Abstract
Optimization of culture conditions and immobilization parameters for alkaline protease production was carried out by employing Bacillus megaterium MTCC2444. The partially purified enzyme was tested for its stability in the presence of oxidants, surfactants and commercial detergents. The optimum temperature, pH, incubation time and inoculum size were 55 ºC, 11, 48 h, 1 %, respectively. Calcium alginate was used as the immobilization matrix and the effects of gel concentration, bead size, age of immobilized cells, solidification period and initial biomass concentration on alkaline protease production and cell leakage were investigated. The results indicated that the immobilization was most effective with 4 % gel concentration, bead size of 3 mm, 24 h aged immobilized cells for a solidification period of 12 h at 1.5 % initial biomass concentration. The enzyme showed good stability in the presence of oxidants, surfactants and commercial detergents.
optimization; immobilized cells; cell leakage; detergent; oxidant stability
ENGINEERING, TECHNOLOGY AND TECHNIQUES
Immobilization of Bacillus megaterium MTCC 2444 by Ca-alginate entrapment method for enhanced alkaline protease production
Soma Mrudula* * Author for correspondence: somamrudula@hotmail.com ; Nidhi Shyam
Department of Microbiology; M. G. R. College; Dr. M. G. R. Nagar; Hosur-635109; Tamil Nadu -India
ABSTRACT
Optimization of culture conditions and immobilization parameters for alkaline protease production was carried out by employing Bacillus megaterium MTCC2444. The partially purified enzyme was tested for its stability in the presence of oxidants, surfactants and commercial detergents. The optimum temperature, pH, incubation time and inoculum size were 55 ºC, 11, 48 h, 1 %, respectively. Calcium alginate was used as the immobilization matrix and the effects of gel concentration, bead size, age of immobilized cells, solidification period and initial biomass concentration on alkaline protease production and cell leakage were investigated. The results indicated that the immobilization was most effective with 4 % gel concentration, bead size of 3 mm, 24 h aged immobilized cells for a solidification period of 12 h at 1.5 % initial biomass concentration. The enzyme showed good stability in the presence of oxidants, surfactants and commercial detergents.
Key words: optimization, immobilized cells, cell leakage, detergent, oxidant stability
INTRODUCTION
Proteases are among the commercially most viable enzymes which constitute about 65 % of the total industrial enzyme market (Oskouie et al. 2008). They find multiple applications in the industrial sectors such as food, pharmaceutical, leather and detergent (Gupta et al. 2002). Proteases are classified as acidic, neutral or alkaline depending on their activity at different pH (Al-Shehri et al. 2004). Today, the largest share of the enzyme market has been held by the detergent alkaline proteases that are active at alkaline pH range. The feasibility of protease application in the detergents depends on its compatibility and stability with all commonly used detergent components such as surfactants and oxidizing agents (Cheng et al. 2010).
Microorganisms are the predominant producers of the proteases, among which Bacillus sp. has been commercialized for alkaline protease production (Abo-Aba et al. 2006). Microbial enzymes are usually obtained from free or immobilized cells. Whole cell immobilization technique has several advantages over free cell systems such as higher yield of enzyme activity, higher operational stability, greater resistance to environmental perturbations, lower enzyme cost and repeated use of biocatalyst (Mamo and Gessesse 2000; Nampoothiri et al. 2005; Konsoula and Liakopoulou 2006). The most common immobilization matrix used nowadays is alginate, since encapsulation in Ca-alginate gels occurs under very mild conditions and is characterized by the low cost (Bladino et al. 2001; Dey et al. 2003).
The present work focused on the screening of B. megaterium MTCC2444 for alkaline protease production. The optimization of physical and immobilization parameters for enhanced production of alkaline protease was carried out. Further the enzyme was tested for its possible application as a detergent additive.
MATERIALS AND METHODS
Microorganism and culture maintenance
The microorganism used in this study was Bacillus megaterium MTCC2444 supplied from Microbial Type Culture Collection (MTCC) and Gene Bank, Institute of Microbial Technology, Chandigarh, India. The strain was maintained at 4 ºC on nutrient agar slants and was sub-cultured fortnightly.
Screening of Bacillus megaterium MTCC 2444 for enzyme production
The strain was plated on skim milk agar medium (Norazizah et al. 2005). The inoculated plates were then incubated at 35 ºC for 48 h. After this, the plates were flooded with HgCl2-HCl solution for 5 min. The organism was screened for the production of alkaline protease by growing at pH 10.0.
Cultivation of B. megaterium
The strain was grown in 250 ml Erlenmeyer flasks containing 25 ml of culture medium at 55 ºC for 48 h. The culture medium used for growing the strain contained (g/l) in distilled water (pH 7.0):Casein, 10.0; glucose, 10; yeast extract, 3; peptone, 5; NaCl, 5; CaCl2 . 2H2O, 0.4 and MgCl2, 0.2.
Enzyme extraction
At the end of the fermentation, the broth was centrifuged at 6000 relative centrifugal force (RCF) for 20 min at 4 ºC and the cell free supernatant obtained was used as the source of extracellular enzyme.
Enzyme activity
Enzyme extract (1.0 mL) was mixed with 1.0 mL of casein solution (1 %, w/v) prepared in glycine-NaOH buffer (0.1 M, pH 10.0) and incubated at 50 ºC for 30 min. After incubation, the reaction was terminated by the addition of 5.0 ml of trichloroacetic acid (TCA) (5 %, v/v) and was further allowed to stand for 10 min to precipitate the undigested protein. The contents were then centrifuged at 10,000 RCF for 10 min. The amount of amino acids released was estimated using a standard curve of tyrosine (Lowry et al. 1951). A suitable control (prepared by adding TCA to the substrate prior to the addition of the enzyme) was run simultaneously.
One unit of protease is defined as that amount of enzyme that releases 1 µg of tyrosine per minute under specific conditions of assay.
Optimization of physical parameters
The parameters selected for the optimization were temperature (25-60 ºC), pH (4.0-13.0), incubation period (24-96 h) and inoculum size (0.5-3 %).
Cell immobilization
Wet cells were prepared by growing the organism in 250 ml Erlenmeyer flasks containing 100 ml culture medium at 55 ºC for 48 h. After fermentation, the cells were harvested by centrifuging the broth at 6000 RCF for 20 min at 4 ºC. The cells were then washed thrice with sterile saline and re-centrifuged. The washed cells were used with same concentration for the immobilization and in experiments with the free cells. The entrapment of cells in calcium alginate beads was carried out by extruding through a syringe a mixture of alginate slurry (3 %, w/v) and wet weight (0.6 % w/v) into a gently stirred 0.2 M CaCl2 solution at room temperature. The beads formed were kept for curing at 4 ºC for 1 h. The cured beads were washed with sterile distilled water for three to four times and was then subjected to fermentation.
Fermentation
The immobilized cells were added to 250 ml Erlenmeyer flasks containing 50 ml of production medium. The composition of the production medium was same as that of growth medium, except the difference in the concentration of CaCl2 added. In the production medium 0.5 g/l of CaCl2 was added in order to keep the beads intact during the prolonged operation. Batch fermentations with free and immobilized cells were carried out by incubating the flasks at 55 ºC for 120 h. The samples were withdrawn at regular intervals of 24 h and assayed for cell leakage and alkaline protease activity. The effectiveness factor of the immobilized system was defined as the ratio of alkaline protease activity of the the immobilized systems to that of the free cells (Anisha and Prema 2008).
Cell growth and cell leakage
The cell growth in freely suspended cultures and cells leaked from the gel matrix were determined as cell wet weight (CWW) by measuring the optical density at 660 nm. The optical density value was then converted into mg CWW/l using a standard curve (Reyed 2007).
Optimization of bead characteristics
For the preparation of the beads with proper permeability and rigidity, the following parameters were optimized: gel concentration (2, 4 and 6 %), bead size (3, 5 and 7 mm), initial biomass concentration (0.3-2.0 %), solidification period (3, 12 and 24 h) and age of immobilized cells (24, 48 and 72 h). The fermentation was carried out at 55 ºC for 72h.
Repeated batch fermentation
Optimized conditions of immobilization (gel concentration, 4 %; bead diameter, 5 mm; biomass concentration, 1.5 %; age on immobilized cells, 24 h and solidification time, 24 h) were selected for effective cell encapsulation in repeated batch fermentation. After attaining the maximum production of alkaline protease (72 h), the beads collected from the spent medium were washed with sterile saline solution and transferred aseptically to fresh production medium (50 ml). The process was repeated for ten cycles until the beads started disintegrating. The enzyme titres and cell leakage of each cycle were determined. The operational stability of the immobilized system was determined by the standard procedures reported in literature (Konsoula and Liakopoulou 2006; Anisha and Prema 2008).
Partial purification by precipitation and dialysis
Enzyme in the cell free supernatant portion of the culture was precipitated by ammonium sulphate upto 80 % saturation and kept overnight at 4ºC (Venugopal and Saramma 2006). The precipitate was recovered by centrifugation at 6000 RCF for 10 min and re-suspended in 0.1 M glycine-NaOH buffer of pH 10.0 (Qader et al. 2009). This was dialyzed against the same buffer. The dialyzed fraction was centrifuged at 6000 RCF for 10 min and the alkaline protease activity was tested in the supernatant. The purified extract was further used for applications in the detergents.
Detergent application
The protease (0.5 U/ml) was incubated with varying concentrations of oxidizing agent such as H2O2 (1 to 5 %), surfactants such as SDS (0.05, 0.1, 0.2, 0.4 and 0.6 %) and Triton X-100 (0.1, 0.4, 0.7, 1 and 4 %) for 30 min. The residual activity was measured by standard assay procedure. The stability of the protease in the commercial detergents were tested by incubating the enzyme (0.5 U/ml) with the solutions of different commercial detergents such as Ariel, Tide, Henko, Surf excel and Wheel at a concentration of 7 mg/ml for 1 h. Suitable aliquots were withdrawn at different time intervals and the residual activities were measured by the standard assay procedure. It was compared with the control incubated under similar conditions without any detergent. Also, to rule out the possibility of any protease (if at all present) contained as ingredient of the detergent, the detergent solutions at a concentration of 7mg/ml were assayed for alkaline protease activity without incubating with the enzyme.
RESULTS AND DISCUSSION
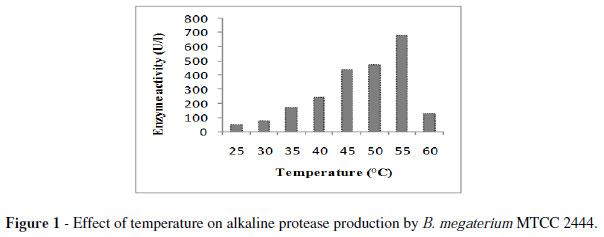
The strain MTCC2444 produced transparent circular zones around the colonies in an opaque white background detected protease production. Initially, the strain produced 471 U/l enzyme at 55 ºC for 48 h.The enzyme production was maximum at 55 ºC (Fig. 1). Some authors have reported optimum temperature as 50 ºC (Shaheen et al. 2008; Boominadhan et al. 2009). On the other hand, 37 ºC has been reported as the optimum temperature for protease production by B. proteolyticus CFR3001, B.pumilus SG2 and Bacillus sp. (Bhaskar et al. 2007; Sangeetha et al. 2008; Nagalakshmi and Ramesh 2009). It has been noted that the important characteristic of most microorganisms is their strong dependence on the extracellular pH for cell growth and enzyme production (Kumar and Tagaki 1999).
The results of pH studies showed that the strain MTCC2444 produced maximum yield at pH 11.0 (Fig. 2). Similar results were reported for the protease production by B. subtilis BS1 (Shaheen et al. 2008), whereas the optimum pH of 9.0 and 10.5 were recorded for B. cereus MTCC6840 and B. circulans, respectively (Joshi et al. 2007; Jaswal et al. 2008). The optimum incubation time was 48 h (Fig. 3). This was in agreement with the findings of Shumi et al. (2004) for protease production by Listeria monocytogenes. In contrast, 36 and 40 h of incubation time was found maximum for protease production by B. pumilus SG2 (Sangeetha et al. 2008) and B. circulans (Jaswal et al. 2008), respectively.
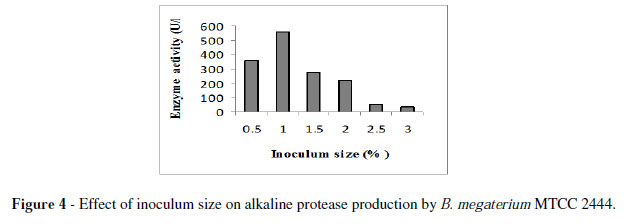
The enzyme yield of B. megaterium MTCC2444, reached maximum at 1 % inoculum size (Fig. 4). These results were comparable with that of Nagalakshmi and Ramesh (2009) for alkaline protease production by Bacillus sp. Under these conditions, the strain MTCC2444 produced 699 U/l enzyme.
The observations of the present experiment revealed that there was 1.5-fold increase in the enzyme yield by the immobilized cells when compared to free cells. The production of α- galactosidase by Streptomyces griseoloalbus showed that there was a 2-fold increase in the enzyme yield by the immobilized cells than that of free cells (Anisha and Prema 2008). The study showed higher production of protease because the immobilized cell displayed better operational stability (Fortin and Vuillemard 1990; Kukubu et al. 1981), higher efficiency of catalysis (Ramakrishna et al. 1992). They are reusable (Mamo and Gessesse 2000) and greater resistance to environmental perturbations (Konsoula and Liakopoulou 2006).
Gel concentration has a significant effect on the enzyme production. The optimum concentration of alginate may differ with respect to the organism and the product of interest (Nampoothiri and Pandey 1998). In order to elucidate the effect of alginate used for the entrapment of B. megaterium, three different sodium alginate concentrations were tried. The production of alkaline protease was maximum with 4 % alginate as compared to other concentrations used (Fig. 5). These results were in agreement with the investigations of Shinmyo et al. (1982) and Dobreva et al. (1996) for the production of α-amylase by B. amyloliquefaciens and B. licheniformis, respectively. It has been reported that 2 % of gel concentration showed maximum production of lactic acid from pineapple waste using immobilized Lactobacillus delbrueckii (Idris and Suzana 2006). It has been shown that at higher gel concentration, the enzyme yields decreased due to poor diffusion of nutrients through the beads. However when 2 % gel concentration was used, the beads produced were too soft and fragile, resulting in cell leakage from the beads.
The maximum alkaline protease yields was attained for 3 mm bead diameter by the strain MTCC2444. A further increase in bead diameter beyond this value did not improve the enzyme production (Fig. 6). Smaller bead diameter produced higher protease production. This could be due to an increase in the surface volume ratio (Guoqiang et al. 1991; El-Katany et al. 2003). Similar pattern was also reported by Idris and Suzana (2006) for lactic acid production at 1 mm bead size for higher yield. An optimum bead diameter of 5.2 mm have been reported for alkaline protease production (Kocher and Mishra 2009).
In the present, study the optimum initial biomass was 1.5 % (Fig. 7). As the biomass concentration in the gel beads increased, the cell leakage into the fermentation medium also increased correspondingly. This could be attributed to the fact that when the cell loading in the beads increased, the nutrient/cell ratio decreased, which might become limiting (Beshay 2003). These results seemed to be in agreement with work performed by Anisha and Prema (2008) for αgalactosidase by S .griseoloalbus. Dobreva et al. (1996), working with immobilized B. licheniformis found that an optimum biomass concentration of 0.4 % for α-amylase production.
Prolongation of solidification time improves the stability of beads. The optimum solidification time for the maximum protease production by B. megaterium MTCC2444 was 12 h with subsequent decrease in cell leakage (Fig. 8). In contrast, the solidification time of 24 h, 1 h and 10 h improved the stabilities of gel beads entrapped with B. licheniformis (Dobreva et al. 1996), A. niger (Ellaiah et al. 2004) and S. griseoloalbus (Anisha and Prema 2008) for the production of α-amylase, lipase and α-galactosidase, respectively. Results for the different ages of beads showed that 24 h yielded maximum when compared with other aged beads (Fig. 9). Dobreva et al. (1996) found 36 h aged beads best for α-amylase production.
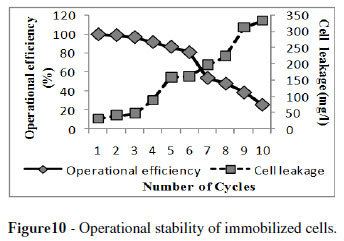
Whole cell immobilization of alkaline protease offered the advantage of using the immobilized cells in repeated batch fermentation processes. Gel concentration, bead diameter, biomass concentration, solidification time and age of immobilized cells are highly dependent on the operational stability and efficiency of the immobilized cells. The optimum conditions selected for the effective immobilization of B megaterium MTCC 2444 were 4 % gel concentration, 3 mm bead diameter, 1.5 % biomass concentration, solidification time of 12 h and 24 h aged immobilized cells. Under these conditions, alginate beads retained upto 95 % of their operational efficiency upto three successive fermentation cycles and beads retained 50 % ability of enzyme production upto eight cycles of fermentation (Fig. 10). The immobilized beads of S. griseoloalbus for α-galactosidase production could be reused efficiently without any loss of enzyme upto three cycles and beads had good stability and retained 75 % ability of enzyme production after eight cycles of repeated batch fermentation (Anisha and Prema 2008).
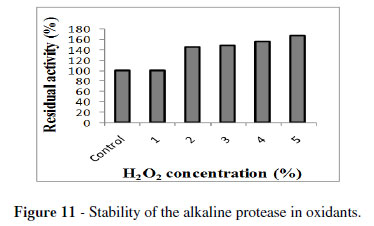
The enzyme showing stability towards the oxidizing agents are more promising in detergent industries as peroxides are common ingredients of modern bleach-based detergent formulations. In the present study, H2O2 at a concentration of 1 % showed 100 % residual activity. Whereas at 5 % concentration, there was 168 % increase in the residual activity (Fig.11). Previous reports on the stability of alkaline protease indicated that B. clausii I-52 protease exhibited residual activity upto 114 % after the treatment with 1 % H2O2 (Joo et al. 2003). While an alkaline protease from Vibrio fluvialis VM10 strain showed 132 % activity after incubation with 4 % H2O2 (Venugopal and Saramma 2006). A good detergent protease should have ability to work in the presence of surfactant active agents. The treatment with SDS in the concentration range of 0.05 to 0.6 % retained the protease activity from B. megaterium MTCC 2444 in the range of 70 to 75 % (Fig.12). These results were similar to the activity of protease of B. cereus MTCC6840 at 1 % concentration of SDS (Joshi et al. 2007). In contrast, VM10 protease showed 60 to 65 % activity towards 0.05 to 0.4 % SDS concentration (Venugopal and Saramma 2008). The results obtained in this study revealed that the enzyme was slightly inactivated and retained the activity in the range of 60 to 82 % at a concentration of 0.1 to 4 % Triton X-100 (Fig.13). Similar results were observed for VM10 protease (Venugopal and Saramma 2006). Among the various detergents tested in the present study, the maximum stability was obtained for Surf excel (46 %) after 1 h of incubation (Fig. 14). Similar results were obtained for VM10 protease with Surf retaining activity of 61 % (Venugopal and Saramma 2006). The studies on alkaline protease production by Bacillus sp. APR-4 on detergent stability showed that Fena and Farishta detergents retained stability of 78 % upto 24 h of incubation (Kumar and Bhalla 2004).
CONCLUSION
In the present study, the optimization of physical conditions and cell immobilization for alkaline protease production by B. megaterium was carried out. Under the optimum conditions, the enzyme yields were higher. Sodium alginate served as a good matrix for the entrapment of B. megaterium cells for alkaline protease production. In addition, the enzyme was much promising with regard to stability in the oxidants, surfactants and commercial detergents. These properties could make this protease as an ideal choice for application in the detergent formulations.
ACKNOWLEDGEMENT
The authors wish to acknowledge the Management, M.G.R.College, Hosur for providing laboratory facilities.
Received: Julho 28, 2010; Revised: January 02, 2011; Accepted: November 23, 2011.
- Abo-Aba SEM, Soliman EAM, Nivien AA. Enhanced production of extracellular alkaline protease in Bacillus circulans through plasmid transfer. Res J Agr Biol Sci 2006;2:526-530.
- Al-Shehri LM, Abdul-Rahman M, Yasser S. Production and some properties of protease produced by Bacillus licheniformis isolated from Tihamet Aseer, Saudi Arabia. Pak J Biol Sci 2004; 7:1631-1635.
- Anisha GS, Prema P. Cell immobilization technique for the enhanced production of α-galactosidase by Streptomyces griseoloalbus. Bioresour Technol. 2008; 99:3325-3330.
- Beshay U. Production of alkaline protease by Teredinobacter turnirae cells immobilized in Caalginate beads. Afr J 2003;2:60-65.
- Bhaskar N, Sudeepa ES, Rashmi HN, Tamil Selvi A. Partial purification and characterization of protease of Bacillus proteolyticus CFR 3001 isolated from fish processing waste and its antibacterial activities. Bioresour Technol 2007;98:2758-2764.
- Bladino A, Macias M, and Cantero D. Immobilization of glucose oxidase with calcium alginate gel capsules. Process Biochem 2001;36:601-606.
- Boominadhan U, Rajakumar R, Sivakumar PKV, Melvin MJ. Optimization of protease enzyme production using Bacillus sp. isolated from different wastes. Bot Res Intl. 2009;2:83-87.
- Cheng K, Lu FP, Li M, Liu LL, Liang XM. Purification and biochemical characterization of a serine alkaline protease TC4 from a new isolated Bacillus alcalophilus TCCC11004 in detergent formulations. Af J Biotech 2010;9:4942-4953.
- Dey G, Singh B, Banerjee R. Immobilization of αamylase produced by Bacillus circulans GRS 313. Braz Arch Biol Technol 2003;46:167-176.
- Dobreva E, Ivanova V, Tonkova A, Radulova E. Influence of the immobilization conditions on the efficiency of α-amylase production by Bacillus licheniformis. Process Biochem 1996;31:229-234.
- El-Katany MH, Ahmed MH, Gehan MS, El-Komy HM. Enzyme production by alginate encapsulated Trichoderma sp. Food Technol Biotech 2003;41:219-225.
- Ellaiah P, Prabhakar T, Ramakrishna B, Thaer Taleb A, Adinarayana K. Production of lipase by immobilized cells of Aspergillus niger. Process Biochem 2004;39:525-528.
- Fortin C, Vuillemard JC. Culture fluorescence monitoring of immobilized cells. Physiology of Immobilized Cells 1990;34:45-55.
- Guoqiang D, Kaul R, Mattiasson B. Evaluation of alginate immobilized Lactobacillus casei for lactate production. Appl Microbiol Biot. 1991;36:309-314.
- Gupta R, Beg QK, Khan S, Chauhan B. An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biot 2002;60:381-395.
- Idris A, Suzana W. Effect of sodium alginate concentration, bead diameter, initial pH and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochem 2006;41:1117-1123.
- Jaswal RK, Kocher GS, Virk MS. Production of alkaline protease by Bacillus circulans using agricultural residues: a statistical approach. Indian J Biotechnol 2008;7:356-360.
- Joo HS, Kumar CG, Park GC, Paik SR, Chang CS. Oxidant and SDS-stable alkaline protease from Bacillus clausii I-52: production and some properties. J Appl Microbiol 2003;95:267-272.
- Joshi GK, Kumar S, Sharma V. Production of moderately halotolerant, SDS-stable alkaline protease from Bacillus cereus MTCC 6840 isolated from lake Nainital, Uttaranchal state, India. Braz J Microbiol 2007;38:773-779.
- Kocher GS, Mishra S, Immobilization of Bacillus circulans MTCC 7906 for enhanced production of alkaline protease under batch and packed bed fermentation conditions. Internet J Microbiol 2009;7:359-378.
- Konsoula Z, Liakopoulou MK. Starch hydrolysis by the action of an entrapped in alginate capsules α-amylase from Bacillus subtilis. Process Biochem 2006;41:343-349.
- Kukubu T, Karube I, Suzuki S. Protease production by immobilized mycelia of Streptomyces fradiae. Biotechnology and Bioengineering 1981;23:29-37.
- Kumar CG, Tagaki H. Microbial alkaline proteases: from a bioindustrial viewpoint. Biotech Adv. 1999;17:561-594.
- Kumar D, Bhalla TC. Bacillus sp. APR-4 protease as a laundry additive. Indian J Biotechnol 2004;3:563-567.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 1951;193:265-273.
- Mamo G, Gessesse A. Immobilization of alkaliphilic Bacillus sp. Cells for xylanase production using batch and continuous culture. Appl Biochem Biotechnol 2000;87:95-101.
- Nagalakshmi R, Ramesh S. Studies on the production of protease by Bacillus sp. Recent Research in Science and Technology 2009;1:250-254.
- Nampoothiri KM, Pandey A. Immobilization of Brevibacterium cells for the production of L-glutamic acid. Bioresour Technol. 1998;63:101-106.
- Nampoothiri KM, Roopesh K, Chacko S, Pandey A. Comparative study of amidase production by free and immobilized Escherichia coli cells. Appl Biochem Biotechnol 2005;120:97-108.
- Norazizah S, Sayangku N, Abd-Rahman RNZ, Basri M, Salleh AB. Optimization of environmental and nutritional conditions for the production of alkaline protease by a newly isolated bacterium Bacillus cereus strain 146. J Appl Sci Res 2005;1:1-8.
- Oskouie SFG, Tabandeh F, Yakhchali B, Eftekhar F, Response surface optimization of medium composition for alkaline protease production by Bacillus clausii. Biochem Eng J. 2008;39:37-42.
- Qader SA, Iqbal S, Niazi Z. Partial purification and characterization of intracellular alkaline phosphatase from newly isolated strain of Bacillus subtilis KIBGE-HAS. Internet J Microbiol 2009;7:401-404.
- Ramakrishna SV, Jamuna R, Emery AN. Production of ethanol by immobilized yeast cells. Appl Biochem Biotechnol 1992;37:275-282.
- Reyed MR. Biosynthesis and properties of extracellular amylase by encapsulation of Bifidobacterium bifidum in batch culture. Aust J Basic Appl Sci. 2007;1:7-14.
- Sangeetha R, Geetha A, Arulpandi I. Optimization of protease and lipase production by Bacillus pumilus SG2 isolated from an industrial effluent. Internet J Microbiol 2008;5:1-9.
- Shaheen M, Aamer AS, Abdul H, Fariha H. Influence of culture conditions on production and activity of protease from Bacillus subtilis BS1. Pak J Bot 2008;40:2161-2169.
- Shinmyo A, Kimura H, Okada H. Physiology of αamylase production by immobilized Bacillus amyloliquefaciens. Eur J Appl Microbiol Biotechnol. 1982;14:7-12.
- Shumi W, Towhid Hossain MD, Anwar MN. Production of protease from Listeria monocytogenes. Int J Agr Biol. 2004;6:1097-1100.
- Venugopal M, Saramma AV. Characterization of alkaline protease from Vibrio fluvialis strain VM10 isolated from a mangrove sediment sample and its application as a laundry detergent additive. Process Biochem 2006;41:1239-1243.
Publication Dates
-
Publication in this collection
29 Mar 2012 -
Date of issue
Feb 2012
History
-
Received
28 July 2010 -
Accepted
23 Nov 2011 -
Reviewed
02 Jan 2011