Abstracts
Our research has focused on the main design features and release performances of time-dependent colon-specific (TDCS) delivery tablets, which relies on the relative constancy that is observed in the small intestinal transit time of dosage forms. But inflammatory bowel disease(IBD)can affect the transit time, and usually results in watery stool. Compared to the TDCS and wax-matrix TDCS tablet, a promising time-dependent colon-specific delivery system was investigated. In our study, a suppository-base-matrix coated tablet was evaluated. Water soluble suppository-base helps the expansion of tablet, facilitates uniform film dissolution and achives high osmotic pressure. Combining the expansion of carboxymethyl starch sodium (CMS-Na) and the moisture absorption of NaCl, the coated TDCS tablet obtained a burst and targeted drug delivery system. A very good correlation between in vitro drug release and in vivo outcome was observed. This TDCS coated tablet provides a promising strategy to control drug release to the desired lower gastrointestinal region.
Inflammatory bowel disease; Lonicera japonica Thunb/extract; Suppository/colon-specific delivery system; Drugs/colon-specific delivery system; Tablets/colon-specific delivery system
Nossa pesquisa focou-se nas principais características de planejamento e de desempenho de liberação cólon-específica tempo-dependente (TDCS) de comprimidos, que leva em conta a constância relativa observada no tempo de trânsito intestinal das formas de dosagem. A doença inflamatória do intestino (IBD) pode afetar o tempo de trânsito e, geralmente, resulta em fezes aquosas. Comparando ao TDCS e a comprimidos TDCS com matriz-cerosa, investigou-se sistema promissor de liberação cólon-específica tempo-dependente. Em nosso estudo, avaliou-se comprimido revestido com matriz base de supositório. A base de supositório solúvel em água auxilia a expansão do comprimido, facilita a dissolução uniforme do filme e atinge alta pressão osmótica. Associando a expansão do carboximetil amido sódico (CMS-Na) à absorção de umidade do NaCl, o comprimido revestido TDCS originou sistema de liberação direcionado e de erupção. Observou-se correlação muito boa entre a liberação in vitro e a in vivo do fármaco. Este comprimido revestido TDCS representa estratégia promissora para o controle da liberação do fármaco na região gastrintestinal mais baixa.
Doença inflamatória do intestino; Lonicera japonica Thunb./extrato; Supositório/liberação cólon-específica; Fármaco/liberação cólon-específica; Comprimidos/revestidos/liberação cólon específica
INTRODUCTION
To treat localized colonic diseases, especially IBD, we always have to deal with the abnormal colon function of the frequent, liquid stools. IBD is a term primarily used to refer to two diseases of the intestines: Crohn's disease and ulcerative colitis. In Crohn's disease, the inflammation may involve any area throughout the entire digestive tract, often affecting the last part of the small intestine. In some cases, ulcerative colitis can involve the entire large intestine too. The most commonly seen symptom of IBD is diarrhea. Inflammation can affect transit time, which usually causes food to pass through more quickly and allows less time for drug absorption and increases elimination of a drug (Gastrointestinal Society Canadian Society of intestinal Research, 2012GASTROINTESTINAL SOCIETY CANADIAN SOCIETY OF INTESTINAL RESEARCH. What is IBD?: symptoms/complications, diagnosis, management. Available at: http://www.badgut.org/information-centre/inflammatory-bowel-disease.html. Accessed on: 20 Dec. 2012.
http://www.badgut.org/information-centre...
).
There are currently several strategies to achieve colonic specificity, such as bacterially triggered colon-specific delivery system (Zou et al., 2005; Soodabeh, Jalal, Abbas, 1999SOODABEH, D.; JALAL, H.; ABBAS, K. Release of 5-ASA from acrylic type polymeric prodrugs designed for colon-specific drug delivery. J. Control. Release, v.58, n.3, p.279-287, 1999.), pressure-controlled colon-specific delivery system (Yang, Chu, Fix, 2002YANG, L.; CHU, J.S.; FIX, J.A. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int. J. Pharm., v.235, n.1-2, p.1-15, 2002.), pH-dependent colon-specific delivery system (Krogars et al., 2000KROGARS, K.; HEINAMAKI, J.; VESALAHTI, J.; MARVOLA, M.; ANTIKAINEN, O.; YLIRUUSI, J. Extrusion- spheronization of pH-sensitive polymeric matrix pellet for possible colonic drug delivery. Int. J. Pharm., v.199, n.2, p.187-194, 2000.), time-dependent colon-specific delivery system (Halsas et al., 2001HALSAS, M.; PENTTINEN, T.; VESKI, P.; JURJENSON, H.; MARVOLA, M. Time-controlled release pseudoephedrine tablets: bioavailability and in vitro/in vivo correlations. Pharmazie, v.56, n.9, p.718-723, 2001.; Cheng et al., 2004CHENG, G.; AN, F.; ZOU, M.J.; SUN, J.; HAO, X.H.; HE, Y.X. Studies on the diclofenac sodium time-dependent and 5-aminosalicylic acid pH dependent oral colon- specific drug delivery systems. World J. Gastroenterol., v.10, n.12, p.1769-1774, 2004.), and a combination of these methods (Gupta, Beckert, Price, 2001GUPTA, V.K.; BECKERT, T.E.; PRICE, J.C. A novel pH- and time-based multi-unit potential colonic drug delivery system. I. Development. Int. J. Pharm., v.213, n.1-2, p.83-91, 2001.). However, bacterially triggered approach is criticized for the concern of the highly variable composition of the microflora, which can be heavily affected by diet modifications, stress, drug intake, and IBD. As with pH-dependent methods, even accumulated short-chain fatty acids metabolism might account for the erratic performances that have been obtained from enteric-coated systems (Gazzaniga et al., 2006GAZZANIGA, A.; MARONI, A.; FOPPOLI, A.; PALUGAN, L. Oral colon delivery: rationale and time-based drug design strategy. Discov. Med., v.36, n.6, p.223-228, 2006.).
As far as the time-dependent delivery approaches are concerned, a dosage form with a quick burst of drug release and a 2-3 h lag time in small intestinal tract is relied on for the direct release of bioactive compounds at the colon or the last part of the small intestine.
Lonicera japonica Thunb is a medicinal plant usually used to effectively treat colitis and cancer in clinical practice (Zhu, Hu, 2006ZHU, S.C.; HU, H.Q. The chemical constituent and pharmaceutical action of Lonicera japonica Thunb. Heilongjiang Med. J., n.4, p.299, 2006.; Xia, 2005XIA, R. Study on determination method and pharmacokinetics of chlorogenic acid in animals after mainline. Sichuan, 2005. p.18-20. (Thesis of Master degree. Sichuan University.). ; Zhang Shao, Wang, 2006ZHANG, X.T.; SHAO, W.H.; WANG, W. The preparation and application of extract of Flos loniceare. CN Pat. 1730015A, 2006.). The purpose of this study was to explore the feasibility of the time-dependent colon-specific suppository-base-matrix (TDCS-SBM) tablet with the extract of Lonicera japonica Thunb. It is well known that PEG can melt and expand at 37 °C and ethylcellulose is soluble in PEG. Also, being a flexible, water-soluble polymer, PEG can be used to create very high osmotic pressures (Money, 1989MONEY, N.P. Osmotic pressure of aqueous polyethylene glycols. relationship between molecular weight and vapor pressure deficit. Plant Physiol., v.91, n.2, p.766-769, 1989.; Xia, 2005XIA, R. Study on determination method and pharmacokinetics of chlorogenic acid in animals after mainline. Sichuan, 2005. p.18-20. (Thesis of Master degree. Sichuan University.). ). These properties make PEG one of the most suitable molecules for TDCS tablet, particularly when used for the pulse release system. Utilizing the CMS-Na expanding property and all the properties of PEG, the TDCS- SBM tablet was designed to achieve not only time-dependent but also osmotic film-dissolution controlled drug delivery system. A more stable release profile could be obtained as compared to the conventional TDCS tablet.
The TDCS-SBM coated tablets consisted of a tablet, an inner water-insoluble coating layer, and an enteric outer layer. Figure 1 showed the schematic diagram of TDCS-SBM tablet. During its transit through the GIT, the PEG in the core of TDCS-SBM tablet melted. The enteric-coating layer dissolved in the small intestine, and water was imbibed into the core. The aqueous environment caused swelling, high osmotic pressure, film dissolution, and then the coating layer was ruptured. Melted PEG core enabled the drug to be released in a pulsatile manner.
The schematic diagram of time-dependent colonspecific suppository- base-matrix (TDCS-SBM) tablet.
MATERIALS AND METHODS
Materials
The extract of Lonicera japonica Thunb was provided by Ningbo Shuanglin Chinese Traditional Medicine Pharmaceutical Co., (chorogenic acid 24%, batch No 081102 Ningbo, China). Standard chorogenic acid was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). PEG 400, PEG 600, PEG 1000, and PEG 6000 were obtained from Beijing Haidian Huiyou Fine Chemical Industry Factory (CP 2010 Beijing, China). Carboxymethyl starch sodium (CMS-Na) USP-NF was purchased from Shanghai Yung Zip Pharm Trading Co., Ltd. (Shanghai, China). Surelease(r) aqueous ethylcellulose dispersions and Opadry OY-P-7171 were supplied by Shanghai Colorcon Co., Ltd. (Shanghai, China). Talc and sodium chloride were purchased from Tianjin Kemiou Pharmaceutical Co. Ltd. (Tianjin, China). Sucrose was provided by Tianjin Bodi Chemical Industry Co. Ltd. (Tianjin, China). Microcrystalline cellulose was obtained from Asahikasei Chemical Industry Co. Ltd. (MCC, Avicel PH101,Tokyo, Japan). Acetonitrile was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All other chemicals and solvents were of analytical grade, and deionized double-distilled water was used throughout the study.
Apparatus and chromatographic conditions
The Shimadzu HPLC system (Kyoto, Japan) consisted of a LC-10AT pump, a SPD-10A UV detector, and a sample injector. The data was acquired and processed using an Anastar(r) software (Shimadzu Kyoto, Japan). The analytical column used was Hypersil ODS-2 (4.6 mm×250 mm, 5 μm ID) from Dalian Elite Analytical Instruments Co., Ltd. (Dalian, China). The chromatographic analysis was performed at room temperature at a flow rate of 0.8 mL• min−1 with injection volume of 20 µL. The UV absorbance was monitored at 362 nm. The mobile phase consisted of acetonitrile- double-distilled water-triethyl ammonium-glacial acetic acid (13:87:0.01:0.1, v/v).
Preparation of the tablets
The basic tablets consisted of 40% (w/w) dry extract of Lonicera japonica Thunb, 20% (w/w) CMS-Na, 20% (w/w) PEG, 8% (w/w) sodium chloride, 10% (w/w) MCC and 2% (w/w) talc. PEG 6000 and PEG 600 or PEG 400 were melted for mixture and cooled. Drug and excipients (except talc) were sieved (mesh 80) and blended by sieving; the mixtures were granulated with 95% alcohol (mesh 20) and were dried for 2 h at 30 °C. Core tablets with average weight of 310 mg were prepared using a single punch tablet machine (Type-TDP, 1st Pharm Machine Manu. Co., Shanghai, China). The diameter of core tablets was 8.5 mm with round beveled. The hardness of the tablet was controlled at 5-7 kg/mm2. The influence of the types of binder or moistening and PEG was investigated. Similarly, the effect of the amount of CMS-Na, NaCl, and PEG was also evaluated with sucrose as diluents to adjust tablets weight to 310 mg.
Preparation of the coated tablets
The core tablets were coated in a conventional rotating pan. The basic inner- coating formulation was 15% Surelease(r) which contained PEG 400 (1.0%, w/w) as channeling agent with 7% weight gain (theoretical solid content of Surelease consumed). Operating conditions were as follows: the rotation speed, 30-45 rpm; coating pan angle, 45°; the nozzle port size, 0.8 mm; inlet air temperature, 35 °C; tablet bed temperature, 25 °C; sprayed flow rate, 0.7 mL/min. The outer-coating material was Opadry OY-P-7171 in 90% ethanol. The weight gain of outer enteric layer material was 8% w/w. Operating conditions were the same as the above except for the spray rate at 1.0 mL/min with inlet temperatureat 30 °C and outlet temperature at 25 °C.
In vitro dissolution test
All dissolution tests were performed using USP type II dissolution apparatus (ZRD6-B dissolution tester, Shanghai Huanghai Instrument Factory, Shanghai China). Place 750 mL of 0.1 N hydrochloric acid in the vessel, and assemble the apparatus. Allow the medium to equilibrate to a temperature of 37±0.5 °C. Place 6 tablets in the apparatus, cover the vessel, and operate the apparatus for 2h at the rate of 75 rpm. After 2 h of operation in 0.1 N HCl, withdraw an aliquot of the solution and immediately add 250 mL of 0.20 M tribasic sodium phosphate equilibrated to 37±0.5 °C to the solution in the vessel. Adjust, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8±0.05. For the release study of the formulation screening, withdraw an aliquot of the fluid at the film rupture time, and 30, 60, 90, 120, 150, 180 min after film rupture. The film rupture time was defined as the time at which amount of drug release was greater than 10%. For the optimized formulation, withdraw a specimen at 3, 4, 4.5, 5, 5.5, 6, 6.5, 7 and 7.5 h. After each sampling, lost volume was replaced with equal volume of the fresh medium to maintain constant total volume. The solutions were immediately filtered through a 0.45 μm membrane and determined by HPLC.
Dissolution testing conditions were selected based on a screening study using USP apparatus 2. According to USP, dissolution medium may be water, or a buffered aqueous solution (typically pH 4.0 to 8.0). After 2 hours of operation in 0.1 N HCl, release profiles under different dissolution media (double distilled water, pH 6.8, pH 7.0, pH 7.4, and pH 7.8 phosphate buffer) were evaluated. Effect of varying paddle speeds of 50, 75, 100, and 150 rpm were also investigated with water media.
Statistic analysis
One-way analysis of variance test (ANOVA) was performed to check whether there was significant difference among the formulations. Release profiles of TDCS tablet, TDCS-SBM tablet, and WM-TDCS tablet were compared using 'the fit factors', which included the calculation of similarity factor f 2. According to this theory, two release profiles were considered to be similar if f 2 value was greater than 50 (between 50 and 100) (Jeffrey, Henry, 1996JEFFREY, W.M.; HENRY, H.F. Mathematical comparison of dissolution profiles. J. Pharm. Technol., v.20, p.64-74, 1996.; Xia, Liu., 2000XIA, J.H.; LIU, C.X.; The statistical evaluation and analysis of in vitro dissolution of solid preparation. J. Chin. Pharm., v.35, p.1-2, p.345-349, 2000.).
Experimental protocol in vivo
Technetium-99 m pertechnetate eluate containing approximately 370 MBq of activity was dropped on the top of the two sides of the core tablets (a few tablets made manually). Then the core tablets were coated as described in previous section.
All studies were performed according to the Guidelines that were approved by the Ethics Committee on Human Subjects of the General Military Hospital. Two healthy, male volunteers were selected for the in vivo studies. First of all, a 99Tc labeling coated tablet was administered. After administration, the volunteers immediately were laid on their backs under the monitor of Sophy DSX-NXT Spect system (Sophy, Buc, France). The movement of the coated tablet was followed by gamma scintigraphy. The image was collected at appropriate intervals for a period of 0.5 min. Energy was selected as 140 Kev; window width was 20%; collecting matrix was 256×256. Regions of interest were drawn around the site of movement and the total activity in this area representing the amount of released drug substance was characterized (George, 1996GEORGE, A.A. Evaluation of the gastric retention properties of a cross-linked polymer coated tablet versus those of a nondisintegrating tablet. Int. J. Pharm., v.75, n.2-3, p.241-247, 1996.).
RESULTS
Validation of assay method
The retention time of chorogenic acid in HPLC was about 6.3 min. The linear regression equation of the calibration curve was A=18 004 c +55.886, (r=0.999)over the concentration range of 0.5~20.0 μg/mL. The limit of quantification (LOQ) was 0.25 μg/mL. The criteria of precision, accuracy and recovery for analyzing samples were fulfilled in the developed analytical method.
Effect of different tablet formulations
Influence of binders or moistening agent
The formulations granulated using 95% ethanol and 5% PVP in 95% ethanol solution gave a suitable hardness of tablets and the appearance of the tablets was uniform. However, formulations granulated with 5% ethylcellulose in 95% ethanolic solution did not yield the required hardness. To keep the formulation simple, 95% ethanol was selected as moistening agent.
Effect of amount of CMS-Na and NaCl on drug release
For the effect of CMS-Na and NaCl on drug release, same results were obtained as studied by Zou et al. (2009). It showed that the combination of CMS-Na and NaCl had significant influence on film rupture time for TDCS tablet; and the former exhibited higher effect. The influence of sodium chloride on drug release could be due to the osmotic pressure.
Effect of the types of PEG on drug release
Polymers with appropriate expanding, osmotic pressure, and also film- dissolution property can be used for colon-specific delivery system. Dissolutions of the three types of PEG core coated tablets (with basic inner layer) showed that there were no significant differences for the release profile. The volume expansion degree of three types of PEG was evaluated. 10 g of PEG 1000, PEG 6000: PEG 400(1:2.1), or PEG 6000: PEG 600(1:1) was placed into a graduated test tube and then was melted. The melting (37 °C) and room temperature volumes were determined. It showed that the increases in the melting volume of PEG 1000, PEG 6000-PEG 400 (1:2.1), and PEG 6000-PEG 600 (1:1) were 17.2 ± 0.5%, 15.7±0.7%, and 16.4±0.4%, and exhibited no significant difference. Hence PEG 1000 was selected as the suppository-base-matrix of the tablet for the future study.
Effect of amount of PEG on drug release compared with the WM-TDCS tablet
The tablet formulations (Table 1) containing 0%, 10%,20%,30% PEG 1000 with basic inner- and outer-coating layer were investigated. The drug release profiles of each formulation were presented in Figure 2. It was evident that the mean film rupture of TDCS-SBM tablet containing 10%,20% PEG 1000 was similar with TDCS. TDCS- SBM tablet containing 30% PEG1000 showed more rapid film rupture time. Also the drug release from TDCS-SBM and WM-TDCS tablet was better reproducible than that of TDCS tablet with higher variability. Furthermore, TDCS-SBM tablet exhibited a burst drug release. It showed that the drug release from TDCS-SBM tablet reached about 70% within 0.5 to 1.0 h.
One-way ANOVA was performed to check whether there was significant difference of drug release among the same formulations; and the value of p was not statistically significant. Comparing the release of each other within the same formulation (with 9 release points), the 'similarity factor' f 2 of TDCS tablet was 44.2 ± 7.4 and others were more than 50 by the fit factors method. The results in Table IIsuggested that the release of tablets from TDCS-SBM and WM-TDCS was considered to be similar to each other.
The 2 of the dissolution profile of tablets between the same formulations with 9 release points
Effect of the pore forming agent and the inner-coating membrane on drug release
With this study, it is concluded that core tablets coated with Surelease(r) containing no pore forming agent with weight gain 7% had no drug release within 24 h. While the amount of PEG 400 was 2.0%, tablets coated with weight gain 12% achieved about 3 h lag time. Finally, 1.0% PEG 400 was selected. The in vitro release behaviors of the inner-coated tablets for basic core tablet with different inner-coating levels (3%, 5%, 7%, 9%, 10% and 11% w/w) of Surelease(r) were studied. As shown in Table III, the tablets with a weight gain of 7% of Surelease(r) were selected as the optimized formulation.
Influence of weight gain (observed value) of inner layer with PEG 0.1% on film rupture time in pH 6.8 phosphate buffer (n=6)
Effect of different dissolution conditions
After 2 hours of operation in 0.1 N HCl, release profiles under different dissolution media (distilled water, pH 6.8, pH 7.0, pH 7.4, and pH 7.8 phosphate buffer) were evaluated. It was demonstrated that the drug release lag time and the drug release were independent of the dissolution media since the inner coating material, Surelease(r), was pH-independent. The release characteristics of the formulation were to be evaluated over the physiologic pH range, pH 7.4.
The release profile showed no difference in the liberation of the active substance at paddle speed of 50, 75, 100, and 150 rpm. At the latter stage of the drug release, the greater the paddle speed was, the faster the release rate with no significant difference. For these studies, the paddle speed was selected at 75 rpm.
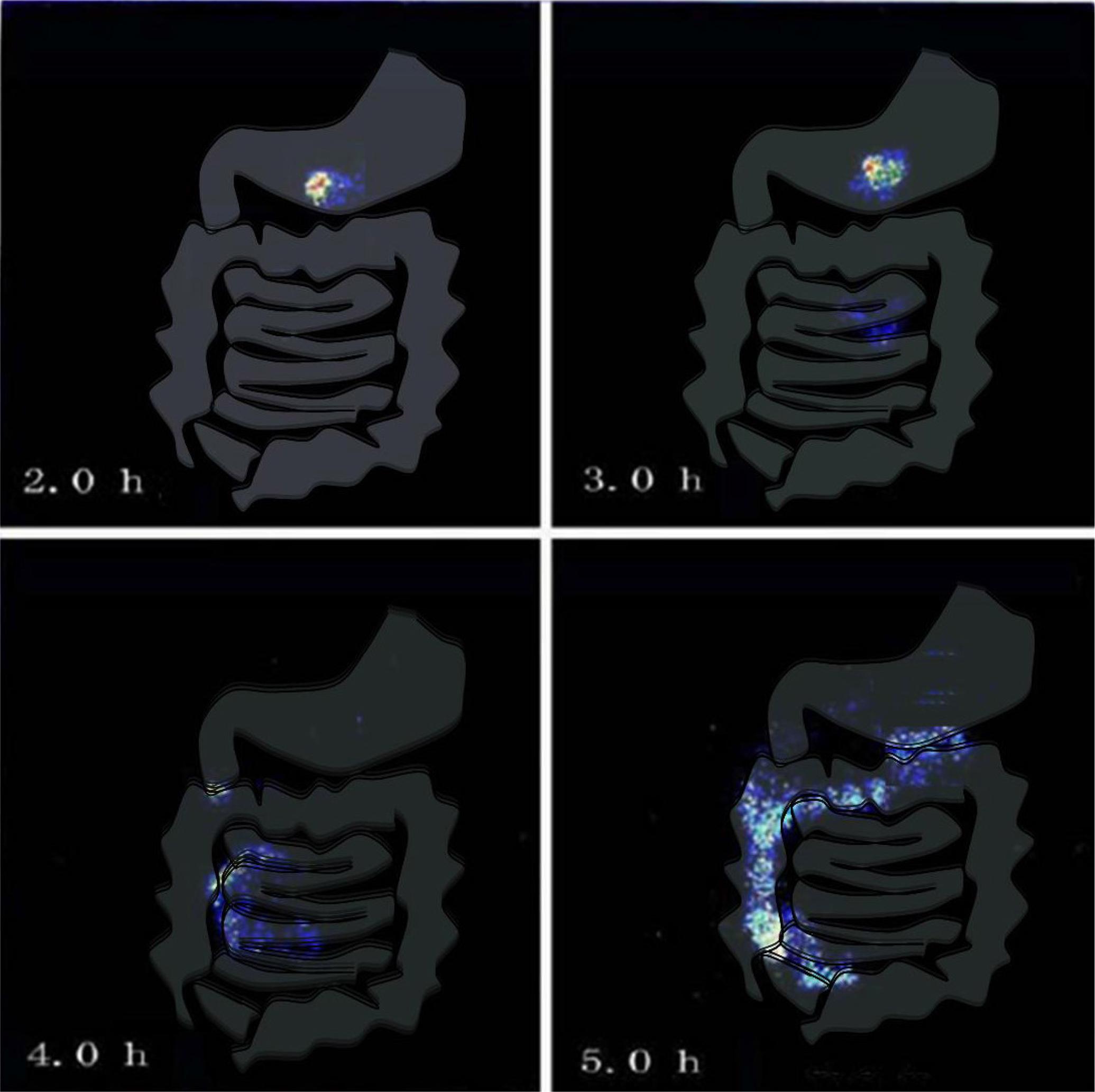
γ-Scintigraphy photos in vivo
Consequently, gamma scintigraphy was employed to study the colon release of the drug in our two volunteers. Figure 3 showed the movement traces of the tablet after administration of the extract of Lonicera japonica Thunb coated tablets. As shown in the γ-scintigraphy photos, it was suggested that the break-up of the film coated tablets occurred in the human colon. The photographs revealed that radioactivity from the coated tablets remained in the stomach at 2 h after administration. At the 3rd hour it reached small intestinal. At the 4th hour it was still in small intestine with limited drug release. When it reached ascending colon at the 5th hour, drug was released completely for both of the two volunteers.
Results of γ-scintigraphy technique for release of technetium-99 coated time-dependent colon-specific suppository-base-matrix (TDCS-SBM) tablet.
DISCUSSIONS
It is well documented that gastric emptying of a preparation varies. In contrast, the transit time in small intestine for the preparation is surprisingly constant at 3±1 h and appears to be independent of the types of the preparations and whether subjects are fed or not (Halsas et al., 2001HALSAS, M.; PENTTINEN, T.; VESKI, P.; JURJENSON, H.; MARVOLA, M. Time-controlled release pseudoephedrine tablets: bioavailability and in vitro/in vivo correlations. Pharmazie, v.56, n.9, p.718-723, 2001.; Muraoka, Shimokawa, 1998MURAOKA, M.; HU, Z.; SHIMOKAWA, T. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J. Control. Release, v.52, n.1-2, p.119-129, 1998.). Based on these understandings, we designed the enteric time-dependent colon-specific drug delivery system to achieve a considerable drug release after the preparation's arrival into the colon.
A new time-dependent colon-specific suppository-base-matrix delivery system of extract of Lonicera japonica Thunb was developed based on the volume expanding of core tablet, high osmotic pressure, film dissolution, and time-dependent properties. The in vitro and in vivo studies indicated that it is feasible to use TDCS-SBM tablet to achieve stable drug release in colon independent of pH values and paddle agitation speed. Comparing TDCS-SBM with TDCS and other time-controlled release system (Halsas et al., 2001HALSAS, M.; PENTTINEN, T.; VESKI, P.; JURJENSON, H.; MARVOLA, M. Time-controlled release pseudoephedrine tablets: bioavailability and in vitro/in vivo correlations. Pharmazie, v.56, n.9, p.718-723, 2001.), the lag time and drug release were more stable. The drug release of TDCS-SBM tablet showed pulsatile manner, which is different from the WM-TDCS tablet. It is beneficial for the treatment of IBD.
The drug release profiles of TDCS-SBM tablet with inner coated layer were not affected by the pH values of the media. This was reasonably expected, since the channeling agent PEG 400 was pH-insensitive and the formation of the micro-channels was not affected by pH value. The hydrophobic Surelease(r) membrane was relatively impermeable to water molecules; hence it controlled the rate of the water molecules diffusion and water uptake. The weight gain of the coating layer certainly determined the rate of water uptake, by controlling the formation rate of the aqueous channels. The ability of the disintegrating agent to expand was also one of the key factors for time-dependent controlled release (Fan et al.,2001FAN, T.Y.; WEI, S.L.; YAN, W.W.; CHEN, D.B.; LI, J. An investigation of pulsatile release tablets with ethylcellulose and Eudragit L as film coating materials and cross- linked polyvinylpyrrolidone in the core tablets. J. Control Release, v.77, 3p.245-251, 2001.).
CONCLUSIONS
From the results of in vitro and in vivo, this study showed that TDCS-SBM tablet achieved a drug release in pulsatile manner with a 2-3 h lag time in small intestine. PEG has film-dissolution property and is water soluble, the drug release of TDCS-SBM hence showed a pulsatile manner different from the WM-TDCS tablet. It is beneficial for the treatment of IBD. Since PEG can melt and expand at 37 °C, dissolve ethyl cellulose and create osmotic pressures, TDCS-SBM tablet can obtain a quick burst of drug release after 2-3 h lag time in small intestine. This study provides a promising strategy for colon-specific drug delivery using "a time delay" as the release trigger.
ACKNOWLEDGMENT
This study was supported by National Key Technology R&D Program in the 11th Five year Plan of China (No. 2010ZX09401-304-413).
REFERENCES
- CHENG, G.; AN, F.; ZOU, M.J.; SUN, J.; HAO, X.H.; HE, Y.X. Studies on the diclofenac sodium time-dependent and 5-aminosalicylic acid pH dependent oral colon- specific drug delivery systems. World J. Gastroenterol., v.10, n.12, p.1769-1774, 2004.
- FAN, T.Y.; WEI, S.L.; YAN, W.W.; CHEN, D.B.; LI, J. An investigation of pulsatile release tablets with ethylcellulose and Eudragit L as film coating materials and cross- linked polyvinylpyrrolidone in the core tablets. J. Control Release, v.77, 3p.245-251, 2001.
- GASTROINTESTINAL SOCIETY CANADIAN SOCIETY OF INTESTINAL RESEARCH. What is IBD?: symptoms/complications, diagnosis, management. Available at: http://www.badgut.org/information-centre/inflammatory-bowel-disease.html. Accessed on: 20 Dec. 2012.
» http://www.badgut.org/information-centre/inflammatory-bowel-disease.html - GAZZANIGA, A.; MARONI, A.; FOPPOLI, A.; PALUGAN, L. Oral colon delivery: rationale and time-based drug design strategy. Discov. Med., v.36, n.6, p.223-228, 2006.
- GEORGE, A.A. Evaluation of the gastric retention properties of a cross-linked polymer coated tablet versus those of a nondisintegrating tablet. Int. J. Pharm., v.75, n.2-3, p.241-247, 1996.
- GUPTA, V.K.; BECKERT, T.E.; PRICE, J.C. A novel pH- and time-based multi-unit potential colonic drug delivery system. I. Development. Int. J. Pharm., v.213, n.1-2, p.83-91, 2001.
- HALSAS, M.; PENTTINEN, T.; VESKI, P.; JURJENSON, H.; MARVOLA, M. Time-controlled release pseudoephedrine tablets: bioavailability and in vitro/in vivo correlations. Pharmazie, v.56, n.9, p.718-723, 2001.
- JEFFREY, W.M.; HENRY, H.F. Mathematical comparison of dissolution profiles. J. Pharm. Technol., v.20, p.64-74, 1996.
- KROGARS, K.; HEINAMAKI, J.; VESALAHTI, J.; MARVOLA, M.; ANTIKAINEN, O.; YLIRUUSI, J. Extrusion- spheronization of pH-sensitive polymeric matrix pellet for possible colonic drug delivery. Int. J. Pharm., v.199, n.2, p.187-194, 2000.
- MONEY, N.P. Osmotic pressure of aqueous polyethylene glycols. relationship between molecular weight and vapor pressure deficit. Plant Physiol., v.91, n.2, p.766-769, 1989.
- MURAOKA, M.; HU, Z.; SHIMOKAWA, T. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J. Control. Release, v.52, n.1-2, p.119-129, 1998.
- SOODABEH, D.; JALAL, H.; ABBAS, K. Release of 5-ASA from acrylic type polymeric prodrugs designed for colon-specific drug delivery. J. Control. Release, v.58, n.3, p.279-287, 1999.
- XIA, R. Study on determination method and pharmacokinetics of chlorogenic acid in animals after mainline. Sichuan, 2005. p.18-20. (Thesis of Master degree. Sichuan University.).
- XIA, J.H.; LIU, C.X.; The statistical evaluation and analysis of in vitro dissolution of solid preparation. J. Chin. Pharm., v.35, p.1-2, p.345-349, 2000.
- YANG, L.; CHU, J.S.; FIX, J.A. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int. J. Pharm., v.235, n.1-2, p.1-15, 2002.
- ZHANG, X.T.; SHAO, W.H.; WANG, W. The preparation and application of extract of Flos loniceare. CN Pat. 1730015A, 2006.
- ZHU, S.C.; HU, H.Q. The chemical constituent and pharmaceutical action of Lonicera japonica Thunb. Heilongjiang Med. J., n.4, p.299, 2006.
- ZOU, M.J.; OKAMOTO, H.; CHENG, G.; HAO, X.; SUN, J.; CUI, F.; DANJO, K. Synthesis and properties of polysaccharide prodrugs of 5-aminosalicylic acid as potential colon-specific delivery systems. Eur. J. Pharm. Biopharm., v.59, n.1, p.155-160, 2005.
- ZOU, M.J.; WANG, Y.; XU, C.H.; CHENG, G.; REN, J.G.; WU, G.L. Wax-matrix tablet for time-dependent colon-specific delivery system of Sophora flavescens Aiton: preparation and in vivo evaluation. Drug Dev. Ind. Pharm., v.35, n.2, p.224-233, 2009.
Publication Dates
-
Publication in this collection
Jul-Sep 2014
History
-
Received
17 June 2013 -
Accepted
16 Dec 2013