Abstracts
OBJECTIVE: To evaluate the effectiveness of the Amplatzer™ septal occluder in the treatment of ostium secundum atrial septal defects (OS ASDs). METHODS: Retrospective cohort study conducted between November 1998 and September 2005 involving 101 OS ASD transcatheter occlusion procedures in our institution. All procedures were conducted in the hemodynamic laboratory under general anesthesia with transoesophageal echocardiographic monitoring (TEE). Clinical and echocardiography assessments of the patients were conducted at 30 days, six months and on an annual basis. The results are presented as averages, standard deviations and percentages. Event-free survival was estimated using the Kaplan-Meier curve. RESULTS: From the 101 patients, 60 (59.4%) were females. Mean age, weight, height, body mass index and body surface area were, respectively: 24.3 ± 18.31 years, 51.88 ± 23.76kg, 140.59 ± 39.3cm, 23.18 ± 18.9kg/m² and 1.24 ± 0.21m². The prevalence of interatrial septum aneurysms was 4.95%, and 98 cases had an isolated defect. ASD diameters were 21.47 ± 6.96mm using an angiography and 21.22 ± 7.93 mm using a TEE. The average size of the implanted devices was 23.92 ± 7.25mm, ranging from 9mm to 40mm. The procedure time was 90.47 ± 26.67 minutes and the average hospital stay was 2.51 ± 0.62 days. Clinical and echocardiography follow-up was conducted at 12.81 ± 8.41 months and all devices were securely anchored without any residual shunts. The procedure success rate was 93% (94/101). In five cases adequate deployment of the device was not possible and 2 patients presented residual ASD. No major complications occurred. CONCLUSION: The Amplatzer™ septal occluder is an effective OS ASD transcatheter treatment device.
Atrial septal defect; Amplatzer™; transcatheter closure
OBJETIVO: Avaliar a efetividade da prótese de Amplatzer® para tratamento de comunicação interatrial tipo ostium secundum (CIA OS). MÉTODOS: Estudo de coorte histórica entre novembro de 1998 e setembro de 2005, em que foram realizados 101 procedimentos para oclusão percutânea de CIA OS em nossa instituição. Os procedimentos foram efetuados no laboratório de hemodinâmica, sob anestesia geral e com monitorização por ecocardiografia transesofágica (ETE). Os pacientes foram acompanhados clinicamente e com ecocardiografia em 30 dias, seis meses e depois anualmente. O resultados são apresentados em média, desvio padrão e porcentual, e a sobrevida livre de eventos foi estimada pela curva de Kaplan-Meier. RESULTADOS: Dos 101 pacientes, 60 (59,4%) eram mulheres. As médias para idade, peso, altura, índice de massa corporal e superfície corporal foram, respectivamente, de 24,3 + 18,31 anos, 51,88 + 23,76 kg, 140,59 + 39,3 cm, 23,18 + 18,9 kg/m², e 1,24 + 0,21 m². A prevalência de aneurisma do septo interatrial foi de 4,95%, e 98 casos eram de defeito único. O diâmetro das CIAs foi de 21,47 + 6,96 mm pela angiografia e de 21,22 + 7,93 mm pela ETE. As próteses implantadas mediam 23,92 + 7,25 mm, variando de 9 mm a 40 mm. O tempo de procedimento foi de 90,47 + 26,67 minutos e a média de internação hospitalar, de 2,51 + 0,62 dias. Os seguimentos clínico e ecocardiográfico ocorreram com 12,81 + 8,41 meses e todas as próteses estavam bem ancoradas e sem shunt residual. O sucesso do procedimento foi de 93% (94/101). Em cinco casos não se conseguiu liberação adequada do dispositivo e dois pacientes apresentaram CIA residual. Não foram registradas complicações maiores. CONCLUSÃO: A prótese de Amplatzer® mostrou-se efetiva para o tratamento percutâneo de CIA OS.
Comunicação interatrial; Amplatzer®; tratamento percutâneo
ORIGINAL ARTICLE
Effectiveness of the Amplatzer ™ device for transcatheter closure of an ostium secundum atrial septal defect
Cristiano Oliveira Cardoso; Raul Ivo Rossi Filho; Paulo Renato Machado; Lisia M. Galant François; Estela S. K. Horowitz; Rogério Sarmento-Leite
Programa de Pós-graduação do Instituto de Cardiologia do Rio Grande do Sul Fundação Universitária de Cardiologia Porto Alegre, RS
Mailing address Mailing address: Raul Ivo Rossi Filho Av. Princesa Isabel, 370 U. Pesquisa 90620-001 Porto Alegre, RS E-mail: rossi.pesquisa@cardiologia.org.br
SUMMARY
OBJECTIVE: To evaluate the effectiveness of the Amplatzer septal occluder in the treatment of ostium secundum atrial septal defects (OS ASDs).
METHODS: Retrospective cohort study conducted between November 1998 and September 2005 involving 101 OS ASD transcatheter occlusion procedures in our institution. All procedures were conducted in the hemodynamic laboratory under general anesthesia with transoesophageal echocardiographic monitoring (TEE). Clinical and echocardiography assessments of the patients were conducted at 30 days, six months and on an annual basis. The results are presented as averages, standard deviations and percentages. Event-free survival was estimated using the Kaplan-Meier curve.
RESULTS: From the 101 patients, 60 (59.4%) were females. Mean age, weight, height, body mass index and body surface area were, respectively: 24.3 ± 18.31 years, 51.88 ± 23.76kg, 140.59 ± 39.3cm, 23.18 ± 18.9kg/m2 and 1.24 ± 0.21m2. The prevalence of interatrial septum aneurysms was 4.95%, and 98 cases had an isolated defect. ASD diameters were 21.47 ± 6.96mm using an angiography and 21.22 ± 7.93 mm using a TEE. The average size of the implanted devices was 23.92 ± 7.25mm, ranging from 9mm to 40mm. The procedure time was 90.47 ± 26.67 minutes and the average hospital stay was 2.51 ± 0.62 days. Clinical and echocardiography follow-up was conducted at 12.81 ± 8.41 months and all devices were securely anchored without any residual shunts. The procedure success rate was 93% (94/101). In five cases adequate deployment of the device was not possible and 2 patients presented residual ASD. No major complications occurred.
CONCLUSION: The Amplatzer septal occluder is an effective OS ASD transcatheter treatment device.
Key words: Atrial septal defect; Amplatzer; transcatheter closure.
Introduction
Atrial septal defects (ASDs) are one of the most common cardiac congenital defects, accounting for roughly 5% to 10% of all cases. It predominates in females with a 1.5 - 3.5:1 female/male ratio1. Ostium secundum ASD (OS ASD) accounts for roughly 75% of this pathology and the remaining 25% are due to ostium primum ASD, sinus venosus ASD and coronary sinus ASD2. Since 1948, when Murray described the first atrioseptoplasty3, it has been the gold standard surgical therapy offering excellent immediate results. Konstantinides4, Murphy5, Groundstrem6 and Ross-Hessenlink7 reported high success rates and very few complications during late postoperative follow-up of the surgical closure. During the past few decades, various devices have been used in percutaneous OS ASD closure attempts, however the results were not encouraging. In 1997, Sharafudin and associates8 published an experimental study using a new integrated, self centering and self-expandable prosthesis called Amplatzer® (AGA Medical Corporation, Golden Valley, USA). Subsequently, clinical studies have been demonstrating that the results with this device are similar to surgery9-10. In adults with this pathology, various study series with late postoperative follow-up have demonstrated favorable clinical evolution11-17.
The objective of this study is to evaluate the effectiveness of the Amplatzer® device (AGA Medical Corporation, Golden Valley, USA) for transcatheter treatment of OS ASD.
Methods
Delineation - Uncontrolled retrospective cohort study.
Study population - Between November 1998 and September 2005, 101 OS ASD closure procedures were performed in the hemodynamic department of our institution. The inclusion criterion was the presence of OS ASD confirmed by a transthoracic (TTE) or transesophageal (TEE) echocardiograph, with indication for defect closure due to clinical repercussions. Patients with ostium primum ASD, sinus venosus ASD, coronary sinus ASD or other associated congenital defects were excluded.
Statistical analysis - The results are expressed as averages, standard deviations and percentages. The Kaplan-Meier survival curve was calculated and differences were considered statistically significant when p<0.05. The data were analyzed using the computer program SPSS 11.0.
Amplatzer® Septal Occluder
The Amplatzer® septal occluder (AGA Medical Corporation, Golden Valley, USA) is made with a 0.004 0.0075 inch thick nitinol metal alloy (55% nickel and 45% titanium) comprised of two disks linked together by a short connecting waist forming an integrated unit which is self- expandable and self-centering. One disk remains attached to the septum on the right atrial side and the other, which is larger, on the left atrial side. The size of the prosthesis is determined by the waist of the device that corresponds to the ASD diameter. The device is available in various sizes (4 - 40mm) and is filled with polyester fiber that has high thrombogenic properties.
Transcatheter procedure
The procedures were performed in the hemodynamic laboratory under general anesthesia and TEE monitoring. During the femoral vein catheterization, right and left cavity pressures were recorded; blood samples were taken for oximetry and to calculate pulmonary and systemic blood flow. Next a sizing balloon was placed across the defect and the stretched diameter was measured guided by echocardiography and cineangiography. The size of the Amplatzer® device was determined using the stretched balloon echocardiography and cineangiography measurements plus 1 or 2mm. The device implantation technique followed the method described by Fontes and associates18.
The procedure was monitored by TEE in order to compare the defect size, guide the device positioning in the septum, verify defect occlusion, evaluate residual flow and assess any possible risk to the adjacent structure. The margins, the distance between the ASD margins and the atrioventricular valves, inferior and superior vena cava, coronary sinus and aorta, were considered ideal when they exceeded 5 mm.
All patients were given a 5 mg/kg dose of acetylsalicylic acid (ASA) before the procedure and prophylactic antibiotic therapy with cefazolin (50 mg/kg) upon closure as well as 8 and 16 hours after closing, in addition to the recommendation of prophylactic treatment for infectious endocarditis and use of ASA for 6 months after the implant.
On the following day, the patients were clinically evaluated with electrocardiogram, chest x-ray and TEE. After discharge, clinical and echocardiographic assessments were conducted at 30 days, 6 months and 1 year.
The patients or their designated representatives were briefed on the procedure and signed a consent form approved by the research and ethics committee of our institution.
Results
In this study 101 patients underwent Amplatzer® septal occluder implantations to treat OS ASD in our institution. The clinical characteristics of the patients are shown in Table 1.
The electrocardiography findings before the procedure demonstrate that 96.03% (97/101) of the patients presented sinus rhythm, 2.97% (3/101) atrial fibrillation and 0.99% (1/101) atrial flutter. In relation to conduction, 29.7% (30/101) presented normal conduction, 12.87% (13/101) complete right bundle branch block and 57.42% (58/101) right bundle branch conduction disorders.
The TEE, during the procedure, revealed that 98 (97.02%) of the patients presented isolated OS ASD and only five patients (4.95%) had septal aneurysms.
Using cardiac catheterization it was confirmed that the mean pulmonary artery systolic pressure was 23.83 ± 1.15 mmHg, the mean pulmonary artery diastolic pressure was 11.27 ± 7.14 mmHg, and the pulmonary to systemic blood flow ratio (Qp/Qs) was 4.93.
Procedure, fluoroscopy and hospital stay durations are shown in Table 2.
Mean septal occluder size was 23.92 ± 7.25 mm, of which the smallest was 9mm and the largest 40mm. Defect size was estimated by the stretched balloon size using TEE and cineangiography and the respective sizes were 21.22 ± 7.93 mm and 21.47 ± 6.96 mm. There was no significant statistical difference in relation to the measurements taken by the echocardiography technician and the hemodynamic technician (p=0.89).
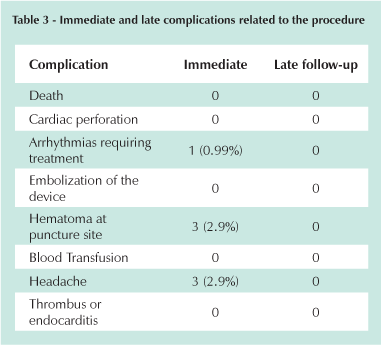
During deployment there were no immediate complications such as death, perforations, embolization of the device or neurological complications. Three patients (2.97%) had a hematoma at the femoral puncture site and 1 presented supraventricular tachycardia that was reverted with adenosine during the procedure. The success rate was 93% (94/101) with 7 unsuccessful procedures. In 2 cases, the device did not completely occlude the defect leaving a slight residual ASD and in 5 other cases the device did not deploy due to coronary sinus or vena cava compression. In these cases, the devices were removed with no complications and the patients were referred for subsequent surgery. One of the 2 patients that had a residual shunt after closure was referred to surgery and the other was given clinical treatment. Three patients suffered from headaches during the first 30 postoperative days, a known symptom after implantation of this type of device, that was spontaneously resolved thereafter. Immediate and late complications are shown in Table 3.
Long term follow-up is currently at 12.81 ± 8.41 months and 98.9% (100/101) of the patients are asymptomatic (Figure 1). One patient that was 70 years old at the time of the implantation with pulmonary artery hypertension has been admitted to the hospital twice for heart failure decompensation. Echocardiographic monitoring showed adequate device positioning, without a residual shunt and complete occlusion of the defect. In our series, no cases of thrombi, endocarditis or other late complications related to the Amplatzer® device were observed.
Discussion
Even though this study was a cohort with a small number of patients, it demonstrated that occlusion of OS ASD using the Amplatzer® device is very safe and effective. From the 101 patients included in the study, there were no deaths, cardiac perforations or other serious complications during the hospital phase, which is consistent with the reports in literature19,20. During the late evolution, currently at 12.81 ± 8.41 months, no cases of thrombi, embolization or endocarditis related to the device have been recorded even though these complications have been reported in literature21-23. The Amplatzer® prothesis has proven to be a safe and effective device and currently can be considered an alternative to surgical treatment for selected patients.
Another important and essential aspect is TEE monitoring during the procedure. This guidance offers adequate visualization of the margins and safe deployment of the device as well as confirmation that the vital structures such as vena cavae, coronary sinus and aortic rim are not compressed. It has been often reported in medical literature that deficient rims are intimately related to the success of the procedure and adequate attachment of the device to the septum24,25. If the device is deployed without this critical evaluation the chance of incomplete defect occlusion or embolization of the prosthesis increases significantly. In our study, five devices were not deployed due to compression of the vital heart structures and were removed without harming the patients. This approach confirms that the adequate visualization during device implantation using TEE, is extremely important.
Our series confirmed medical literature findings that the Amplatzer® prosthesis is a safe alternative to treat ostium secundum atrial septal defects. The procedure presents a low rate of hospital complications, reduced hospital stays and safety, based on late follow-up26-28. Besides the device's proven safety, the transcatheter procedure avoids a thoracotomy and offers the benefit of not submitting the patient to aortic clamping and extracorporeal circulation, crucial timeframes in atrioseptoplasty. These timeframes are directly related to surgical morbidity. In a prospective study comparing the Amplatzer® device with surgical treatment in children, Bialbowski and associates29 found similar closure success rates (95.5% surgery vs. 97.9% transcatheter, p=NS), however the hospital stays for surgery were longer (7.5 vs. 2.2 days, p<0.001). In addition, all surgical complications (pericardial hemorrhage, arrhythmias, bleeding, pneumonia and repeat surgery) were observed in 68.2% of the patients in comparison to 6.4% in the Amplatzer® group. Blood transfusion requirements were 40.9% in the surgical group and 2.12% in the transcatheter group (p<0.001). In another study, Zhong-Dong DU and associates30 also compared surgical and transcatheter closure results for OS ASD treatment and found a success rate of 100% for surgery in comparison to 95.7% for Amplatzer® (p=0.006). In regard to outcomes, major complication rates (cerebral embolism, cardiac perforation, endocarditis, repeat surgery, death related to the procedure, embolization of the prosthesis or permanent pacemaker requirements) were 0.2% for the transcatheter procedure and 5.2% for surgery (p<0.001). The surgical group presented greater anesthesia times (159.7 ± 54.1 vs. 105.7 ± 43.2 minutes, p<0.001) and hospital stays (3.4 ± 1.2 vs. 1 ± 0.3 days, p<0.001). No differences were found in regard to mortality during the hospital stay or late follow-up. The results from our cohort coincide with surgical outcomes reported in international literature.
Study limitations - The present study presents limitations that should be considered. This was an uncontrolled retrospective cohort study of a group submitted to surgery. Even though our results are similar to the surgical results published in literature (in relation to success rates, complications and late success), we cannot confirm that one method is superior to the other since the ideal delineation would be a randomized clinical trial (RCT). Additionally, the Single Health Care System (SUS) does not cover the prosthesis and only patients with private health care plans have access to this type of treatment.
Future prospects - The use of OS ASD transcatheter closures has been on the rise in our area since it offers similar success rates, lower morbidity and less expressive complications than surgery. Additionally, in the majority of cases the procedure is corrective and less aggressive for the patient. In some developed countries, the percutaneous procedure has surpassed surgery. However, in developing countries cost is a limiting factor for routine use. Vladimiro Vida and associates31, in a non-randomized study conducted in Guatemala, compared the effectiveness and cost of the two treatments. The two therapies proved to be effective, however the Amplatzer® presented a higher cost than surgery. Without any doubt, in the near future, the prospect is that the use of the transcatheter treatment will be more widespread in our area since it presents similar corrective success and lower morbidity. It is evident that a RCT is required to definitely confirm the superiority of one treatment over the other.
Conclusion
The Amplatzer® device is safe and effective for OS ASD transcatheter closure offering high success rates, few complications and adequate long term maintenance.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
Manuscript received May 31, 2006; revised manuscript received August 29, 2006; accepted November 3, 2006.
- 1. Braunwald E, Zipes D, Libby P. Heart Disease. 6th ed. Philadelphia: Saunders Company; 2001.
- 2. Ho YS, Baker EJ, Rigby ML, Anderson RH. Congenital heart disease, morphologic and clinical correlations. Barcelona: Mosby-Wolfe; 1995.
- 3. Murray G. Closure of defects in cardiac septa. Ann Surg. 1948;128:843.
- 4. Konstantinides S, Geibel A, Olschewski M, Görnandt L, Roskamm H, Spillner G, et al. A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med. 1995;333:469-73.
- 5. Murphy JG, Gersh BJ, McGoon MD, Mair DD, Porter CJ, Ilstrup DM, et al Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. 1990;323:1645-50.
- 6. Groundstroem KWE, Iivainen TE, Talvensaari T, Lahtela JT. Late postoperative follow-up of ostium secundum defect. Eur Heart J. 1999;20:904-9.
- 7. Ross-Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, van Rijen EH, Utens EM, et al. Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21-31 years. Eur Heart J. 2003;24:190-7.
- 8. Sharafudin MJA, Xiaoping GU, Titus JL, Urness M, Cercera-Ceballos JJ, Amplatz K. Transvenous closure of secundum atrial septal defects. Circulation. 1997;95:2162-8.
- 9. Thomson JD, Aburawi EH, Watterson KG, Van Doorn C, Gibbs JL. Surgical and transcatheter (Amplatzer) closure of atrial septal defects: a prospective comparison of results and cost. Heart. 2002;87:466-9.
- 10. Cowley CG, Lloyd TR, Bove EL, Gaffney D, Dietrich M, Rocchini AP. Comparison of results of closure of secundum atrial septal defect by surgery versus amplatzer septal occluder. Am J Cardiol. 2001;88:589-91.
- 11. Purcell IF, Brecker SJ, Ward DE. Closure of defects of the atrial septum in adults using the Amplatzer device: 100 consecutives patients in a single center. Clin Cardiol. 2004;27(9):509-13.
- 12. Fischer G, Stieh J, Uebing A, Hoffmann U, Morf G, Kramer HH. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single center study in 236 consecutive patients. Heart. 2003;89:199-204.
- 13. Chan KC, Godman MJ, Walsh K, Wilson N, Redington A, Gibbs JL. Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (Amplatzer septal occluder): a multicenter UK experience. Heart. 1999;82:300-6.
- 14. Fischer G, Kramer HH, Stieh J, Harding P, Jung O. Transcatheter closure of secundum atrial septal defects with the new self-centering Amplatzer septal occluder. Eur Heart J. 1999;20:541-9.
- 15. Cao QL, Du ZD, Joseph A, Koenig P, Heitschmidt M, Rhodes J, et al. Immediate and six-month results of the profile of the Amplatzer septal occluder as assessed by transesophageal echocardiography. Am J Cardiol. 2001;88:754-9.
- 16. Butera G, De Rosa G, Chessa M, Rosti L, Negura DG, Luciane P, et al. Transcatheter closure of atrial septal defect in young children: results and follow-up. J Am Coll Cardiol. 2003;42:241-5.
- 17. Rossi RI, Cardoso CO, Machado PR, et al. Transcatheter closure of atrial septal defect with Amplatzer® prostesis in young children. Catheter Cardiovasc Interv. 2005;66(1):128.
- 18. Fontes VF, Pedra CAC, Pedra SRFF, Esteves CA, Braga SLN, Assef JE, et al. Experiência inicial no fechamento percutâneo da comunicação interatrial com prótese de Amplatzer. Arq Bras Cardiol. 1998;70:147-53.
- 19. Braga LNB, Sousa AGMR, Pedra CAC, Esteves SRF, Esteves CA, Fontes VF. Efetividade clínica e segurança do tratamento percutâneo da comunicação interatrial tipo ostium secundum com prótese de Amplatzer®. Arq Bras Cardiol. 2004;83:7-13.
- 20. Herrmann HC, Silvestry FE, Glaser R, See V, Kasner S, Bradbury D, et al. Percutaneous patent foramen ovale and atrial septal defect closure in adults: results and device comparison in 100 consecutive implants at a single center. Cathet Cardiovasc Interv. 2005;64:197-203.
- 21. Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, et al. Incidence and clinical course of thrombus formation on atrial septal defect and patients foramen ovale closure device in 1000 consecutive patients. J Am Coll Cardiol 2004; 43: 302-9.
- 22. Vojacek J, Mates M, Popelová J, Pavel P. Perforation of the right atrium and the ascending aorta following percutaneous transcatheter atrial septal defect closure. Interact Cardiovasc Thorac Surg. 2005;4:157-9.
- 23. Anzai H, Child J, Natterson B, Krivokapich J, Fishbein MC, Chan VK, et al. Incidence of thrombus formation on the Cardioseal and the Amplatzer interatrial closure devices. Am J Cardiol. 2004;93:426-31.
- 24. Du ZD, Koenig P, Cao QL, Waight D, Heitschmidt M, Hijazi ZM. Comparison of transcatheter closure of secundum atrial septal defect using the Amplatzer septal occluder associated with deficient versus sufficient rims. Am J Cardiol. 2002;90:865-9.
- 25. Podnar T, Martanovic P, Gavora P, Masura J. Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Cathet Cardiovasc Intervent. 2001;53:386-91.
- 26. Yew G, Wilson NJ. Transcatheter atrial septal defect closure with Amplatzer septal occluder: five years follow-up. Cathet Cardiovasc Interv. 2005;64(2):193-6.
- 27. Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505-7.
- 28. Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002;39:1061-5.
- 29. Bialkowski J, Karwot B, Szkutnik M, Banaszak P, Kusa J, Skalski J. Closure of atrial defect in children. Surgery versus Amplatzer® device implantation. Tex Heart Inst J. 2004;31:220-3.
- 30. Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K, Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults. J Am Coll Cardiol. 2002;39:1836-44.
- 31. Vida VL, Barnoya J, O'Connell M, Leon-Wyss J, Larrazabal LA, Castañeda AR. Surgical versus percutaneous occlusion of ostium secundum atrial septal defects: results and cost-effective considerations in a low-income country. J Am Coll Cardiol. 2006;47:326-31.
Mailing address:
Publication Dates
-
Publication in this collection
29 May 2007 -
Date of issue
Apr 2007
History
-
Accepted
03 Nov 2006 -
Received
31 May 2006 -
Reviewed
29 Aug 2006