Abstract
Excessive activation of osteoclasts during bone infections can result in destructive bone complications, including non-union and delayed fracture healing. Enterococcus faecalis and Streptococcus pyogenes are known pathogens associated with bone and joint infections, which can lead to severe complications and the deterioration of tissue. This study aimed to investigate the potential of mechano-bacteria intervention in combating these bacteria. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) tests were conducted on Polycaprolactone (PCL) and PCL/graphene (G) scaffolds containing different concentrations of graphene, namely 0.5, 1.5, and 2.5 wt%, to evaluate the thermal impact on the activity of E. faecalis and S. pyogenes. Furthermore, the Kirby Bauer Method was used to assess the antibacterial activity against both bacteria. The analysis of the average inhibition zone showed a correlation between the antibacterial effect and the concentration of G within the scaffolds. The highest inhibition zone was observed when 2.5 wt% G was used for both E. faecalis and S. pyogenes. The higher Tmax from Thermogravimetric Analysis showed PCL/G with 2.5 wt% G was due to the greater heat energy required to break down sp2 hybridized carbon atoms in a hexagonal framework. The TGA results indicate that higher graphene content (2.5 wt%) requires more energy for thermal decomposition compared to lower concentrations (0.5 wt% and 1.5 wt%) and PCL while the DSC results also showed that presence of G had a significant impact on Tg (glass transition temperature), Tc (crystallization temperature), and Tm (melting temperature), as the temperature increased with the addition of G. Based on the result of this study, it was concluded that G had potential for inhibiting bacteria growth.
Keywords:
polycaprolactone; graphene; scaffold; Enterococcus faecalis; Streptococcus pyogenes
Resumo
A ativação excessiva dos osteoclastos durante infecções ósseas pode resultar em complicações ósseas destrutivas, incluindo não união e cicatrização retardada de fraturas. Enterococcus faecalis e Streptococcus pyogenes são patógenos conhecidos associados a infecções ósseas e articulares, que podem levar a complicações graves e deterioração dos tecidos. Este estudo teve como objetivo investigar o potencial da intervenção mecanobacteriana no combate a essas bactérias. Análises Termogravimétricas (TGA) e Calorimetria Exploratória Diferencial (DSC) foram realizadas em estruturas de Policaprolactona (PCL) e PCL/grafeno (G) contendo diferentes concentrações de grafeno, a saber, 0,5%, 1,5% e 2,5% em peso, para avaliar o impacto térmico na atividade de E. faecalis e S. pyogenes. Além disso, o Método de Kirby Bauer foi utilizado para avaliar a atividade antibacteriana contra ambas as bactérias. A análise da média da zona de inibição mostrou uma correlação entre o efeito antibacteriano e a concentração de G nas estruturas. A maior zona de inibição foi observada quando 2,5% em peso de G foi usado tanto para E. faecalis quanto para S. pyogenes. O maior Tmax da Análise Termogravimétrica mostrou que PCL/G com 2,5% em peso de G foi devido à maior energia térmica necessária para quebrar átomos de carbono hibridizados sp2 em uma estrutura hexagonal. Os resultados da TGA indicam que um maior teor de grafeno (2,5% em peso) requer mais energia para decomposição térmica em comparação com concentrações mais baixas (0,5% e 1,5% em peso) e PCL, enquanto os resultados da DSC também mostraram que a presença de grafeno teve um impacto significativo em Tg (temperatura de transição vítrea), Tc (temperatura de cristalização) e Tm (temperatura de fusão), à medida que a temperatura aumentou com a adição de G. Com base nos resultados deste estudo, concluiu-se que o G tinha potencial para inibir o crescimento bacteriano.
Palavras-chave:
policaprolactona; grafeno; estrutura; Enterococcus faecalis; Streptococcus; pyogenes
1. Introduction
Bone remodeling is a complex process including the resorption of old or damaged bone followed by the deposition of new material. This dynamic process is regulated by various factors and plays a significant role in bone growth and repair, as well as the regulation of calcium and phosphate metabolism in the body. Previous studies have shown that osteoclasts and osteoblasts are responsible for bone resorption and formation, respectively. Abnormal activation of osteoclasts during bone infections leads to pathological destruction and complications, such as non-union and delayed fracture healing (Borggaard et al., 2020). Streptococcus pyogenes is a bacteria strain recognized for its propensity to cause bone infection, which result in severe complications and the destruction of bone tissue. Furthermore, osteoclasts, which are responsible for removing old bone, play a significant role in this process (Yokohata et al., 2023). Previous studies found that Streptolysin O (SLO), produced by most clinical isolates of S. pyogenes, inhibits the activation of osteoclasts and induces cell death in mature osteoclasts. This inhibition helps to prevent osteoclast activation during Group A Streptococcal (GAS) infections (Cobos et al., 2020).
According to (Yi et al., 2019), bone remodeling is a process that includes the resorption of old or damaged bone, followed by the deposition of new material. This dynamic process is regulated by various factors and plays a significant role in bone growth, repair, and the regulation of calcium and phosphate metabolisms in the body. Osteoclasts and osteoblasts are responsible for bone resorption and formation, respectively. Abnormal activation of osteoclasts during bone infections results in pathological bone destruction and complications, such as non-union and delayed fracture healing. A previous study showed that S. pyogenes is a bacteria strain known to cause bone infections (Martin A. et al., 2023) resulting in severe complications and destruction (Rennz et al., 2019). Osteoclasts, the cells responsible for bone resorption, play a significant role in this process. Furthermore, SLO, produced by most clinical isolates of S. pyogenes, inhibits the activation of osteoclasts and induces apoptosis in mature osteoclasts. This process blocks the activation of osteoclast during GAS infection, as reported by a previous study (Yi et al., 2019).
Enterococcus faecalis was identified as a pathogen responsible for infections in the joints surrounding prosthetic devices. These infections often manifest as a part of larger components including multiple micrograms. The treatment of E. faecalis poses significant challenges due to low virulence, slow ability to kill bacteria through antimicrobial agents, tolerance to antimicrobials, and growing resistance (Diez-Pascual, 2021).
Heat can be used to kill bacteria through a process known as thermal disinfection. When a material is subjected to high temperatures, the heat energy is transferred to the bacteria, causing the denaturation of proteins and cell membranes, thereby rresulting in destruction (Lemme et al., 2022). The mechanism of graphene (G) heat to kill bacteria includes several key processes and interactions. The references provided offer valuable insights into the diverse mechanisms through which G and graphene-based materials exert antibacterial effects. These mechanisms include oxidative and membrane stress, electron transfer, direct contact destruction, and the generation of reactive oxygen species. Additionally, the sharp edges and unique properties of G contribute to the disruption of bacteria membranes, leading to cell death (Diez-Pascual, 2021; Li et al., 2022).
Graphene is highly versatile in various fields due to the exceptional electrical, mechanical, and thermal properties. The high electrical conductivity and thermal stability make G an ideal material for integration with polycaprolactone (PCL) to develop membranes with enhanced antimicrobial properties. By dispersing G within the PCL matrix, the resulting membrane gains the ability to generate localized heat upon exposure to an external source. This process can be precisely controlled, allowing for targeted disruption of bacteria membranes while minimizing damage to surrounding healthy tissues. According to a previous study, the combination of PCL and G offered a unique method of combating bacterial infection (Anitasari et al., 2023).
The antimicrobial activity of G was also attributed to the inducing ability of oxidative stress and disrupting the structural integrity of bacteria cells, leading to destruction (Nasker et al., 2023). Furthermore, the incorporation of G into PCL, has been shown to enhance antimicrobial activity through several mechanisms, including physical destruction, oxidative stress, and lipid extraction (Budi et al., 2023). The antimicrobial properties of G have been explored in diverse applications, including wound dressing, dental materials, and orthopaedic implants (Mohammed et al., 2020). However, only limited data is available, on tolerance to antimicrobials and growing resistance. Limited data is also available on antibiotics that are effective against S. pyogenes and E. faecalis, which have been previously classified as difficult to treat. Several previous studies also showed high rates of treatment failure. In this study, a mechano-bacteria were explored as a method of combating bacteria. Specifically, the characteristics of the PCL/G were used to disrupt the bacteria growth.
2. Materials and Methods
2.1. Fabrication PCL/G
The scaffolds PCL and PCL/G were created using a method called solvent casting and particulate leaching method. The PCL obtained from Sigma-Aldrich in the USA, was dissolved in trichloromethane (Honeywell, USA) in a ratio of 1:10 w/v at room temperature for 12 hours. Graphene with varying weight percentages, such as 0.5%, 1.5%, and 2.5%, as well as NaCl were added to the blend and mixed for 2 hours. After the preparation, the blend was poured into a mold and left to dry at room temperature for 1 day. Afterward, the scaffolds were subjected to evaporation in a drying vacuum oven (Deng Yng, Taiwan) at 35°C for 24 hours to ensure complete evaporation of trichloromethane. The scaffold was then soaked in deionized water and placed in a water bath (BH-130D, Taiwan) to remove the porogen for 24 hours. Then drying in oven with temperature 50°C for 24 hours (Anitasari et al., 2023).
2.2. Antibacterial analysis (Kirby Bauer Method)
The antibacterial properties of PCL/G were evaluated in the laboratory of Medical Microbiology at the Medical Faculty of Universitas Mulawarman. The study followed the CLSI M02 (Zimmer, 2022) which 30 µg tetracycline disc and chloroform (0.001wt%) were used as positive and negative controls, respectively. Antibacterial analysis commenced with the preparation of inoculum and adjusted to a concentration of 108 CFU/mL, using McFarland standard 0.5 as a reference. The S. pyogenes and E. faecalis bacteria were then spread on Mueller-Hinton Agar (MHA). Subsequently, the PCL/G samples were impregnated on the agar. The plates were incubated at 37°C overnight, allowing the bacteria to grow. The diameters of the inhibition zone surrounding the samples were measured (Yuniati et al., 2018).
2.3. Thermal analysis
Thermal properties were analyzed using Thermogravimetric Analysis (TGA) and Different Scanning Calorimetry (DSC) with a Linseis instrument (Germany). A 5 mg sample was heated at a rate of 10 /min in an air atmosphere. The temperature range was from 0 to 1000 °C, with the sample held at the target temperature for 2 minutes to remove any prior thermal effects. Various parameters were derived from the resulting thermodynamic curves (Babaie et al., 2019).
2.4. Statistical analysis
All experiments in this study were repeated at least three times to ensure reliability and accuracy. Each experiment was conducted in triplicate and the error bars in the graphs represented the standard error (SE). Statistical analyses were carried out using SPSS software. The obtained data for different samples were compared using a one-way ANOVA test at a significance level of p< 0.05 (Unagolla and Jayasuriya, 2019).
3. Results
3.1. Antibacterial analysis
The impact of G presence in the PCL/G scaffold was assessed by testing various concentrations to determine the effect on the activity of E. faecalis and S. pyogenes bacteria. The result showed a clear relationship between the antibacterial effect and the concentration of G in the scaffolds, as indicated in Table 1 and Figure 1. Specifically, the highest inhibition zone was observed when using 2.5 wt% G for both E. faecalis (p<0.05) and S. pyogenes (p<0.05), while the smallest was found with 1.5 wt% and 0.5 wt%, respectively. The average inhibition zone for 2.5 wt% G was 19.5 0.3 mm in E. faecalis and 14.3 2.2 in S. pyogenes.
Zone of inhibition of E. faecalis and S. pyogenes. The data showed that there was significantly different activity between various concentrations of graphene in E. faecalis and S. pyogenes.
Zone of inhibition of E. faecalis and S. pyogenes. The data showed that PCL/G scaffolds had antibacterial activity, especially 2.5 wt% of G.
3.2. Thermal analysis
Thermal weight loss analysis was conducted on three samples at a heating rate of 20 °C/min. The TGA and DSC curves were obtained, as shown in Figure 2 and Figure 3. These graphs illustrate that the thermal degradation of PCL and PCL/G occurs completely in an air atmosphere below 1000 °C. Key mass loss events of G can be identified by their decomposition temperatures in the TGA graphs: RI (<100 °C) corresponds to water evaporation, RII (100-360 °C) to the decomposition of oxygen-containing groups and RIII (360-1000 °C) to carbon combustion.
TGA graphs from PCL and PCL/G containing different concentration 0.5 wt%, 1.5 wt% and 2.5 wt%, showing their typical signatures with characteristics TGA peaks and peak position (Tmax).
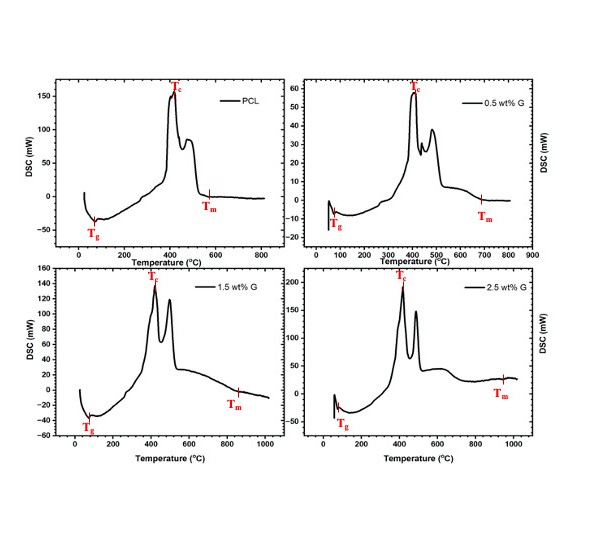
DSC graphs of PCL and PCL/G containing different concentrations (0.5 wt%, 1.5 wt%, and 2.5 wt%) of graphene were analyzed.
Notably, the first and second TGA peaks correspond to the loss of water and oxygen functional groups. The third mass loss step in PCL and PCL/G was attributed to carbon combustion in air. These TGA peaks vary significantly when different sizes of carbonaceous materials were analyzed, which is the primary focus of our study and will be elaborated on in subsequent sections. As depicted in the TGA graphs (Figure 2), the carbon combustion temperature was marked by the temperature of maximum mass change rate (Tmax), with PCL showing the earliest carbon combustion (Tmax = 440 ± 0.3) and PCL/G varying G concentration: 0.5 wt% G (Tmax= 421 ± 0.5), 1.5 wt% (Tmax= 440 ± 0.0), 2.5 wt% G (Tmax= 460 ± 0.3). The differences in Tmax can be attribute to the maximum external heat energy needed to break down non-graphitic sp3 hybridized carbon with high defects density following rigorous oxidation, compared to PCL and PCL/G with 2.5 wt% G required higher Tmax (p<0.05).
The obtained TGA results and Tmax values can be explained as 2.5 wt% G require the highest temperature and energy for its thermal decomposition compared to PCL, 0.5 wt%, and 1.5 wt% G which need less energy and experience more rapid degradation. The RIII region represents the combustion of carbon in air and these pronounced TGA peaks can be correlated to their respective carbon lattice structures with respects to the external heat energy supplied to oxidize the carbon framework (Farivar et al., 2021; Rodriguez-Estupinan et al., 2022).
The relationship between the flow of heat and the temperature of materials was examined using DSC. In this study, DSC test was conducted on PCL and PCL/G scaffolds with varying amount of G, such as 0.5, 1.5, and 2.5 wt% G. Based on the data in Table 2 and Figure 3, it was evident that the introduction of 2.5 wt% G increased Tg (glass transition temperature) from 70°C for PCL to 90°C for the 2.5 wt% G. This result showed that G had a significant impact on Tc (crystallization temperature) as well, as Tc increased with the addition of G. Furthermore, the Tm (melting temperature) of scaffolds containing 2.5 wt% of G was measured at 950°C, which was 59% higher than that of PCL (p<0.05). This increased lamellae thickness within the scaffold structure.
DSC result of PCL/G scaffolds. The data showed that graphene had an effect on the thermal properties of PCL.
4. Discussion
The thermal behavior of PCL/G scaffolds and the ability to release antibacterial agents at specific temperatures have attracted significant attention due to the potential impact on bacteria growth in real-world applications (Yaragalla et al., 2021). TGA and DSC plays a significant role in characterizing the thermal conditions that trigger the release of antibacterial agents from PCL/G scaffolds, thereby having an indirect effect on bacteria growth. Furthermore, the interaction between the thermal properties of the material and the susceptibility of bacteria membranes to temperature changes contributes to the complex nature of the thermal reaction between PCL/G scaffolds and bacteria membranes (Huang et al., 2020).
Graphene, known for its distinctive physical properties, can potentially disrupt bacterial membranes. Its extensive specific surface area and robust structural stability enhance its role as a thermal interface material, facilitating heat transfer to bacterial membranes. At elevated temperatures, the interaction between G and bacterial membranes may become more pronounced, leading to localized heating and subsequent destabilization of the membranes (Troncoso and Torres, 2020). Evaluating the thermal stability of scaffolds is crucial when examining the interaction between PCL/G and bacterial membranes. Scaffolds that retain their structural integrity at higher temperatures have a more pronounced impact on bacterial membranes during thermal reactions (Huang et al., 2020; Memisoglu et al., 2023).
In materials science, the relationship between weight and thermal properties concerning antibacterial characteristics has been a subject of significant research interest. Several studies have delved into the intricate interplay between these factors to develop materials with enhanced antibacterial efficacy while maintaining thermal stability. For instance, study by Zheng et al. (2019) have highlighted the importance of thermal stability in materials imbued with antibacterial agents. Zheng et al. (2019) demonstrated that antibacterial agents exhibited good thermal stability, a crucial aspect in ensuring the durability and functionality of antibacterial plastics. Some PCL/G scaffolds are specifically designed to exhibit antibacterial effects triggered by temperature. This process releases antibacterial agents or engages in targeted interactions with membranes at specific temperatures, thereby impacting viability (Yaragalla et al., 2021). The impact of thermal reactions on bacteria membranes varies depending on the specific species and strains, as bacteria exhibit different sensitivity levels to changes in temperature (Huang et al., 2020).
E. faecalis and S. pyogenes are two bacteria that commonly infect humans and cause a range of health issues. E. faecalis is a leading cause of hospital-acquired infections, particularly in patients with compromised immune systems. Furthermore, S. pyogenes, also known as group A Streptococcus, is responsible for a variety of infections, such as skin and strep throat (Tadesse et al., 2023). These bacteria possess virulence factors that facilitate the invasion of the immune system and the establishment of infections. E. faecalis can form biofilms, which are communities of bacteria encased in a protective extracellular matrix, thereby enabling high resistance to antibiotics. S. pyogenes produces several toxins that contribute to tissue damage and the spread of infection (Alangari et al., 2023).
In the development of effective strategies to combat pathogens, it is important to understand the mechanisms of bacteria growth and survival. According to a previous study, the integration of PCL and graphene-dispersed membranes presents a potential solution to disrupting the growth and survival of E. faecalis and S. pyogenes (Kim et al., 2023).
Several studies have investigated the effects of PCL/G on E. faecalis. (Martini et al., 2020) showed that PCL/G membranes effectively inhibited the growth of E. faecalis in vitro. A significant reduction in bacteria viability was observed when exposed to the PCL/G membrane compared to the control. The findings are consistent with the results from the study on PCL/G scaffolds, which also noted a marked antibacterial effect against E. faecalis. However, the study on PCL/G scaffolds delves deeper into the thermal properties of the material, indicating that the heat generated by the membrane upon exposure to an external hat source plays a crucial role in disrupting bacterial membranes, leading to cell damage and death (Liu et al., 2020)This thermal mechanism is less emphasized in Martini et al. (2020) findings, which primarily focus on the direct antibacterial effects of the PCL/G membranes.
Building on this, Kumar et al. (2019) further elucidated the role of thermal properties in the antibacterial efficacy of PCL/G membranes, suggesting that the heat generated can induce structural changes in the bacterial cell wall, contributing to the observed antibacterial effects (Tollu and Ekin, 2021). This aspect of thermal disruption is a significant addition to the understanding of how PCL/G scaffolds operate against E. faecalis, as it highlights a dual mechanism of action; the inherent antibacterial properties of graphene and the thermal effects induced by the PCL matrix. This duality is not commonly addressed in earlier studies, which often focus on either the chemical on either the chemical or physical properties of the materials without integrating both perspectives.
Similarly, Nguyen et al. (2022) also contributed to the discourse on the antibacterial properties of graphene, nothing its effectiveness in inhibiting E. faecalis growth through mechanisms that involve membrane disruption and oxidative stress (Venanzio et al., 2019). This finding aligns with the conclusions drawn in the study on PCL/G scaffolds, reinforcing the idea that graphene’s intrinsic properties significantly enhance the antibacterial efficacy of the composite material. However, the study on PCL/G scaffolds uniquely emphasizes the importance of thermal stability and the resultant heat generation, which is a relatively novel perspective in the context of antibacterial materials.
In contrast, previous research has often focused on alternative antibacterial agents or methods, such as the use of novobiocin, which inhibits membrane synthesis in E. faecalis protoplasts (Xiang et al., 2022). While these studies provide valuable insights into the mechanisms of action against E. faecalis, they do not explore the thermal properties of materials like PCL/G, which may offer a more comprehensive approach to combating bacterial infections. The incorporation of thermal dynamics into the evaluation of antibacterial materials represents a significant advancement in the field, as it opens new avenues for enhancing the efficacy of existing materials.
In addition to studies on E. faecalis, PCL/G scaffolds have shown promising results against S. pyogenes. Lee et al. (2018) conducted an experiment to evaluate the antimicrobial activity of PCL/G scaffolds against S. pyogenes. The result showed a significant reduction in bacteria viability when exposed to the membrane compared to the control.
The thermal disruption mechanism of the PCL/G scaffolds played a significant role in inhibiting S. pyogenes growth. Furthermore, the heat generated upon exposure to an external source disrupted the bacteria cell membrane, leading to cell death. The antimicrobial properties of G also enhanced the effectiveness of the scaffolds in combating S. pyogenes infections. Wang et al. (2019), investigated the ability of PCL/G membranes to eradicate S. pyogenes biofilms. The result showed that the membranes effectively disrupted the biofilm structure and prevented the reformation of biofilms. Based on this result, it was concluded that PCL/G membranes have the potential to eradicate persistent S. pyogenes infections and prevent recurrence.
Comparing the effectiveness of PCL/G disruption against E. faecalis and S. pyogenes requires consideration of individual properties and synergistic effects. PCL provides an efficient method of thermal disruption due to the low melting point and biocompatibility (Papanikolaou et al., 2023). Furthermore, the ability to transition from a solid to a liquid state at relatively low temperatures allows for the penetration of bacteria cell walls, leading to membrane damage and death. PCL also offers the advantage of controlled heat generation, minimizing the risk of damage to surrounding healthy tissues (Williams et al., 2023). Graphene enhances the overall effectiveness of the scaffold due to the high electrical conductivity and antimicrobial properties. This electrical conductivity allows for the generation of localized heat upon exposure to an external source, thereby disrupting bacteria membranes. Additionally, the antimicrobial properties of G contribute to the inhibition of bacteria growth and the prevention of biofilm formation (Bhatt et al., 2023; Nasker et al., 2023).
The combination of PCL/G in the scaffold produced a synergistic effect and maximized disruption, as well as increased antimicrobial efficacy (Ding et al., 2020). The electrical conductivity and antimicrobial properties of G were complemented by the thermal properties of PCL, resulting in a highly effective scaffold for combating bacterial infections (Hitscherich et al., 2018).
5. Conclusion
The integration of PCL and graphene-dispersed scaffolds presented a promising solution to the persistent threat of bacterial infections. Through the harnessing of thermal properties and the conductivity of G, scaffolds effectively disrupted the growth and survival of E. faecalis and S. pyogenes. Furthermore, the thermal disruption mechanisms of PCL, combined with the antimicrobial properties of G, produced a synergistic effect that maximized the disruption of bacteria membranes. This disruption resulted in the death of the bacteria, thereby preventing the establishment and spread of infections.
Acknowledgements
We express our gratitude to all those who contributed to this research. This research was supported by Medical Faculty, Universitas Mulawarman.
References
-
ALANGARI, A., MATEEN, A., ALQAHTANI, M., SHAHID, M., SYED, R., SHAIK, M., KHAN, M., ADIL, S. and KUNIYIL, M., 2023. Antimicrobial, anticancer, and biofilm inhibition studies of highly reduced graphene oxide (HRG): in vitro and in silico analysis. Frontiers in Bioengineering and Biotechnology, vol. 11, pp. 1149588. http://doi.org/10.3389/fbioe.2023.1149588 PMid:37025362.
» http://doi.org/10.3389/fbioe.2023.1149588 -
ANITASARI, S., WU, C. and SHEN, Y.K., 2023. PCL/Graphene scaffolds for the osteogenesis. Bioengineering (Basel, Switzerland), vol. 10, no. 3, pp. 305. http://doi.org/10.3390/bioengineering10030305 PMid:36978696.
» http://doi.org/10.3390/bioengineering10030305 -
BABAIE, A., REZAEI, M. and SOFLA, R., 2019. Investigation of the effects of polycaprolactone molecular weight and graphene content on crystallinity, mechanical properties and shape memory behavior of polyurethane/graphene nanocomposites. Journal of the Mechical Behavior of Biomedical Materials, vol. 96, pp. 53-68. http://doi.org/10.1016/j.jmbbm.2019.04.034 PMid:31029995.
» http://doi.org/10.1016/j.jmbbm.2019.04.034 -
BHATT, S., PATHAK, R., PUNETHA, V. and PUNETHA, M., 2023. Recent advances and mechanism of antimicrobial efficacy of graphene-based materials: a review. Journal of Materials Science, vol. 58, no. 19, pp. 7839-7867. http://doi.org/10.1007/s10853-023-08534-z PMid:37200572.
» http://doi.org/10.1007/s10853-023-08534-z -
BORGGAARD, X.G., PIRAPAHARAN, D.C., DELAISSE, J.M. and SOE, K., 2020. Osteoclasts’ ability to generate trenches rather than pits depends on high levels of active cathepsin K and efficient clearance of resorption products. International Journal of Molecular Sciences, vol. 21, no. 16, pp. 5924. http://doi.org/10.3390/ijms21165924 PMid:32824687.
» http://doi.org/10.3390/ijms21165924 -
BUDI, H.S., ANITASARI, S., SHEN, Y.K., TANGWATTANACHULEEPORN, M., NURAINI, P. and SETIABUDI, N.A., 2023. Novel application of 3D scaffolds of poly(E-caprolactone)/graphene as osteoinductive properties in bone defect. European Journal of Dentistry, vol. 17, no. 3, pp. 790-796. http://doi.org/10.1055/s-0042-1755550 PMid:36351454.
» http://doi.org/10.1055/s-0042-1755550 -
COBOS, M., PINTA, I.D.L., QUINDOS, G., FERNANDEZ, M.J. and FERNANDEZ, M.D., 2020. Graphene oxide-silver nanoparticle nanohybrids: synthesis, characterization, and aantimicrobial properties. Nannomaterials, vol. 10, no. 2, pp. 376. http://doi.org/10.3390/nano10020376 PMid:32098083.
» http://doi.org/10.3390/nano10020376 -
DIEZ-PASCUAL, A.M., 2021. State of the art in the antibacterial and antiviral appliactions of carbon-based polymeric nanocomposites. International Journal of Molecular Sciences, vol. 22, no. 19, pp. 10511. http://doi.org/10.3390/ijms221910511 PMid:34638851.
» http://doi.org/10.3390/ijms221910511 -
DING, Z., HE, F., LI, Y., JIANG, Z., YAN, H., HE, R., FAN, J., ZHANG, K. and YANG, W., 2020. Novel shape-stabilized phase change materials based on parafilm/EPDM@graphene with high thermal conductivity and low leakage rate. Energy & Fuels, vol. 34, no. 4, pp. 5024-5031. http://doi.org/10.1021/acs.energyfuels.9b04000
» http://doi.org/10.1021/acs.energyfuels.9b04000 - FARIVAR, F., YAP, P., KARUNAGARAN, R. and LOSIC, D., 2021. Thermogravic Analysis (TGA) of graphene materials: effect of particle size of graphene, graphene oxide and graphite on thermal parameters. Carbon, vol. 7, no. 2, pp. 41.
-
HITSCHERICH, P., APHALE, A., GORDAN, R., WHITAKER, R., SINGH, P., XIE, L., PATRA, P. and LEE, E., 2018. Electroactive graphene composite scaffolds for cardiac tissue engineering. Journal of Biomedical Materials Research. Part A, vol. 106, no. 11, pp. 2923-2933. http://doi.org/10.1002/jbm.a.36481 PMid:30325093.
» http://doi.org/10.1002/jbm.a.36481 - HUANG, L., XU, S., WANG, Z., XUE, K., SU, J., SONG, Y., CHEN, S., ZHU, C., TANG, B., and YE, R., 2020. Self-reporting and photothermally enhanced rapid bacterial killing on a laser-induced graphene mask. ACS nano Journal, vol. 14, no. 9, pp. 12045-12053.
-
KIM, M., ROSA, V. and MIN, K., 2023. Effect of two graphene derivatives on Enterococcus faecalis biofilms and cytotoxicity. Dental Materials Journal, vol. 42, no. 2, pp. 211-217. http://doi.org/10.4012/dmj.2022-095 PMid:36543190.
» http://doi.org/10.4012/dmj.2022-095 -
KUMAR, P., HUO, P., ZHANG, R. and LIU, B., 2019. Antibacterial properties of graphene-based nanomaterials. Nanomaterials (Basel, Switzerland), vol. 9, no. 5, pp. 737. http://doi.org/10.3390/nano9050737 PMid:31086043.
» http://doi.org/10.3390/nano9050737 -
LEE, E.Y., WONG, G.C.L. and FERGUSON, A.L., 2018. Machine learning-enabled discovery and design of membrane-active peptides. Bioorganic & Medicinal Chemistry, vol. 26, no. 10, pp. 2708-2718. http://doi.org/10.1016/j.bmc.2017.07.012 PMid:28728899.
» http://doi.org/10.1016/j.bmc.2017.07.012 -
LEMME, M.C., AKINWANDE, D., HUYGHEBAERT, C. and STAMPFER, C., 2022. 2D materials for future heterogenous electronics. Nature Communications, vol. 13, no. 1, pp. 1392. http://doi.org/10.1038/s41467-022-29001-4 PMid:35296657.
» http://doi.org/10.1038/s41467-022-29001-4 -
LI, M., CHEN, Z., YANG, L., LI, J., XU, J., CHEN, C., WU, Q., YANG, M. and LIU, T., 2022. Antibacterial activity and mechanisms of GO/Cu2O/ZnO coating on ultrafine glass fiber. Nanomaterials (Basel, Switzerland), vol. 12, no. 11, pp. 1857. http://doi.org/10.3390/nano12111857 PMid:35683713.
» http://doi.org/10.3390/nano12111857 -
LIU, F., PANPAN, J., GONG, H., SUN, Z., DU, L. and WANG, D., 2020. Antibacterial and antibiofilm activities of thyme oil against foodborne multiple antibiotics-resistant Enterococcus faecalis. Poultry Science, vol. 99, no. 10, pp. 5127-5136. http://doi.org/10.1016/j.psj.2020.06.067 PMid:32988551.
» http://doi.org/10.1016/j.psj.2020.06.067 -
MARTIN, A., LOUBET, P., SALIPANTE, F., LAFFONT-LOZES, P., MAZET, J., LAVIGNE, J., CELLIER, N., SOTTO, A. and LARCHER, R., 2023. Clinical features and outcomes of Enterococcal bone and joint infections and factors associated with treatment failure over a 13-year period in a French teaching hospital. Microorganisms, vol. 11, no. 5, pp. 1213. http://doi.org/10.3390/microorganisms11051213 PMid:37317187.
» http://doi.org/10.3390/microorganisms11051213 -
MARTINI, C., LONGO, F., CASTAGNOLA, R., MARIGO, L., GRANDE, N., CORDARO, M., CACACI, M., PAPI, M., PALMIERI, V., BUGLI, F. and SANGUINETTI, M., 2020. Antimicrobial and antibiofilm properties of graphene oxide on Enterococcus faecalis. Antibiotics (Basel, Switzerland), vol. 9, no. 10, pp. 692. http://doi.org/10.3390/antibiotics9100692 PMid:33066198.
» http://doi.org/10.3390/antibiotics9100692 -
MEMISOGLU, G., MURUGESAN, R., ZUBIA, J. and ROZHIN, A., 2023. Graphene nanocomposite membranes: fabrication and water treatment applications. Membranes, vol. 13, no. 2, pp. 145. http://doi.org/10.3390/membranes13020145 PMid:36837648.
» http://doi.org/10.3390/membranes13020145 -
MOHAMMED, H., KUMAR, A., BEKYAROVA, E., AL-HADEETHI, Y., ZHANG, X., CHEN, M., ANSARI, M.S., COCHIS, A. and RIMONDINI, L., 2020. Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Frontiers in Bioengineering and Biotechnology, vol. 8, pp. 465. http://doi.org/10.3389/fbioe.2020.00465 PMid:32523939.
» http://doi.org/10.3389/fbioe.2020.00465 -
NASKER, S., AJAYAN, P. and NAYAK, S., 2023. Emerging trends and future direction of graphene family of materials as potential antimicrobials: a critical review. ACS Materials Letter, vol. 5, no. 3, pp. 673-693. http://doi.org/10.1021/acsmaterialslett.2c01116
» http://doi.org/10.1021/acsmaterialslett.2c01116 - NGUYEN, V., TO, D., PHAN, T., THANH, B., NGUYEN, T., NGUYEN, T.A., NGUYEN, T., DOAN, D., NGUYEN, T., NGUYEN, M., TRAN, V. and NGUYEN, T., 2022. Antimicrobial properties of distichochlamys citrea M.F. Newman rhizome n‐hexane extract against streptococcus pyogenes: experimental evidences and computational screening. ChemistrySelect, vol. 7, no. 17, pp. e202200680.
-
PAPANIKOLAOU, E., SIMOS, Y., SPYROU, K., ALATZOGLOU, C., TSAMIS, K., VEZYRAKI, P., STAMATIS, H., GOURNIS, D., PESCHOS, D. and DOUNOUSI, E., 2023. Does green exfoliation of graphene produce more biocompatible structures? Pharmaceutics, vol. 15, no. 3, pp. 993. http://doi.org/10.3390/pharmaceutics15030993 PMid:36986854.
» http://doi.org/10.3390/pharmaceutics15030993 -
RENNZ, N., TREBSE, R., AKGUB, D., PERKA, C. and TRAMPUZ, A., 2019. Enterococcal periprosthetic joint infection: clinical and icrobiological fndings from an 8-year retrospective cohort study. BMC Infectious Diseases, vol. 19, no. 1, pp. 1083. http://doi.org/10.1186/s12879-019-4691-y PMid:31881851.
» http://doi.org/10.1186/s12879-019-4691-y -
RODRIGUEZ-ESTUPINAN, P., MIRANDA-CARVAJAL, I., CAMPOS, P., GUERRERO-FAJARDO, A., GIRALDO, L. and MORENO-PIRAJAN, J., 2022. Graphene-based materials: analysis through calorimetric techniques. Journal of Thermal Analysis and Calorimetry, vol. 147, no. 17, pp. 9301-9351. http://doi.org/10.1007/s10973-022-11206-w
» http://doi.org/10.1007/s10973-022-11206-w -
TADESSE, M., HAILU, Y., BISET, S., FEREDE, G. and GELAW, B., 2023. Prvalence, antibiotic susceptibility profile and associated factors of group A Streptococcal pharingitis among pediatric patients with acute pharyngitis in Gondar, Northwest Ethiopia. Infection and Drug Resistance, vol. 16, pp. 1637-1648. http://doi.org/10.2147/IDR.S402292 PMid:36992964.
» http://doi.org/10.2147/IDR.S402292 -
TOLLU, G. and EKIN, I., 2021. Biotyping and antimicrobial susceptibility of Enterococcus faecalis and E. faecium isolated from urine and stool samples. Jundishapur Journal of Microbiology, vol. 13, no. 10, pp. e105136. http://doi.org/10.5812/jjm.105136
» http://doi.org/10.5812/jjm.105136 -
TRONCOSO, O.P. and TORRES, F.G., 2020. Bacterial cellulose-graphene based nanocomposites. International Journal of Molecular Sciences, vol. 21, no. 18, pp. 6532. http://doi.org/10.3390/ijms21186532 PMid:32906692.
» http://doi.org/10.3390/ijms21186532 - UNAGOLLA, J. M. and JAYASURIYA, A. C., 2019. Enhanced cell functions on graphene oxide incorporated 3D printed polycaprolactone scaffolds. Materials Science and Engineering C, vol. 102, pp. 1-11.
-
VENANZIO, G., FLORES-MIRELES, A. and CALIX, J., 2019. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nature Communications, vol. 10, no. 1, pp. 2763. http://doi.org/10.1038/s41467-019-10706-y PMid:31235751.
» http://doi.org/10.1038/s41467-019-10706-y -
WANG, B., CHEN, X., AHMAD, Z., HUANG, J. and CHANG, M., 2019. 3D eletrohydrodynamic printing of highly aligned dual-core graphene composite matrices. Carbon, vol. 153, pp. 285-297. http://doi.org/10.1016/j.carbon.2019.07.030
» http://doi.org/10.1016/j.carbon.2019.07.030 -
WILLIAMS, A., MOORE, E., THOMAS, A. and JOHNSON, J., 2023. Graphene-based materials in dental applications: antibacterial, biocompatible, and bone regenerative properties. International Journal of Biomaterials, vol. 2023, pp. 8803283. http://doi.org/10.1155/2023/8803283 PMid:36819211.
» http://doi.org/10.1155/2023/8803283 - XIANG, D., DONG, P. and CHEN, L., 2022. Antagonistic interaction between two key endodontic pathogens Enterococcus faecalis and Fusobacterium nucleatum. Journal of Oral Microbiology, vol. 15, no. 1, pp. 2149448. PMid:36452179.
-
YARAGALLA, S., BHAVITHA, K. and ATHANASSIOU, A., 2021. A review on graphene based materials and their antimicrobial properties. Coatings, vol. 11, no. 10, pp. 1197. http://doi.org/10.3390/coatings11101197
» http://doi.org/10.3390/coatings11101197 - YI, J., TANG, R., YANG, J., CHEN, Y. and FEI, J., 2019. Streptolysin O derived from Streptococcus pyogenes inhibits RANKL-induced osteoclastogenesis through the NF-kB signaling pathway. Molecular Medicine Reports, vol. 19, no. 1, pp. 414-422. PMid:30431141.
-
YOKOHATA, S., OHKURA, K., NAGAMUNE, H., TOMOYASU, T. and TABATA, A., 2023. Human serum albumin stabilizes streptolysin S activity secreted in the extracellular milieu by streptolysin S-producing streptococci. Microbiology and Immunology, vol. 67, no. 2, pp. 58-68. http://doi.org/10.1111/1348-0421.13042 PMid:36478453.
» http://doi.org/10.1111/1348-0421.13042 -
YUNIATI, Y., HASANAH, N., ISMAIL, S., ANITASAR, S. and PARAMITA, S., 2018. Antibacterial activity of dracontomelon dao extracts on methicillin-resistant S. aureus (MRSA) and E.coli multiple drug resistance (MDR). African Journal of Infectious Diseases, vol. 12, no. 1, suppl., pp. 62-67. http://doi.org/10.21010/ajid.v12i1S.8 PMid:29619432.
» http://doi.org/10.21010/ajid.v12i1S.8 -
ZHENG, W., HE, J. and LIU, F., 2019. Preparation and properties of antibacterial ABS plastics based on polymeric quaternary phosphonium salts antibacterial agents. Polymers for Advanced Technologies, vol. 30, no. 10, pp. 2515-2522. http://doi.org/10.1002/pat.4653
» http://doi.org/10.1002/pat.4653 - ZIMMER, B., 2022. CLSI M02 performance standards for antimicrobial disk susceptibility tests Wayne, PA: Clinical and Laboratory Standards Institute.
Publication Dates
-
Publication in this collection
07 Feb 2025 -
Date of issue
2024
History
-
Received
24 July 2024 -
Accepted
19 Oct 2024

 A thermal perspective of the advancements in antibacterial polycaprolactone/graphene scaffolds
A thermal perspective of the advancements in antibacterial polycaprolactone/graphene scaffolds