR WORLD IN CONCRETE & STRUCTURES, 30. Singapore, 2005..
In general, only tortuosity and nt are assumed by the authors. All the other input data were obtained in the literature researches, they are: time, external concentration, slag, cement content, C3A and w/c. The test column indicate which method the literature research used to determine the delamination depth, where SEM is Scanning Electron Microscope.
Bellmann et al (2012)BELLMANN, F., ERFURT, W., LUDWIG, H. M. Field performance of concrete exposed to sulfate and low pH conditions from natural and industrial sources. Cement & Concrete Composites, v. 34, p. 86-93, 2012. is related to a real case structure. Hence, not all the boundary conditions required for the model are presented in the article. The missing conditions were estimated based on the author’s description and in the general knowledge about concrete composition. Laboratory studies have their input data defined and just in Brown and Hooton (2002)BROWN, P., HOOTON, R. D. Ettringite and thaumasite formation in laboratory concretes prepared using sulfate-resisting cements. Cement & Concrete Composites, v. 24, p. 361-370, 2002. was it necessary to arbitrate the cement content.
Table 8 shows that the results obtained in the model were satisfactory; they presented a small error percentage and indicate the model’s applicability. Those divergences between the results are expected due to the complexity in simulating the attack. The results also indicate the simplifications made on the model did not imply big losses in accuracy, the average error being 4.005%.
3.2.2 Sensitivity analysis
Furthermore, the analytical approach analyzes the relationship between the parameters and their influence on the determination of the delamination depth, such as external concentration, cement composition, tortuosity, w/c and slag percentage. Then, a sensitivity analysis is done.
The model fixes the C3A content at 7.9% because the relevance of cement composition had already been analyzed in the experimental approach. The threshold concentration was adopted as 0.001 mol/L, since it presented good results according to Table 8. The study does not isolate tortuosity, since its effects are linked with others factors, such as w/c. Table 9 presents the sensitivity analysis, adopting a period of 600 days. A long period to verify the influence and dependence of the factors was not mandatory. As on the quantitative analysis, the tortuosity was that assumed by Ahmad et al (2005)AHMAD, S., AZAD, A. K., LOUGHLIN, K. F. A study of permeability and tortuosity of concrete. In: CONFERENCE ON OUR WORLD IN CONCRETE & STRUCTURES, 30. Singapore, 2005..
All the parameters act in a consistent way showing that the model is in accordance with the mechanisms of attack existent in literature. Some factors, such as w/c, have more influence on the delamination depth, requiring more attention in order to minimize the impacts.
4. Discussion
This study intends to understand the impact of some factors on the mechanisms of sulfate attack on mortars and concrete. In the experimental approach, the results were coherent and associated. It is possible to set a sequence of which cement has the best performance under sulfate attack and as expected, the sulfate resistant cements performed better: CP III-RS > CP IV-RS > CP II-E > CP V-ARI. The specimens that have more expansion (and consequently increases in mass) also have higher strength losses (flexural and compressive).
The cement with high initial strength (CP V-ARI) had the worst performance, since it has a high fineness, losing completely its strength and being possible to break the sample by hand due to its friability. In addition, high C3A and C3S contents are harmful because the first one reacts with gypsum to form ettringite and the second one stimulates the portlandite production. CP III-RS performed best due to its high GGBS addition and low permeability.
The content of C3A are not known, and the cement composition presented in Table 2, indicates how the quantitative phase composition of the cement could be. A high content of CaO indicates that there could be a higher formation of C3A, because more calcium available. Corroborating with this analysis, the cements SR, S1 and S4, ha lower CaO content, respectively, 14.34% and 14.62% lower than the worst result, S3. A scale of CaO content shows: CP III RS<CP IV RS<CP II E< CP V ARI. This range is the inverse of the cements that performed better. The CaO content in CP III and CP IV is similar, even though they present different efficiencies resistance to sulfate.
The Bogue calculation is a recognized method to predict the quantitative phase composition of Portland Cement. However Taylor (1997)TAYLOR, H. F. W. Cement chemistry. (2. ed.). London : Thomas Telford Publishing, 1997. affirms that the equations are invalid for cement or one with mineral addition. As a result, this estimation is just possible for CP V ARI, the only one that meets the requirement. The quantitative phases for the brand of CP V ARI, series S3, studied the phases composition is: C3S 65.00%; C2S 9.72%; C3A 8.44% e C4AF 8.66%. It could be observed that this value is higher than the 8% limited for SR cements by the standard NBR 5735:1992______________________. NBR 5737: Cimento Portland Resistente a Sulfatos. Rio de Janeiro, 1992.
The cements CP III 40 RS and CP IV 32 RS present a non-standard mineral addition content. The CP IV presents a higher amount of pozzolana and the CP III, a lower content of slag. However, this does not affect the sulfate attack resistance of this cement.
The analytical model verified the performance of concrete with different slag content. This factor affects the cement by reducing the sulfate attack on concrete, although an increase on slag content from 20% to 60% (200% increase) showed a decrease of delamination depth of 37.04%. The w/c factor reveals a high impact on sulfate attack. The variation of w/c from 0.6 to 0.4 (reduction of 33.33%) shows a reduction on delamination of 92.88%. This result represents the importance of a high quality control in the construction field to prevent these variations. Despite simplifications in the model, it presented good results when compared with literature, with an error of under 7%.
Experimental tests show mass increase while the analytical approach evaluates the mass loss (represented by delamination depth). In short-time periods, it is common to have a mass gain, due to the formation of ettringite because it just fills the voids with not enough strength to crack the concrete/mortar. Another study also shows these mass increases with a less than 2-month period (Nehdi et al, 2014NEHDI, M. L., SULEIMAN, A. R., SOLIMAN, A. M. Investigation of concrete exposed to dual sulfate attack. Cement and Concrete Research, v. 64, p. 42-53, 2014.).
5. Conclusion
This article analyzed the visual, dimensional and mechanical property differences between four Portland cement types commercialized in Brazil, immersed in sodium sulfate attack. Also, it presented an analytical model performing quantitative and qualitative analysis.
-
The efficiency of the tested two brands of sulfate resistant cement types has been proven. Since all the Portland cement types follow national standards, the behavior will probably be the same.
-
Tests indicate that the slag is more effective for the sulfate attack when added in large quantities, since CP III-RS performed better than CP II E. The higher the amount of substitution by slag in the cement, the lower the clinker content. Consequently, there are less hydration products to react with the deleterious agent, such as sodium sulfate. The analytical model is consistent with the experiments demonstrating that small additions are not as expressive, as observed in CP II-E.
-
The experimental analysis showed that the cements with a lower content of CaO had a better performance in resisting a sulfate attack.
-
The w/c factor is very sensitive to the attack, since it is proportional to permeability. In addition, it reduces the tortuosity, facilitating the diffusion of aggressive ions. The model indicates that an increase of 25.00% in w/c ration led to an increment of 244.90% in delamination depth. It shows the importance of having a field quality control to prevent an increase of the susceptibility to the deleterious agent.
-
The specimens made with RS cement immersed in sulfate demonstrated a higher compressive strength when compared to specimens immersed in gypsum solution at 105 days - an increase in 18.2% and 17.3% for S1 and S4 respectively. A probable reason is that ettringite has been formed but its concentration was lower than the threshold one, so it filled the voids and increased the compressive strength, to affirm that further analysis is needed.
Acknowledgements
We gratefully acknowledge the agencies CAPES, FAPEMIG and CNPq for providing financial support. We are also grateful for the infrastructure and collaboration of the Laboratório de Construção Civil - UFJF.
References
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 5733: Cimento Portland de alta resistência inicial. Rio de Janeiro, 1991
- ______________________. NBR 5737: Cimento Portland Resistente a Sulfatos. Rio de Janeiro, 1992
- ______________________. NBR 8802: Concreto endurecido - Determinação da velocidade de propagação de onda ultrassônica. Rio de Janeiro, 2013.
- ______________________. NBR 11578: Cimento Portland Composto. Rio de Janeiro, 1991
- ______________________. NBR 13279: Argamassa para revestimento de paredes e tetos - Determinação da resistência à tração na flexão e à compressão. 2nd edition, Rio de Janeiro, 2013.
- ______________________. NBR 13583: Determinação da variação dimensional de barras de argamassa de cimento Portland expostas à solução de sulfato de sódio. 2nd edition, Rio de Janeiro, 2014.
- AHMAD, S., AZAD, A. K., LOUGHLIN, K. F. A study of permeability and tortuosity of concrete. In: CONFERENCE ON OUR WORLD IN CONCRETE & STRUCTURES, 30. Singapore, 2005.
- BASISTA, M., WEGLEWSKI, W. Micromechanical modelling of sulfate corrosion in concrete: influence of ettringite forming reaction. Theoretical and Applied Mechanics, Belgrade, v. 35, p. 29-52, 2008.
- BELLMANN, F., ERFURT, W., LUDWIG, H. M. Field performance of concrete exposed to sulfate and low pH conditions from natural and industrial sources. Cement & Concrete Composites, v. 34, p. 86-93, 2012.
- BROWN, P., HOOTON, R. D. Ettringite and thaumasite formation in laboratory concretes prepared using sulfate-resisting cements. Cement & Concrete Composites, v. 24, p. 361-370, 2002.
- FREDERIKSEN, J. M., MEJLBRO, L., NILSSON, L. Fick’s 2nd law - complete solutions for chloride ingress into concrete - with focus on time dependent diffusivity and boundary condition Lund: Institute of Technology, 2008.
- HOU, H. et al. Thaumasite sulfate attack: case studies and implications. In: INTERNATIONAL CONFERENCE OF CEMENT MICROSCOPY, 37. 2015.
- IDIART, A. E., LÓPEZ, C. M., CAROL, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: a meso-scale model. Cement and Concrete Composites, v. 33, p. 411-423, 2011.
- IRASSAR, E. F., GONZÁLEZ, M., RAHHAL, V. Sulfate resistance of type V cements with limestone filler and natural pozzolana. Cement and Concrete Composites, n.22, p.361-368, 2000.
- LIU, Z. et al. The effect of MgSO4 on thaumasite formation. Cement and Concrete Composites, n.35, p.102-108, 2013.
- MAES, M., BELIE, N. Resistance of concrete and mortar against combined attack of chloride and sodium sulfate. Cement & Concrete Composites, v. 53, p. 59-72, 2014.
- MONTEIRO, P. J. M., KURTIS, K. E. Time to failure for concrete exposed to severe sulfate attack. Cement and Concrete Research, 33, p. 987-993, 2003.
- NAVARRO, C. R., DOEHNE, E., SEBASTIAN, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cement and Concrete Research, n.30, p.1527-1534, 2000.
- NEHDI, M. L., SULEIMAN, A. R., SOLIMAN, A. M. Investigation of concrete exposed to dual sulfate attack. Cement and Concrete Research, v. 64, p. 42-53, 2014.
- NEVILLE, A. M. The confused world of sulfate attack on concrete. Cement and Concrete Research, v. 34, p. 1275-1296, 2004.
- NEVILLE, A. M. Propriedades do concreto ( 5. ed.). São Paulo: Bookman, 2016.
- SANTHANAM, M., COHEN, M. D., OLEK, J. Sulfate attack research - whither now? Cement and Concrete Research, n.31, p. 845-851, 2001.
- SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 1. Summary of experimental results. Cement and Concrete Research, n.32, p. 915-921, 2002.
- SANTHANAM, M., COHEN, M. D., OLEK, J. Mechanism of sulfate attack: a fresh look Part 2. Proposed mechanisms. Cement and Concrete Research, v. 33, p. 341-346, 2003.
- SKALNY, J., MARCHAND, J., ODLER, I. Sulfate attack on concrete London, New York: Spon Press, 2002.
- SUN, C., CHEN, J., ZHU, J., ZHANG, M., YE, J. A new diffusion model of sulfate ions in concrete. Construction and Building Materials, v. 39, p. 39-45, 2013.
- TAYLOR, H. F. W. Cement chemistry (2. ed.). London : Thomas Telford Publishing, 1997.
- WHITTAKER, M., BLACK, L. Current knowledge of external sulfate attack. Advances in Cement Research, 2015. ISSN 0951-7197.
- ZHOU, Q., HILL, J., BYARS, E. A., CRIPPS, J. C., LYNSDALE, C. J., SHARP, J. H. The role of pH in thaumasite sulfate attack. Cement and Concrete Research, n.36, p.160-170, 2006.
Publication Dates
-
Publication in this collection
Oct-Dec 2018
History
-
Received
09 Feb 2018 -
Accepted
23 June 2018