Abstract
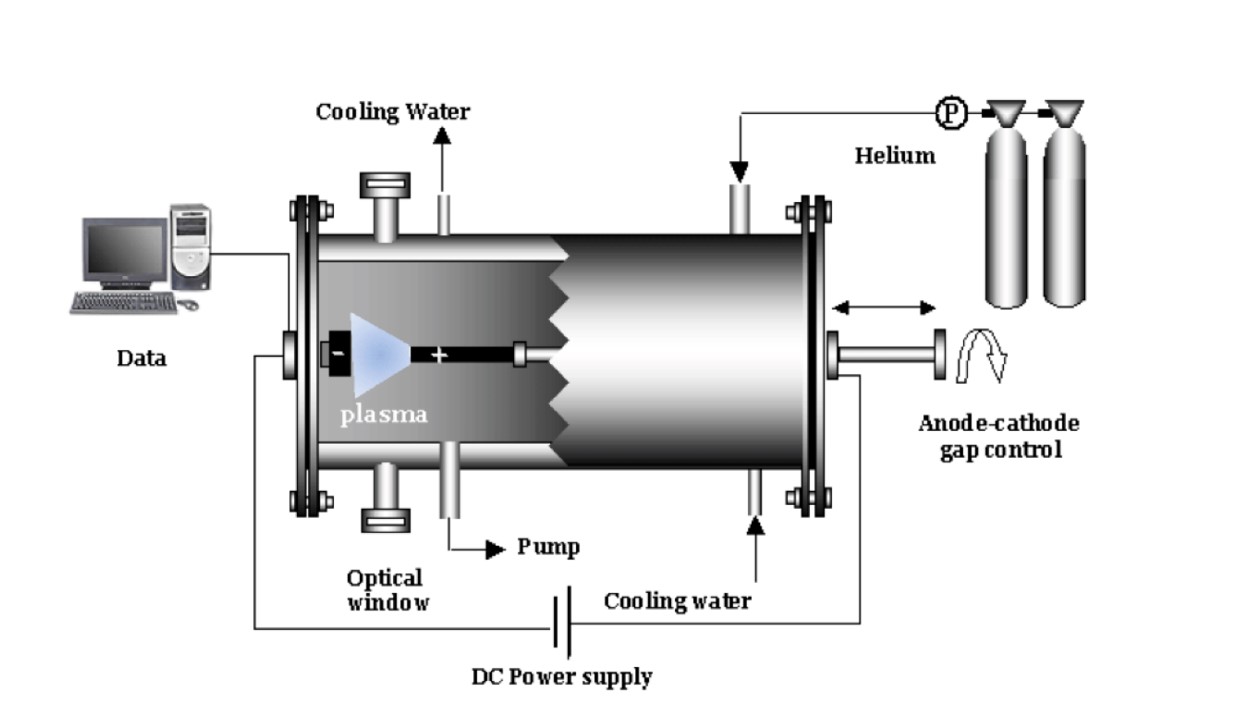
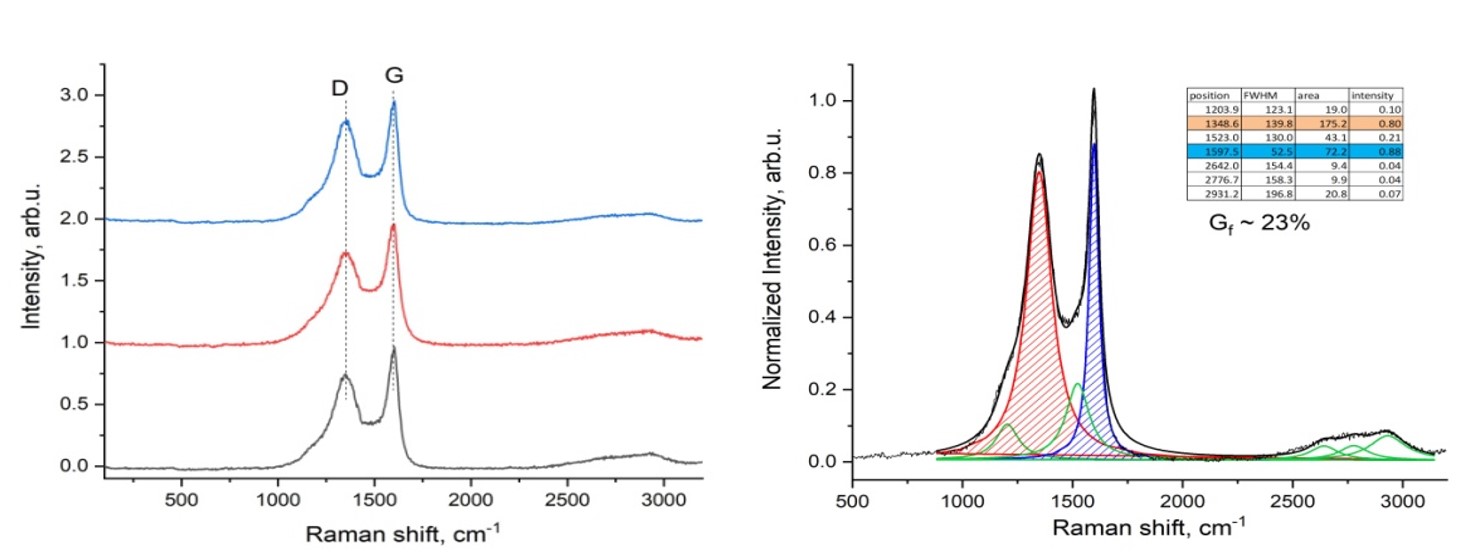
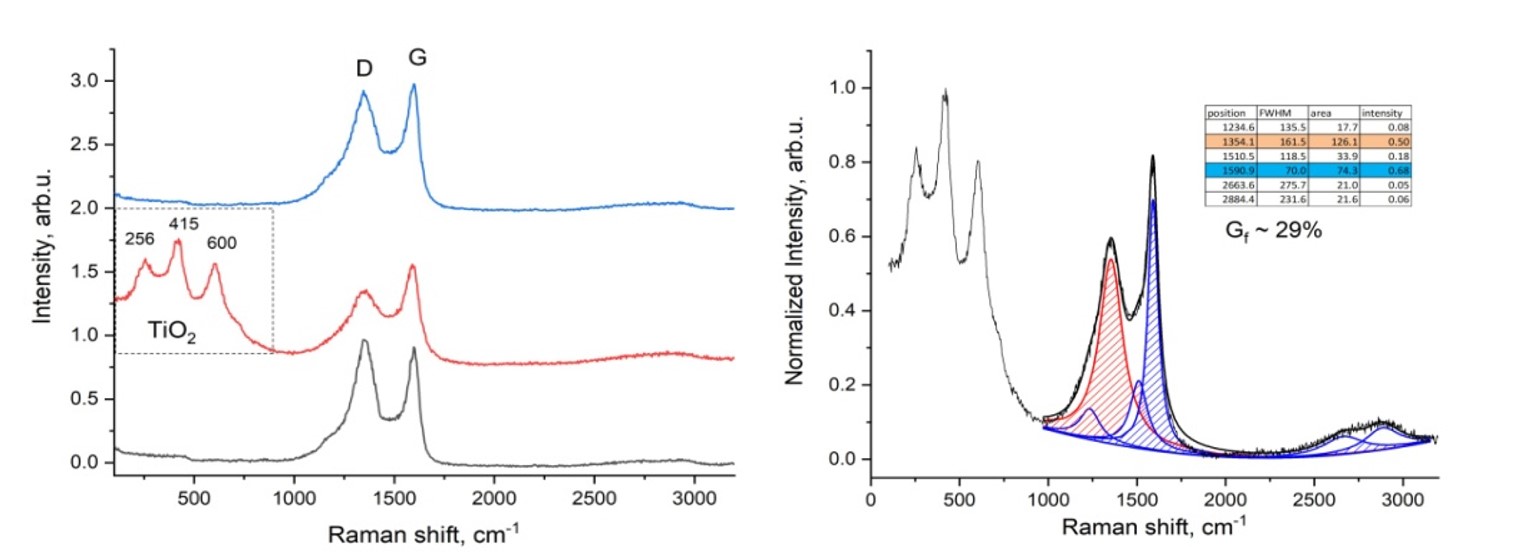
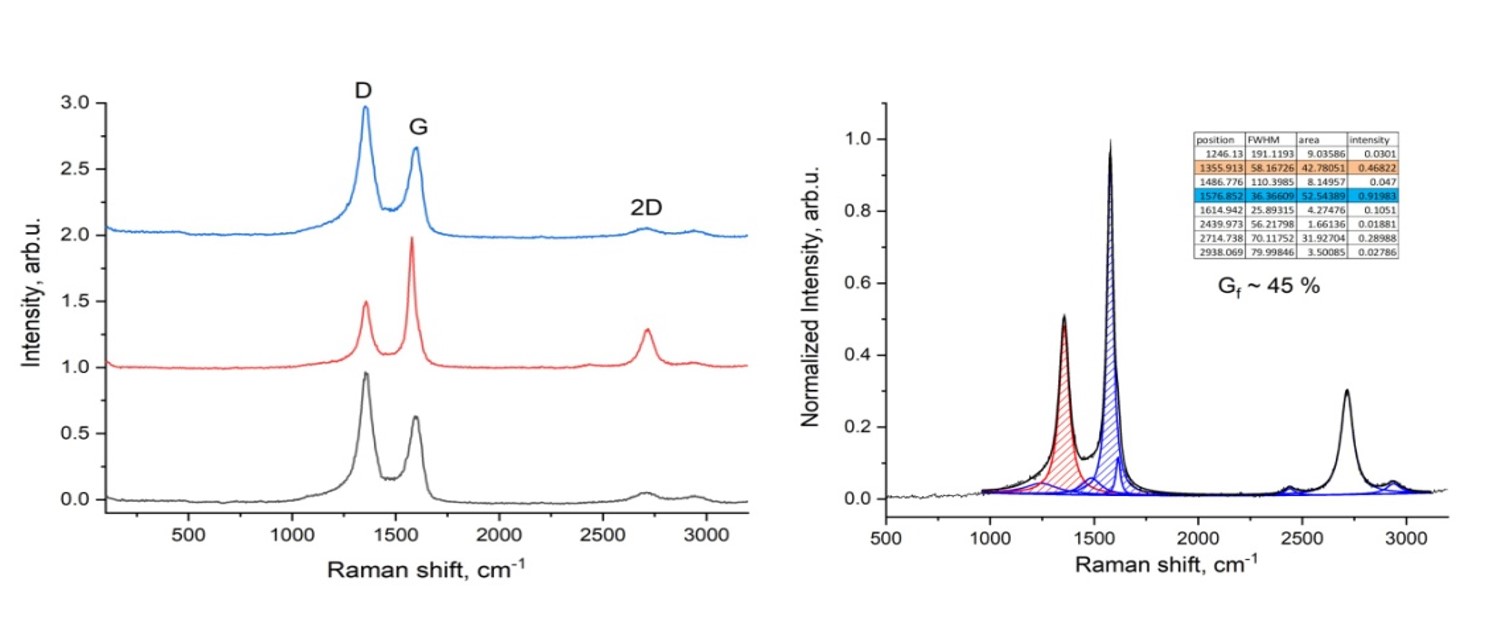
The increasing global consumption of plastic products has resulted in a growing accumulation of plastic waste, posing severe environmental challenges. The study aims to explore methods for recycling plastic macaque waste to produce carbon nanomaterials. Carbon nanomaterials were obtained via electric arc discharge from plastic waste processed at 1173 K in a nitrogen and water vapor environment. Key properties such as moisture, ash, and volatility were analyzed with a Thermoster Eltra analyzer. Pore volume, bulk density, pH, and adsorption activity were also assessed. This study addresses plastic waste pollution by converting it into porous carbon nanomaterials through pyrolysis at 900 °C. These materials, used as electrodes, produce graphene-forming nanomaterials via electric arc discharge. Analysis confirmed the composition using Raman spectroscopy, X-ray diffraction, and gas chromatography. The study reveals that the electrical conductivity of the synthesized carbon nanomaterials is close to that of graphite, with a reduction in electrical resistance of up to 3.6 times compared to the initial carbonized material. The process yields valuable products like nanomaterials, hydrogen, and flammable gases. This research presents an innovative and sustainable approach for the recycling of plastic waste into graphene-forming carbon nanomaterials using electric arc discharge.
Keywords:
plastic; carbonization; electric arc method; graphite; carbon

 Plastic waste recycling for the production of graphene nanomaterials using electric arc discharge
Plastic waste recycling for the production of graphene nanomaterials using electric arc discharge Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail