Abstract
A correlation between cancer and hypercoagulability has been described for more than a century. Patients with cancer are at increased risk for thrombotic complications and the clotting initiator protein, tissue factor (TF), is possibly involved in this process. Moreover, TF may promote angiogenesis and tumor growth. In addition to TF, thrombin seems to play a relevant role in tumor biology, mainly through activation of protease-activated receptor-1 (PAR-1). In the present study, we prospectively studied 39 lung adenocarcinoma patients in relation to the tumor expression levels of TF and PAR-1 and their correlation with thrombosis outcome and survival. Immunohistochemical analysis showed TF positivity in 22 patients (56%), most of them in advanced stages (III and IV). Expression of PAR-1 was found in 15 patients (39%), most of them also in advanced stages (III and IV). Remarkably, no correlation was observed between the expression of TF or PAR-1 and risk for thrombosis development. On the other hand, patients who were positive for TF or PAR-1 tended to have decreased long-term survival. We conclude that immunolocalization of either TF or PAR-1 in lung adenocarcinoma may predict a poor prognosis although lacking correlation with thrombosis outcome.
Lung adenocarcinoma; Thrombosis; Tissue factor; Protease-activated receptor
Braz J Med Biol Res, April 2010, Volume 43(4) 403-408
Increased expression of tissue factor and protease-activated receptor-1 does not correlate with thrombosis in human lung adenocarcinoma

 E. de Meis1,2, D. Azambuja3, J.P. Ayres-Silva3, M. Zamboni4, V.R. Pinheiro5, R.A. Levy6 and R.Q. Monteiro7
E. de Meis1,2, D. Azambuja3, J.P. Ayres-Silva3, M. Zamboni4, V.R. Pinheiro5, R.A. Levy6 and R.Q. Monteiro7
1Serviço de Patologia Clínica, 2Serviço de Hematologia, 3Divisão de Patologia, 4Serviço de Cirurgia Torácica and 5Serviço de Radiologia, Instituto Nacional do Câncer, Rio de Janeiro, RJ, Brasil
6Departamento de Reumatologia, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
7Instituto de Bioquímica Médica, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
Abstract
A correlation between cancer and hypercoagulability has been described for more than a century. Patients with cancer are at increased risk for thrombotic complications and the clotting initiator protein, tissue factor (TF), is possibly involved in this process. Moreover, TF may promote angiogenesis and tumor growth. In addition to TF, thrombin seems to play a relevant role in tumor biology, mainly through activation of protease-activated receptor-1 (PAR-1). In the present study, we prospectively studied 39 lung adenocarcinoma patients in relation to the tumor expression levels of TF and PAR-1 and their correlation with thrombosis outcome and survival. Immunohistochemical analysis showed TF positivity in 22 patients (56%), most of them in advanced stages (III and IV). Expression of PAR-1 was found in 15 patients (39%), most of them also in advanced stages (III and IV). Remarkably, no correlation was observed between the expression of TF or PAR-1 and risk for thrombosis development. On the other hand, patients who were positive for TF or PAR-1 tended to have decreased long-term survival. We conclude that immunolocalization of either TF or PAR-1 in lung adenocarcinoma may predict a poor prognosis although lacking correlation with thrombosis outcome.
Key words: Lung adenocarcinoma; Thrombosis; Tissue factor; Protease-activated receptor
Introduction
Lung cancer is the most frequent and fatal cancer worldwide, being responsible for up to 2 million deaths each year. In the United States, 215,000 new cases with 161,840 deaths were estimated in 2008, while in Brazil 27,000 new cases were estimated for the same year, with only 10% of these patients expected to survive up to 5 years. The smoking habit is considered to be the main cause of lung cancer, and adenocarcinoma is the most frequent histological type (1-3).
Since the work of Trousseau in the 1860’s, there has been increasing evidence of the interference of clotting factors with cancer progression (4,5). In this context, tissue factor (TF) is a 263-amino acid transmembrane glycoprotein that plays a central role in the interface between blood coagulation and tumor biology (6). It is now established that TF expression correlates with the histological grade of malignancy of several types of cancer, being particularly associated with invasive potential and angiogenesis (7-11). There is evidence that TF expression is related to increased blood thrombogenicity in lung cancer (12).
In addition to TF, thrombin seems to play a significant role in cancer progression (13). In fact, the action of thrombin in tumor biology results in part from the proteolytic cleavage of protease-activated receptor-1 (PAR-1) in tumor cells, which elicits several pro-tumoral responses including angiogenesis, cell proliferation and invasion (14). Some studies have demonstrated an increased expression of PAR-1 in patients’ samples including breast, colon, melanoma, and esophageal cancer (15-18).
In the present study, we attempted to evaluate the expression of TF and PAR-1 in human lung adenocarcinoma samples. Moreover, we followed survival and thrombosis outcome in the same set of patients. Our data showed no correlation between the expression of TF or PAR-1 and risk for thrombosis development. On the other hand, patients who were positive for either TF or PAR-1 tended to have decreased long-term survival.
Material and Methods
We studied paraffin blocks (tumor biopsy or tumor tissue extracted for treatment) obtained from 39 lung adenocarcinoma patients prospectively studied in our service. The criterion of patient selection for immunohistochemistry was paraffin block availability. All patients studied, even non-symptomatic ones, were followed for 2 years or until death, with venous Doppler scan of arms and limbs every 3 months. The study was approved by an internal Instituto Nacional do Câncer Ethics in Research Committee and patients signed an informed free consent term before entering the study.
Immunohistochemistry
All samples were fixed in 10% formalin and embedded in paraffin. Serial tissue sections were cut, mounted on glass slides and dried at 56°C before dewaxing in xylene and rehydration in alcohol according to standard histological procedures. Monoclonal antibodies against TF (#4509; 1:400) were from American Diagnostica, Inc. (USA) and antibodies against PAR-1 were from Santa Cruz Biotechnology (USA) (#sc-13503; 1:10). All sections were subjected to heat-induced epitope retrieval in citrate buffer, pH 6.0, for 3 min, followed by inhibition of endogenous peroxidase (peroxidase block, RE7101, Novocastra, UK). Incubation of primary antibodies (anti-TF or anti-PAR-1) was performed overnight at room temperature in TBS containing 2 mM sodium azide and 1 mM BSA. Slides were further submitted to post-primary blockade (RE7111, Novocastra), and then incubated at room temperature with NovoLink™ polymer (RE7112, Novocastra) for 30 min. Slides were further developed with diaminobenzidine chromogen (RE7105, Novocastra) at 1/20 dilution, and finally stained with Mayer hematoxylin, dehydrated and mounted with Canadian balsam.
TF and PAR-1 expression was evaluated semi-quantitatively as percent positive cells after counting 300 cells, being scored as negative (up to 5% of positive cells), or positive (more than 5% cells staining positively). Negative controls were obtained by omitting the primary antibody. Pancreas adenocarcinoma samples were used as positive controls (data not shown).
Determination of thrombogenic factors in plasma
A blood sample was obtained and analyzed at inclusion in the study and every 6 months up to 2 years of follow-up or death. This evaluation involved the determination of procoagulant factors (fibrinogen and factor VIII) known to induce thrombosis when increased, and of D-dimer for evaluation of fibrinolysis. Fibrinogen levels above 500 ng/dL and 200% factor VIII concentration were considered to be thrombogenic. D-dimer up to 250 ng/mL was considered to be normal. Lupus anticoagulant activity was measured by coagulometry using diluted Russel viper venom. All tests were performed using an automated coagulometer device (Sysmex CA1500, DADE Behring, Newark, USA) according to manufacturer instructions.
Statistical analysis
Data are reported as descriptive statistics (median, minimum and maximum limits and percent). For data analysis, we used the chi-square test with Fisher adjustment when necessary, and P < 0.05 was considered to be significant.
Results
Thirty-nine patients diagnosed with lung adenocarcinoma were prospectively evaluated in this study. Patients’ characteristics are described in Table 1.
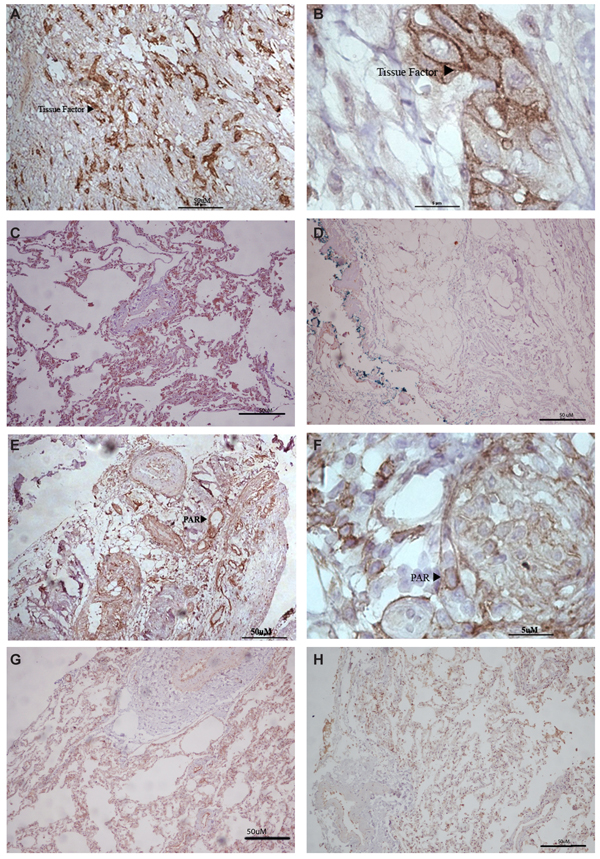
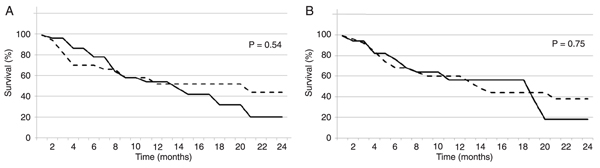
Immunohistochemical analysis of tumor samples showed positive TF staining in 22 patients (56%), as illustrated in Figure 1. Remarkably, TF expression was correlated with disease stage, being more frequent in patients with advanced disease (Table 2). Surprisingly, the frequency of thrombosis showed no correlation with TF expression, as demonstrated in Table 2, with 2 of 5 patients who developed thrombosis demonstrating TF positivity in tumor samples. On the other hand, survival curves showed a trend to decreased survival for TF expression, as demonstrated in Figure 2A.
In addition to TF, immunohistochemistry revealed positivity for PAR-1 expression in a number of patient samples, as illustrated in Figure 1. Table 2 shows that PAR-1 staining was detected in 15 patients (39%), with the expression pattern being also correlated with advanced disease stages. Interestingly, 11 patients were positive for both TF and PAR-1, with this co-expression being more prevalent in the advanced stages of lung adenocarcinoma. Expression of PAR-1 showed no correlation with thrombosis risk (Table 2). Furthermore, as seen with TF, positivity for PAR-1 expression was not statistically significant when we attempted to correlate it with overall survival but clearly demonstrated a trend to decreased long-term survival (Figure 2B). In addition, analysis of thrombogenic risk factors showed no correlation with TF or PAR-1 expression (Table 3).
Immunohistochemistry for tissue factor (arrowhead) in lung adenocarcinoma (A, 100X and B, 1000X), normal lung (C), and negative control (D). Immunohistochemistry for protease-activated receptor-1 (PAR-1; arrowhead) stain in lung adenocarcinoma (E, 100X and F, 1000X), normal lung (G), and negative control (H)
Kaplan-Meier survival curves for lung adenocarcinoma patients regarding TF or PAR-1 expression patterns. A, Survival of patients who stained positive (solid lines; N = 22) compared to negative (dashed lines; N = 17) for TF, as analyzed by immunohistochemistry. B, Survival of patients who stained positive (solid lines; N = 16) compared to negative (dashed lines; N = 23) for PAR-1, as analyzed by immunohistochemistry. TF = tissue factor; PAR-1 = protease-activated receptor-1.
Discussion
A correlation between cancer and blood coagulation activation has been well established, with cancer patients being at increased risk for thrombosis development. In fact, White et al. (19) found that, among the main types of solid cancers, lung cancer showed a high risk for the development of venous thromboembolism. In the present study, we observed a high incidence of thrombosis in a group of lung adenocarcinoma patients. However, it is important to note that the present patients were screened for thrombosis every 3 months even if asymptomatic. This procedure was necessary because it is believed that cancer-related thrombosis is largely undiagnosed.
Several studies have demonstrated that the procoagulant properties of tumor cells mainly derive from the expression of TF, the clotting initiator protein (20,21). Sawada et al. (9) have previously demonstrated increased TF expression in malignant cells from patients with lung cancer. Remarkably, in their study, TF expression levels correlated with the presence of metastatic tumors. Goldin-Lang et al. (12) also demonstrated increased TF expression in non-small cell lung cancer, although no direct correlation between disease stage and TF expression levels was detected. In the present study, we observed an increased TF expression in tumor cells from 22 of 39 lung adenocarcinoma patients, in particular at advanced stages. However, to our surprise, we found no significant correlation between TF expression and the occurrence of thrombosis. There are some possible explanations for this observation. TF expression in lung adenocarcinoma may have no direct correlation with plasma TF levels. Another possibility is that additional thrombogenic factors may have a strong influence on lung adenocarcinoma-related thrombosis. In this regard, we recently observed that positivity for antiphospholipid antibodies is a strong risk factor for thrombosis outcome in lung adenocarcinoma (22). Finally, since post-mortem analyses have not been routinely performed in our Institution, occult pulmonary embolism cannot be excluded in patients with increased TF expression.
In addition to TF, thrombin seems to contribute to tumor progression, mainly through cleavage and activation of PAR-1, a G-protein-coupled receptor (13). Overexpression of PAR-1 has been described in several types of tumor including breast, colon, melanoma, and esophageal cancer (15-18). Moreover, Depasquale and Thompson (23) recently reported PAR-1 expression as a negative prognostic factor in melanomas, which strongly correlated with tumor stage (23). In the present study, we observed increased PAR-1 expression in 15 of 39 lung adenocarcinoma patients. No correlation between PAR-1 expression and thrombosis outcome was detected. However, survival curves showed a trend to decreased long-term survival in PAR-1-expressing patients. It is important to note that 11 patients co-expressed TF and PAR-1, indicating the need for a larger cohort to determine whether these proteins may serve as possible prognostic factors in lung adenocarcinoma.
References
1. Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest 2003; 123: 21-49.
2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA J Clin 2008; 58: 71-96.
3. Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abret CC. Cigarette smoking and subsequent risk of lung cancer in man and women: analysis of a prospective cohort study. Lancet Oncol 2008; 9: 649-656.
4. Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood 1983; 62: 14-31.
5. Palumbo JS. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost 2008; 34: 154-160.
6. Milsom C, Rak J. Tissue factor and cancer. Pathophysiol Haemost Thromb 2008; 36: 160-176.
7. Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg 1995; 82: 1101-1104.
8. Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol 2002; 69: 247-254.
9. Sawada M, Miyake S, Ohdama S, Matsubara O, Masuda S, Yakumaru K, et al. Expression of tissue factor in non-small-cell lung cancers and its relationship to metastasis. Br J Cancer 1999; 79: 472-477.
10. Minamiya Y, Matsuzaki I, Sageshima M, Saito H, Taguchi K, Nakagawa T, et al. Expression of tissue factor mRNA and invasion of blood vessels by tumor cells in non-small cell lung cancer. Surg Today 2004; 34: 1-5.
11. Regina S, Rollin J, Bléchet C, Iochmann S, Reverdiau P, Gruel Y. Tissue factor expression in non-small cell lung cancer: relationship with vascular endothelial growth factor expression, microvascular density, and K-ras mutation. J Thorac Oncol 2008; 3: 689-697.
12. Goldin-Lang P, Tran QV, Fichtner I, Eisenreich A, Antoniak S, Schulze K, et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicates increased blood thrombogenicity and tumor metastasis. Oncol Rep 2008; 20: 123-128.
13. Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006; 10: 355-362.
14. Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest 2003; 124: 58-68.
15. Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion process. Nature Med 1998; 4: 909-914.
16. Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol 2003; 162: 1503-1513.
17. Massi D, Naldini A, Ardinghi C, Carraro F, Franchi A, Paglierani M, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol 2005; 36: 676-685.
18. Ribeiro FS, Simão TA, Amoedo ND, Andreollo NA, Lopes LR, Acatuassu R, et al. Evidence for increased expression of tissue factor and protease-activated receptor-1 in human esophageal cancer. Oncol Rep 2009; 21: 1599-1604.
19. White RH, Chew H, Wun T. Targeting patients for anticoagulant prophylaxis trials in patients with cancer: Who is at highest risk? Thromb Res 2007; 120 (Suppl 2): 29-40.
20. Fernandes RS, Kirszberg C, Rumjanek VM, Monteiro RQ. On the molecular mechanisms for the highly procoagulant pattern of C6 glioma cells. J Thromb Haemost 2006; 4: 1546-1552.
21. Kirszberg C, Lima LG, Da Silva de Oliveira A, Pickering W, Gray E, Barrowcliffe TW, et al. Simultaneous tissue factor expression and phosphatidylserine exposure account for the highly procoagulant pattern of melanoma cell lines. Melanoma Res 2009; 19: 301-308.
22. De Meis E, Monteiro RQ, Levy RA. Lung adenocarcinoma and antiphospholipid antibodies. Autoimmun Rev 2009; 8: 529-532.
23. Depasquale I, Thompson WD. Prognosis in human melanoma. PAR-1 expression is superior to other coagulation components and VEGF. Histopathology 2008; 52: 500-509.
Acknowledgments
We thank V.B. Rumjanek (Instituto de Bioquímica Médica, UFRJ, Rio de Janeiro, RJ, Brazil) for helpful discussions and a critical reading of the manuscript. The authors declare no conflict of interest in the present study. Research supported by Fundação Ary Frauzino (FAF), Instituto Nacional do Câncer (INCA-MS), CNPq, FAPERJ, and FINEP.
 Address for correspondence: E. de Meis, Serviço de Patologia Clínica, Instituto Nacional do Câncer, Praça Cruz Vermelha, 23, 20230-130 Rio de Janeiro, RJ, Brasil. Fax: +55-21-2506-6023. E-mail: edemeisrio@yahoo.com
Address for correspondence: E. de Meis, Serviço de Patologia Clínica, Instituto Nacional do Câncer, Praça Cruz Vermelha, 23, 20230-130 Rio de Janeiro, RJ, Brasil. Fax: +55-21-2506-6023. E-mail: edemeisrio@yahoo.com
Received November 23, 2009. Accepted March 1, 2010. Available online March 12, 2010. Published April 12, 2010. .
The Brazilian Journal of Medical and Biological Research is partially financed by
References
- 1. Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest 2003; 123: 21-49.
- 2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA J Clin 2008; 58: 71-96.
- 3. Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abret CC. Cigarette smoking and subsequent risk of lung cancer in man and women: analysis of a prospective cohort study. Lancet Oncol 2008; 9: 649-656.
- 5. Palumbo JS. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost 2008; 34: 154-160.
- 6. Milsom C, Rak J. Tissue factor and cancer. Pathophysiol Haemost Thromb 2008; 36: 160-176.
- 7. Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg 1995; 82: 1101-1104.
- 8. Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol 2002; 69: 247-254.
- 9. Sawada M, Miyake S, Ohdama S, Matsubara O, Masuda S, Yakumaru K, et al. Expression of tissue factor in non-small-cell lung cancers and its relationship to metastasis. Br J Cancer 1999; 79: 472-477.
- 10. Minamiya Y, Matsuzaki I, Sageshima M, Saito H, Taguchi K, Nakagawa T, et al. Expression of tissue factor mRNA and invasion of blood vessels by tumor cells in non-small cell lung cancer. Surg Today 2004; 34: 1-5.
- 11. Regina S, Rollin J, Bléchet C, Iochmann S, Reverdiau P, Gruel Y. Tissue factor expression in non-small cell lung cancer: relationship with vascular endothelial growth factor expression, microvascular density, and K-ras mutation. J Thorac Oncol 2008; 3: 689-697.
- 12. Goldin-Lang P, Tran QV, Fichtner I, Eisenreich A, Antoniak S, Schulze K, et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicates increased blood thrombogenicity and tumor metastasis. Oncol Rep 2008; 20: 123-128.
- 13. Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006; 10: 355-362.
- 14. Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest 2003; 124: 58-68.
- 15. Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion process. Nature Med 1998; 4: 909-914.
- 16. Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol 2003; 162: 1503-1513.
- 17. Massi D, Naldini A, Ardinghi C, Carraro F, Franchi A, Paglierani M, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol 2005; 36: 676-685.
- 18. Ribeiro FS, Simão TA, Amoedo ND, Andreollo NA, Lopes LR, Acatuassu R, et al. Evidence for increased expression of tissue factor and protease-activated receptor-1 in human esophageal cancer. Oncol Rep 2009; 21: 1599-1604.
- 19. White RH, Chew H, Wun T. Targeting patients for anticoagulant prophylaxis trials in patients with cancer: Who is at highest risk? Thromb Res 2007; 120 (Suppl 2): 29-40.
- 20. Fernandes RS, Kirszberg C, Rumjanek VM, Monteiro RQ. On the molecular mechanisms for the highly procoagulant pattern of C6 glioma cells. J Thromb Haemost 2006; 4: 1546-1552.
- 21. Kirszberg C, Lima LG, Da Silva de Oliveira A, Pickering W, Gray E, Barrowcliffe TW, et al. Simultaneous tissue factor expression and phosphatidylserine exposure account for the highly procoagulant pattern of melanoma cell lines. Melanoma Res 2009; 19: 301-308.
- 22. De Meis E, Monteiro RQ, Levy RA. Lung adenocarcinoma and antiphospholipid antibodies. Autoimmun Rev 2009; 8: 529-532.
- 23. Depasquale I, Thompson WD. Prognosis in human melanoma. PAR-1 expression is superior to other coagulation components and VEGF. Histopathology 2008; 52: 500-509.
Publication Dates
-
Publication in this collection
08 Apr 2010 -
Date of issue
Apr 2010
History
-
Received
23 Nov 2009 -
Accepted
01 Mar 2010

 Increased expression of tissue factor and protease-activated receptor-1 does not correlate with thrombosis in human lung adenocarcinoma
Increased expression of tissue factor and protease-activated receptor-1 does not correlate with thrombosis in human lung adenocarcinoma