Abstract
This article evaluates the efficiency, benefits and limitations of pre-concentration by density separation for process mineralogy studies of low-grade gold ore. The pre-concentration by density aims to generate a product with a high content of gold and to maximize the number of gold-bearing particles characterized, thereby increasing the representativity and reducing the number of polished sections to be analyzed. Pre-concentration by density was carried out for a low-grade sulfide gold ore (0.96 ppm) from the north of Brazil. The sample was ground to below 1.7 mm, sieved down to 0.037 mm and subjected to density separation, amalgamation of the heavy product and cyanide leaching of all products. Gold was assessed in each product to evaluate the separation recovery and distribution. The finer the particle size, the higher the gold distribution in the heavy product, due to a higher degree of liberation of gold and sulfides; these values varied from 65% to 84% in fractions below 0.60 mm and decreased from 40% to 13% in the coarser fractions. Regarding gold distribution, fractions finer than 0.84 mm indicates a notable increase on gold recovery by amalgamation, indication higher surface exposure.
Keywords:
pre-concentration; gold ore; cyanidation and amalgamation
1. Introduction
In sulfidic refractory gold ores, gold particles may be highly disseminated and locked up in sulfides in the form of pyrite and arsenopyrite. All of the largely refractory ores exhibit low rates of gold recovery (typically <20%) by direct cyanidation, gravity separation and diagnostic leaching (Lorenzen, 1995LORENZEN, L. Some guidelines to the design of a diagnostic leaching experiment. Minerals Engineering, v. 8, n. 3, p. 247-256, 1995.; Goodall et al., 2005GOODALL, W. R.; SCALES, P. J.; BUTCHER, A. R. The use of QEMSCAN and diagnostic leaching in the characterisation of visible gold in complex ores. Minerals Engineering, v. 18, n. 8, p. 877-886, 2005.; Soltani et al., 2014SOLTANI, F.; DARABI, H.; BADRI, R.; ZAMANKHAN, P. Improved recovery of a low-grade refractory gold ore using flotation-preoxidation-cyanidation methods. International Journal of Mining Science and Technology, v. 24, n. 4, p. 537-542, 2014.; Dunne, 2016DUNNE, R. Flotation of gold and gold-bearing ores. In: ADAMS, M. D. (ed.). Gold ore processing: project development and operations. Amsterdam: Elsevier, 2016. p. 315-338.; Asamoah et al., 2020ASAMOAH, R. K.; SKINNER, W.; ADDAI-MENSAH, J. Enhancing gold recovery from refractory bio-oxidised gold concentrates through high intensity milling. Mineral Processing and Extractive Metallurgy, v. 129, n.1, p. 64-73, 2020. DOI 10.1080/25726641.2019.1658915.
https://doi.org/10.1080/25726641.2019.16...
) being defined as that for which recovery is less efficient. In reference to the extrapolation of results from bench scale for low-grade gold ores, the pre-concentration may be an alternative, especially when attempting to increase the number of gold grains that can be observed when using scanning electron microscopy coupled with an energy-dispersive X-ray spectrometer (SEM/EDS) in the mineralogical characterization of gold and extraction testing (Laplante et al., 1995LAPLANTE, A. R.; WOODCOCK, F.; NOAPARAST, M. Predicting gravity separation of gold recoveries, Miner. Metall. Process, v.12, p. 74-79, 1995.; Zhou and Cabri, 2004ZHOU, J.; CABRI, L. J. Gold process mineralogy: objectives, techniques, and applications. JOM, v. 56, p. 49-52, 2004.; Lastra et al., 2005LASTRA, R.; PRICE, J.; CABRI, L. J.; RUDASHEVKY, V. N.; McMAHON, G. Gold characterisation of a sample from Malartic East (Quebec) using concentration by hydroseparator. In: INTERNATIONAL SYMPOSIUM ON THE TREATMENT OF GOLD ORES, 5.; ANNUAL CONFERENCE OF METALLURGIST OF CIM, 44., 2005, Calgary, Alberta, Canada. Proceedings […]. Montreal: CIM, 2005. P. 17-29.; Lang et al., 2018LANG, A. M.; AASLY, K.; ELLEFMO, S. L. Mineral characterization as a tool in the implementation of geometallurgy into industrual mineral mining. Mineral Engineering, v. 116, p.114-122, 2018.; Chattopadhyay et al., 2013CHATTOPADHYAY, A.; GORAIN, B. Gold deportment studies on a copper gold ore: a systematic approach to quantitative mineralogy focusing on diagnostic metallurgy. In: ANNUAL CANADIAN MINERAL PROCESSORS OPERATORS CONFERENCE, 45., 2013, Ottawa, Canada. Proceedings […]. Montreal: CIM, 2013. p. 29-42.).
Two typical problems can affect the results of gold ore concentration: variations in the gold grade and in the representativity of the samples (Zhou et al., 2004ZHOU, J.; JAGO, B.; MARTIN, C. Establishing the process mineralogy of gold ores. SGS Minerals - Technical Bulletin, v. 3, p. 1-16, 2004.; Coetzee et al., 2011COETZEE, L. L.; THERON, S. J.; MARTIN, G. J.; VAN DER MERWE, J.; STANEK, T. A. Modern gold deportments and its application to industry. Minerals Engineering, v. 24, n. 6, p. 565-575, 2011.; Ueda et al., 2016UEDA, T.; OKI, T.; KOYANAKA, S. Statistical effect of sampling particle number on mineral liberation assessment. Minerals Engineering, v. 98, p. 204-212, 2016.). Samples that contain high-grade variations may hinder the determination of a suitable number of polished sections to examine. The representativity of the sample may be affected by a biased sample collection in which the properties of the selection must be defined, and the “nugget effect,” caused by sparsely distributed gold grains. The statistical number of grains to be analyzed is another significant negative effect for the mineralogists. The grade of gold in milled products and tailings is usually low, and this leads to a low proportion or a limited number of gold grains in polished sections. Therefore, for low-grade refractory sulfide ore, it is slow and expensive to study sufficient numbers of polished sections to adequately address the possible statistical error.
The effectiveness of mineral separation lies in an understanding of the mineralogy of the ore (Burt, 1984BURT, R. O. Gravity concentration technology. Amsterdam: Elsevier, 1984. 521p. (Developments in Minerals Processing - v.5).; Falconer, 2003FALCONER, A. Gravity separation: old technique/new methods. Physical Separation in Science and Engineering, v. 12, n. 1, p. 31-48. 2003.; Mkandawire et al., 2020MKANDAWIRE, N. P.; McGRATH, T.; BAX, A. EKSTEEN, J. Potential of the dense media cyclone for gold ore preconcentration. Mineral Processing and Extractive Metallurgy, v. 129, n. 1, p. 87-95, 2020. DOI 10.1080/25726641.2019.1669982.
https://doi.org/10.1080/25726641.2019.16...
). Due to its high efficiency, mineral separation by pre-concentration of a product is capable of providing a substantial number of grains or particles to be examined by SEM-based image analysis, which can provide a more rapid and systematic quantitative analysis of mineral grades and textures (Gottlieb, 2000GOTTLIEB, P.; WILKIE, G.; SHUTHERLAND, D.; HO-TUN, E.; SUTHERS, S.; PERERA, K.; JENKINS, B. Using quantitative electron microscopy for process mineralogy applications. JOM, v. 52, n. 4, p. 24-25, 2000.; Gu, 2003GU, Y. Automated scanning electron microscope based mineral liberation analysis: an introduction to JKMRC/FEI mineral liberation analyser. Journal of Minerals and Materials Characterization and Engineering, v. 2, n. 1, p. 33-41, 2003.). In this case, several polished sections were used in gold scanning to determine grain’s composition and associations. However, to ensure these results are accurate and representative, pre-concentration of the samples is often desired.
The methodology describing pre-concentration by hydroseparation has been demonstrated by Lastra et al., (2005LASTRA, R.; PRICE, J.; CABRI, L. J.; RUDASHEVKY, V. N.; McMAHON, G. Gold characterisation of a sample from Malartic East (Quebec) using concentration by hydroseparator. In: INTERNATIONAL SYMPOSIUM ON THE TREATMENT OF GOLD ORES, 5.; ANNUAL CONFERENCE OF METALLURGIST OF CIM, 44., 2005, Calgary, Alberta, Canada. Proceedings […]. Montreal: CIM, 2005. P. 17-29.) and Cabri et al., (2005CABRI, L. J.; BEATTIE, M.; RUDASHEVSKY, N. S.; RUDASHEVSKY, V. N. Process mineralogy of Au, Pd, and Pt ores from the Skaergaard intrusion, Greenland, using new technology. Minerals Engineering, v. 18, p.887-897, 2005.) where the gold grains are concentrated by gravity separation. A similar methodology indicated by Zhou et al., (2004ZHOU, J.; JAGO, B.; MARTIN, C. Establishing the process mineralogy of gold ores. SGS Minerals - Technical Bulletin, v. 3, p. 1-16, 2004.) focuses on pre-concentration of a representative sample using heavy liquid separation techniques and superpanning to separate the populations of gold by density. Both authors focused on concentrate the precious minerals and sulfide by different gravity methods to study polished sections of the concentrates. The enrichment of gold grains enable its characterization by automated or semi-automated microscope techniques for quantifying populations, characterizating forms of occcurence and composition of gold bearing minerals and to provide information for metallurgists and geologists.
This study aims to evaluate the efficiency, benefits and limitations of pre-concentration by density separation by heavy liquid and Mozley table for process mineralogy studies of low-grade gold ore. Besides that, gold recovery was determined by leaching with sodium cyanide, and amalgamation of the heavy product. For this purpose, a sample involving specific issues related to sulfidic refractory gold ore was chosen.
2. Materials and methods
This study was carried out on a gold ore sample collected in the north of Brazil containing 0.96 ppm of Au, a high content of silica (SiO2 - 84.3%) and minor proportions of Al, Fe and S. (Table 1). Major minerals identified by powder X-ray diffraction (XRD) were quartz, mica, chlorite, pyrite and chalcopytite (Figure 1); minerals in minor concentrations identified by scanning electron microscope (SEM) were sulfides (covelite/chalcosite - CuS/Cu2S, esfalerite - ZnS, galena - PbS), feldspar, rutile/anatase (TiO2), bismuth (sulfide and metal) and tellurium minerals. The high mercury contents identified by XRF are related to clusters or agglomerations of minerals identified as traces of matildite (AgBiS2) and volynskite (AgBiTe2).
Sampling was performed using rigid criteria in order to maintain the original characteristics of the sample. The preparation of the sample comprised crushing below 1 inch (25.4 mm), homogenization and sampling of 100 kg and a secondary milling below 1.7 mm and then another homogenization and sampling to obtain an aliquot of 10 kg for this study. The importance of the sampling is clear, particularly for gold ores that may be subject to the nugget effect. Rotating sample splitters were therefore used for sample preparation and chemical analysis.
The technological characterization procedure also involved several steps, as described below:
-
Particle size analysis by wet screening at 1.2, 0.84, 0.60, 0.30, 0.150 and 0.037 mm;
-
Density separation by sink and float method using organic heavy liquid (specific gravity SG = 2.80 g/cm3) for each sieve fraction above 0.037 mm, to obtain the float and sink products (organic heavy liquids are toxic, therefore, separations are carried out in laboratory fume hood with allcollective and personal protective equipment);
-
Mineral separation of the sink product using a Mozley Mineral Separation (Mozley table), giving two products: intermediate (tailings on Mozley separation) and heavy (concentrate on Mozley table);
-
Amalgamation of the heavy product to estimate the proportion of gravitic gold (coarse and accessible gold). For this laboratory study, the reduction of mercury exposure was a priority; elutriation was used to recover the amalgam (Hg+Au alloy) for obtaining the sink amalgamation residue and the amalgam;
-
Cyanidation of the following products: the fraction above 0.037 mm; float (SG<2.8 g/cm3), intermediate (SG>2.8 g/cm3; Mozley tailing) and heavy (SG>2.8 g/cm3; Mozley concentrate; after the removal of the amalgam), giving one liquor and one solid residue for each product and size fraction;
-
Fraction below 0.037 mm was only subjected to chemical analysis (X-ray fluorescence - XRF) since there is a limitation in the liberation studies by SEM regarding sample preparation and particle desaglomeration.
Amalgamation was conducted at a weight proportion of 1:20 (Hg/sample) in a 50% w/w solid suspension with 150 rpm agitation for 15 hours; the sample was then elutriated to separate the amalgam (Hg+Au) from the solid residue.
Cyanidation was performed with a sodium cyanide (NaCN) solution, with an initial concentration of 2.000 ppm of NaCN, in a 50% w/w solid suspension with pH between 10 and 11 balanced with sodium hydroxide (NaOH), under 150 rpm agitation for 48 hours. The final solution and the residue were recovered by filtration.
Sample mineralogical composition and gold association with gangue and bearing minerals was carried out on heavy product using scanning electron microscopy/energy-dispersive spectroscopy (SEM/EDS), automatically detected using Feature/Inca software (Oxford development).
The sink products from heavy liquid separation were processed by the Mozley table in order to enhance the metallurgical recovery (increase gold grades) and decrease mass recovery by concentrating the liberated gold, iron oxides and other heavy mineral particles; the separation efficiency is lower when compared to heavy liquid separation but used, due to limitations of using liquids with higher densities. The Mozley table concentrates (fractions above 0.037 mm) were submitted to amalgamation that is known to be effective to capture coarse gold grains on fractions. The Table 2 presents the analyzed products.
-
Chemical analysis to determine the gold content (for all the obtained products) by atomic aborption.
3. Results and discussion
3.1 Particle size distribution and mineral separation
The size distribution of the sample particles, the gold content and distribution by sieve fraction are shown in Table 3.
The particle size distribution shows that 90.3% of the sample weight is above 0.037 mm. The gold grades show a constant increase from coarse to fine fractions, from 0.55 to 1.81 ppm. It should be emphasized that a notable proportion of gold - 18.9% - is associated to the slimes (fraction below 0.037 mm). The silver grade varies around 3 ppm in fractions coarser than 0.037 mm and increases to 16.1 ppm in the finest fraction.
The grades of gold in the float product vary from 0.49 to 0.21 ppm, and decrease systematically for the fines. For the intermediate product, the grades of gold vary from 0.72 to 1.72 ppm with a slight tendency to increase towards the finer fractions. Conversely, the grades of gold in the heavy product reach a maximum of 5 ppm in the fractions above 0.84 mm and vary from 10 to 17 ppm in the fractions below 0.84 mm.
The results are also shown in Figure 2. Considering the whole sample (fraction 1.7-0.037 mm), 29.8% of gold is associated to the the float product (80.9% of the sample mass), 5.61% to the intermediate (5.2% of the sample mass) and 45.7% to the heavy product (4.26% of the sample mass). Therefore, for a comminution below 1.7 mm, 45.7% of the total gold is associated with 4.26% of the total sample weight (Heavy); aditionally 18.9% of the gold from the whole sample concentrates in the fines (< 0.037 mm). The gold distribution in the heavy product increases toward the fine fractions, suggesting that a finer comminution may increase the gold recovery associated with the heavy products.
Pre-concentration by density separation generates a heavy product capable of providing a concentrate that minimizes the effects of the significant problems identified in gold deportment studies due to statistics and the risk of bias in the sample preparation of the polished section. Pre-concentration by density separation shows that there is a tendency for this content to increase in the finer (and therefore more liberated) fractions.
3.2 Gold association
The application of SEM-EDS microanalysis in the characterization of gold-bearing ore composition indicated that the gold grains were mostly a gold-silver alloy with an average composition of 71% gold and 29% silver (varying from 2.4% to 69.4% Ag and 30.6% to 97.6% Au).
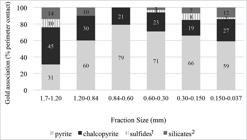
The gold grains exposed, assessed by microscopy, gives an indication of the leachability of the ore. The exposure of the gold grains resulting in the density concentrates showed a trend towards accretion for the finer fractions (Table 4). Most of the locked gold was contained in pyrite and chalcopyrite, with a minor proportion locked in silicates. The exposed perimeter will be defined as the portion of the gold grains that can be extracted per action of a leaching solution, which is in relation to its surface.
The proportion of one mineral that is in contact with another mineral is determined by measuring its interface (perimeter in contact) and is shown in Figure 3. The gold grains identified were mainly locked with pyrite and chalcopyrite and associated with bismuth sulfides, and less frequently with bismuth (metal) and telluride. The gold may also be associated to silicates, such as chlorite, mica and feldspar; in minor proportions, gold locking is also observed with CuS/Cu2S, silver-bismuth telluride, zinc and lead sulfides.
Petruk (2000) described two main textures for gold deposits as: a) gold in fractures and microfractures in rocks and veinlets and microveinlets in minerals (exposed), b) gold in interstitial spaces between mineral grains or occurring between two or more different minerals (intergranular). Another relevant association is gold grain locked in a host mineral (encapsulated gold). The implication of structurally bound gold extraction is that gold in interstitial spaces between sulfide grains will be improved with finer grinding to recovery gold-bearing sulfides by flotation.
3.3 Gold distribution and technological characterization
The gold distribution at attained products (cyanidation liquor, solid residue and amalgam) are shown in Figure 4.
Gold extraction by cyanidation of the float product tends to increased towards the fine fraction (distribution in cyanidation liquor), from around 60 to 95%. On the other hand, gold distribution in the intermediate product shows subtle variations in size fractions, indicating that the particle size does not interfere with the gold accessibility. Gold reporting to the float product is usually fine-grained and associated with light gangue, such as silicates.
The recovery of gold by amalgamation increases significantly from 41% (>1.20 mm) to 76% (<0.037 mm) over the fractions. This behavior may indicate higher effective free coarse gold particles; alternatively, due to surface tension, the amalgam may not interact with the small fraction of ore particles, and consequently the ore must be milled finely enough to expose the gold material (<0.84 mm according to presented data).
In all analyzed products, the gold associated to intermediate and float products may be attributed to being very fine-grained in locked particles with low density gangue (mainly silicates).
The coarser fractions had noticeably lower gold distribution, especially above 0.60 mm (losses greater than 20%). The recovery by amalgam had low performance mainly in fractions above de 0.84 mm, maintaining ~80% (gold recovery) below this fraction.
In tests to determine the amalgamation and sodium cyanide leaching to recover the newly exposed gold, the results indicated that 69.9% was recoverable (Table 5). An alternative of increasing gold recovery mainly locked in pyrite and chalcopyrite and a small amount in silicate would be finer milling required to expose these locked gold grains. Furthermore, the fraction below 0.037 mm was not characterized, corresponding to 18.9% mass.
When comparing gold associations (Table 4 and Figure 3) with gold recovery by amalgamation and cyanidation (Table 5), there is a correlation between increased gold exposure towards the finer fractions and a greater gold recovery (from 6.6 to 16.3%) due to the possibility of access to the cyanide solution, therefore the comparatively low rate of Au recovery is to be related to poor of exposed on the coarse fraction.
4. Conclusions
In literature, there are a limited number of approaches to the separation of dense minerals focused on pre-concentration by density separation; when used along with automated mineralogical techniques, laboratorial separations can provide a very powerful method for quantifying populations of gold fundamental to ore characterization and plant optimization studies in the gold industry.
Several polished sections prepared from the heavy product (sink product from heavy liquid separation and heavy Mozley table product) were analysed by SEM/EDS. The results indicate that the concentrate contains a substantial number of gold grains after removing a significant sulfide and silicate fraction, this enables us to map and quantitatively extract the mineral associations by perimeter contact with other mineral phases, the exposure and associations that are characteristic of gold grains and the characteristics of gold-containing particles, such as their composition and size distribution.
A sample containing low gold content was investigated to evaluate the efficiency of density separation methods for each fraction, and to determine the gold recovery rate by leaching with sodium cyanide. From this perspective, it is shown that concentration methods based on density separation may be a viable alternative, especially when attempting analysis of grains in a polished section for determination of gold in refractory minerals and bulk diagnostic cyanidation using SEM/EDS in subsequent gold characterization and advantages due to the fact that it is a quick, simple and cheaper than other methods. Pre-concentration can be seen as a disadvantage when performed with insufficient mass which compromises the effectivity of analyzing gold distribution and associations in a polished section and the lack of a bulk method to verify results makes the method subject to errors caused by non representative samples. Nevertheless, for an accurate information of the sample, gold distribution on light products must be considered and also its mineralogy since gold association in this producs is not mensured but inferred.
Acknowledgments
We would like to thank the technical team at the Laboratório de Caracterização Tecnológica at Escola Politécnica from the Universidade Federal de Sao Paulo - USP for their analytical support and the scholarship offered by CAPES to F.R. Costa and G. P. Nery. The authors would also like to thank the anonymous referee for reviewing the manuscript and providing valuable comments and suggestions.
References
- ASAMOAH, R. K.; SKINNER, W.; ADDAI-MENSAH, J. Enhancing gold recovery from refractory bio-oxidised gold concentrates through high intensity milling. Mineral Processing and Extractive Metallurgy, v. 129, n.1, p. 64-73, 2020. DOI 10.1080/25726641.2019.1658915.
» https://doi.org/10.1080/25726641.2019.1658915 - BURT, R. O. Gravity concentration technology Amsterdam: Elsevier, 1984. 521p. (Developments in Minerals Processing - v.5).
- CABRI, L. J.; BEATTIE, M.; RUDASHEVSKY, N. S.; RUDASHEVSKY, V. N. Process mineralogy of Au, Pd, and Pt ores from the Skaergaard intrusion, Greenland, using new technology. Minerals Engineering, v. 18, p.887-897, 2005.
- CHATTOPADHYAY, A.; GORAIN, B. Gold deportment studies on a copper gold ore: a systematic approach to quantitative mineralogy focusing on diagnostic metallurgy. In: ANNUAL CANADIAN MINERAL PROCESSORS OPERATORS CONFERENCE, 45., 2013, Ottawa, Canada. Proceedings […]. Montreal: CIM, 2013. p. 29-42.
- COETZEE, L. L.; THERON, S. J.; MARTIN, G. J.; VAN DER MERWE, J.; STANEK, T. A. Modern gold deportments and its application to industry. Minerals Engineering, v. 24, n. 6, p. 565-575, 2011.
- DUNNE, R. Flotation of gold and gold-bearing ores. In: ADAMS, M. D. (ed.). Gold ore processing: project development and operations. Amsterdam: Elsevier, 2016. p. 315-338.
- FALCONER, A. Gravity separation: old technique/new methods. Physical Separation in Science and Engineering, v. 12, n. 1, p. 31-48. 2003.
- GOODALL, W. R.; SCALES, P. J.; BUTCHER, A. R. The use of QEMSCAN and diagnostic leaching in the characterisation of visible gold in complex ores. Minerals Engineering, v. 18, n. 8, p. 877-886, 2005.
- GOTTLIEB, P.; WILKIE, G.; SHUTHERLAND, D.; HO-TUN, E.; SUTHERS, S.; PERERA, K.; JENKINS, B. Using quantitative electron microscopy for process mineralogy applications. JOM, v. 52, n. 4, p. 24-25, 2000.
- GU, Y. Automated scanning electron microscope based mineral liberation analysis: an introduction to JKMRC/FEI mineral liberation analyser. Journal of Minerals and Materials Characterization and Engineering, v. 2, n. 1, p. 33-41, 2003.
- LANG, A. M.; AASLY, K.; ELLEFMO, S. L. Mineral characterization as a tool in the implementation of geometallurgy into industrual mineral mining. Mineral Engineering, v. 116, p.114-122, 2018.
- LAPLANTE, A. R.; WOODCOCK, F.; NOAPARAST, M. Predicting gravity separation of gold recoveries, Miner. Metall. Process, v.12, p. 74-79, 1995.
- LASTRA, R.; PRICE, J.; CABRI, L. J.; RUDASHEVKY, V. N.; McMAHON, G. Gold characterisation of a sample from Malartic East (Quebec) using concentration by hydroseparator. In: INTERNATIONAL SYMPOSIUM ON THE TREATMENT OF GOLD ORES, 5.; ANNUAL CONFERENCE OF METALLURGIST OF CIM, 44., 2005, Calgary, Alberta, Canada. Proceedings […]. Montreal: CIM, 2005. P. 17-29.
- LORENZEN, L. Some guidelines to the design of a diagnostic leaching experiment. Minerals Engineering, v. 8, n. 3, p. 247-256, 1995.
- MKANDAWIRE, N. P.; McGRATH, T.; BAX, A. EKSTEEN, J. Potential of the dense media cyclone for gold ore preconcentration. Mineral Processing and Extractive Metallurgy, v. 129, n. 1, p. 87-95, 2020. DOI 10.1080/25726641.2019.1669982.
» https://doi.org/10.1080/25726641.2019.1669982 - SOLTANI, F.; DARABI, H.; BADRI, R.; ZAMANKHAN, P. Improved recovery of a low-grade refractory gold ore using flotation-preoxidation-cyanidation methods. International Journal of Mining Science and Technology, v. 24, n. 4, p. 537-542, 2014.
- UEDA, T.; OKI, T.; KOYANAKA, S. Statistical effect of sampling particle number on mineral liberation assessment. Minerals Engineering, v. 98, p. 204-212, 2016.
- ZHOU, J.; CABRI, L. J. Gold process mineralogy: objectives, techniques, and applications. JOM, v. 56, p. 49-52, 2004.
- ZHOU, J.; JAGO, B.; MARTIN, C. Establishing the process mineralogy of gold ores. SGS Minerals - Technical Bulletin, v. 3, p. 1-16, 2004.
Publication Dates
-
Publication in this collection
30 Sept 2020 -
Date of issue
Oct-Dec 2020
History
-
Received
09 Mar 2020 -
Accepted
17 June 2020