Abstract
L-theanine has neuromodulatory properties, such as attenuate anxiety disorders and stress. Stress is a situation characterized by the stimulus to cortisol secretion and without specific pharmacological therapies. Thus, this study investigates the effect of L-theanine on the response to acute stress, evaluated by tissue cortisol level, oxidative status markers, and behavioral parameters, in zebrafish. The animals were divided into six groups: G1: control, G2: treated with L-theanine 45 mg/L, G3: treated with L-theanine 100 mg/L, G4: stress, G5: stress+L-theanine 45 mg/L and G6: stress+L-theanine 100 mg/L. Before the execution of the acute stress protocol that consisted of chasing the animals with a net for two minutes, exposure to L-theanine was performed in aquarium water for 1 hour. Tissue cortisol levels were analyzed, as well as cerebral oxidative status (lipoperoxidation and levels of thiolic compounds) and fish behavior (new tank test). L-theanine at a concentration of 45 mg/mL modulated the effects of acute stress on zebrafish, inhibiting increased levels of tissue cortisol. However, it was not able to alter the behavior or oxidative status of the animals. Thus, we conclude that the administration of L-theanine can promote acute stress attenuation, modulating the pathological responses of stress.
Keywords:
Stress; L-theanine; Zebrafish
INTRODUCTION
Stress can manifest in many forms, all posing a threat to general homeostasis; hence, different levels of stress response can serve as indicators of well-being and quality of life (Glienke, Piefke, 2017).
Acute stress necessitates an appropriate stimulus; in mammals, it triggers a stimulatory process in the amygdala and hippocampus (Fiksdal et al., 2019). Known as the hypothalamic-pituitary-adrenal axis (HPA), stress induces the production of corticotropin-releasing hormone (CRH), which affects the anterior pituitary gland and regulates the release of adrenocorticotrophic hormone (ACTH). This, in turn, induces the adrenal glands to release adrenaline and, subsequently, cortisol (Tsigos et al., 2020). Specific effects triggered by cortisol include the stimulation of hepatic gluconeogenesis, increased blood glucose, heightened insulin resistance, elevated blood pressure, endothelial dysfunction with increased coagulation, interference with the inflammatory response, suppression of the immune system, and stimulation of acid secretion in the stomach (Hackett, Steptoe, 2016). In teleosts such as zebrafish, the hypothalamus-pituitary-interrenal axis (IPH) is a homolog of the HPA axis in mammals, making it a suitable experimental model for various stress conditions (Alsop, Vijayan, 2008). Unlike murine models, zebrafish have analogs of most human brain structures and well-expressed neurotransmitter systems and neuroendocrine stress axes, exhibiting robust stress-related behaviors that can be reliably evaluated using various tests (Demin et al., 2021).
Considering that existing therapies present undesirable side effects, the search for new substances that attenuate pathological responses to stress has instigated the scientific community. L-theanine is a non-protein amino acid with nutraceutical properties found mainly in green tea leaves (Sharma, Joshi, Gulati, 2018). Structurally, L-theanine is an analog of glutamate; therefore, it binds to the same receptors, hindering the neuroexcitatory effects triggered by glutamatergic activation (Lardner, 2014). This mechanism has already been linked to its beneficial effects on emotional status, such as improvements in mood, cognition (Camfield et al., 2014), and sleep quality (Williams et al., 2020; Türközü, Şanlier, 2017).
In particular, the consumption of L-theanine can attenuate anxiety disorders and mitigate the negative effects of exposure to acute stress (Kimura et al., 2007). Although studies have demonstrated that supplementation with L-theanine attenuates clinical and behavioral effects in models of acute and chronic stress in humans (Evans et al., 2021; Sakamoto et al., 2019), its effect on cortisol levels has not yet been clarified.
Thus, using zebrafish as an experimental model, this study investigated the efficacy of a single exposure to L-theanine on the acute stress response, as assessed by tissue cortisol levels, oxidative status markers, and behavioral parameters.
MATERIAL AND METHODS
Reagents
Trichloroacetic acid (CAS 76-03-9; Vetec Brazil), Glutathione (CAS 70-18-8, Sigma-Aldrich, Germany), 5,5’-ditiobis-(2-nitrobenzoic acid) (CAS 69-78-3, Sigma-Aldrich, Germany), 1,1,3,3Tetraethoxypropane (MDA) (CAS 122-31-6, Sigma-Aldrich, Germany), and L-theanine (99,91% pure) (CAS N/A, Florien, Brazil). All other chemical reagents were of analytical grade (Vetec, Brazil).
Animals
Adult animals (4-6 months) of the wild variety Danio rerio of both sexes (50% males/50% females) were obtained from a commercial supplier (Casa dos Bichos Pet Shop, RS, Brazil). The animals were acclimatized for at least 14 days under laboratory conditions before the experiments. The zebrafish were kept in dechlorified, aerated water in 40-liter aquariums, with controlled temperature (26°C±2°C), under filtration, and at a density of three animals per liter. The light/dark cycle was set at 14/10 hours, respectively. They were fed twice a day with commercial flocked feed (Alcon BASIC®, Alcon, Brazil). Temperature and pH (7.0 and 7.5) were monitored daily. Ammonia, nitrate, and nitrite levels were monitored weekly and maintained at appropriate levels for each species, following the guidelines by Westerfield (2000).
This study was approved by the Ethics Committee on the Use of Animals (CEUA) at the University of Cruz Alta (protocol number 04/2021) and followed the recommendations of the National Council for Animal Control and Experimentation (CONCEA).
Experimental design
The two highest nonlethal concentrations of L-theanine, determined in the acute toxicity test (OECD, 203, 2019) were selected for treatment (data not shown). Fish acclimatized to laboratory conditions were randomly distributed among six 3 L glass aquariums (n=9) based on the following experimental groups:
-
Group 1: Control (n=36)
-
Grupo 2: L-TH 45 mg/L (n=36)
-
Grupo 3 - L-TH 100 mg/L (n=36)
-
Group 4: Stress (n=36)
-
Group 5: L-TH 45 mg/L+stress (n=36)
-
Group 6: L-TH 100 mg/L+stress (n=36)
For data reliability, four independent batches were tested, each comprising nine animals per group per batch). After 24 hours of acclimatization, the animals were exposed to L-theanine for 1 h (Giacomini et al., 2020). They were then placed in a neutral tank and subjected to an acute stress stimulus involving a two-minute chase by a net (Abreu et al., 2017). Following this, behavioral tests were conducted and euthanized by cryo-euthanasia followed by decapitation. The brains of some animals were collected and stored at -80°C to determine oxidative status parameters.
To measure peak cortisol secretion, death by freezing occurred 15 minutes after the execution of the stress protocol (Abreu et al., 2014), and some animals were immediately frozen to determine tissue cortisol levels.
Behavioral assessment
For the Novel Tank Test (NTT), the animals (n=18) were placed individually in an aquarium (30×15×10 cm). A Logitech® C920 HD Pro webcam recorded 6-minute videos, capturing the entire front part of the apparatus to observe and analyze the animals’ behavior pattern. Behavioral analysis was conducted using ANY-Maze® automated tracking software (Stoelting Co. Wood Dale, IL, USA). Parameters such as total distance traveled, average speed, absolute angle of rotation, and time spent in the lower aquarium were measured. These parameters served as indicators of anxiety and locomotor activity (Levin, Bencan, Cerutti, 2007).
Cortisol assessment
Tissue cortisol was extracted following the method described by Sink, Lochmann and Fecteau (2008). A pool of zebrafish (n=3) was macerated, homogenized, and placed in 5mL test tubes (n=6). A total of 3mL of phosphate saline (PBS, pH 7.3) was added to the tubes and homogenized for 30 seconds. Subsequently, 1 mL was transferred to a 10 mL test tube, and 3 mL of ethyl ether was added. This mixture was homogenized for 30 seconds and immediately submerged in nitrogen, with the procedure repeated three times. After homogenization, tubes containing ethyl ether and extracted cortisol were placed in a water bath (37°C) for 12 hours for complete evaporation. The tubes containing lipid extract with cortisol were kept at −20°C. At the time of cortisol measurement, 200 μL of PBS was added to the sample for enzyme immunoassay (ELISA) reading.
Oxidative status markers
For the determination of oxidative status markers, each brain was homogenized with 150μL of Tris-HCl buffer 50 mM pH 7.4.
Following the method of Buege and Aust (1978), lipoperoxidation levels were evaluated by measuring thiobarbituric acid-reactive substances (TBARS). In this technique, the samples (n=8) were mixed with trichloroacetic acid (TCA, 10%) and thiobarbituric acid (0.67 %), heated, and absorbance was measured at 532 nm using a plate reader. The results were expressed in nmol of malondialdehyde (MDA)/mg of protein based on a standard malondialdehyde curve (MDA).
Antioxidant capacity was evaluated by quantifying non-protein sulfhydryl compounds (NPSH) through the reaction with 5’-5-ditiobis-(2-nitrobenzoic) acid (DTNB), according to Boyne and Ellman (1972). Results were expressed in μmol of GSH/mg of protein, based on standard glutathione curve (GSH) (n=6). Protein concentration in all samples was determined following the Peterson (1977) method.
Statistical analysis
The normality and homogeneity of the data were analyzed using the Kolmogorov-Smirnov test. Considering that the data presented a normal distribution, they were subjected to a two-way analysis of variance (ANOVA), considering treatment and stress as factors. Tukey’s test was used to verify the differences between groups. The results were expressed in±standard error of the mean. For all analyses, a p<0.05 value was considered significant.
RESULTS AND DISCUSSION
Stress is a prevalent factor in various neuropsychiatric diseases, particularly affective disorders such as depression, anxiety, panic, phobias, adaptation disorders, and post-traumatic stress disorder (Demin et al., 2021). Pharmacological stress therapy should be comprehensive to all stress symptoms, with a favorable safety profile. However, anxiolytics and antidepressants are the main medications (Anghelescu et al., 2018) that have undesirable side effects such as drowsiness, loss of concentration, and reflexes (Sharma, Joshi, Gulati, 2018). Although several natural products have beneficial effects against stress-related symptoms or stimulating effects, actions that prevent the effects triggered by an acute stress situation have rarely been investigated.
This study investigates the effects of L-theanine on the response to acute stress in an animal model.
As demonstrated in Figure 1, acute stress triggers an increase in tissue cortisol levels in teleosts, emphasizing the activation of the hypothalamic-pituitary-interstellar axis (IHP), analogous to the human HPA axis. When a stressful stimulus occurs, the hypothalamus, which includes the preoptic nucleus homologous to the paraventricular nucleus in mammals, produces CRH, which acts on the anterior pituitary. Mediated by the CRFR1 receptor, ACTH is released into the bloodstream, which travels to the steroidogenic cells of the interrenal tissue, where it promotes the activation of melanocortin receptor 2 (MC2R), which stimulates the cortisol synthesis cascade (Demin et al., 2021). The similarity in hormonal regulation between zebrafish and mammals makes this experimental model superior to murine models that synthesize corticosterone (Tsigos et al., 2020).
Tissue cortisol level in zebrafish treated with L-theanine and subjected to acute stress. Data are expressed as mean±standard error of mean, n=6. *p<0,05 different from Stress Control group.
Furthermore, we verified that pretreatment of animals with L-theanine at a concentration of 45 mg/L prevented the stress-induced increase in cortisol without altering levels in non-stressed controls. Cortisol is one of the most widely used stress biomarkers because it is important in animal responses to dangerous situations. It is involved in adaptive processes, and because of its ease of diffusion into tissues, its levels increase within a few minutes (Lunkes et al., 2021).
Clinical studies have evaluated the effects of L-theanine on cortisol levels. Although it has been demonstrated that the administration of L-theanine promotes a reduction in salivary cortisol levels in stressful situations (Evans et al., 2021; White et al., 2016), other studies have not reported the same results (Hidese et al., 2019). Considering this inconsistency, studies related to stress in humans present methodological and ethical difficulties, such as the consumption of other types of tea, medications, diet, nutrient intake, and the type of work (Hidese et al., 2019), making experimental animal models powerful tools in stress-related research. To the best of our knowledge, this is the first experimental study to demonstrate a reduction in tissue cortisol levels after a single exposure to L-theanine, confirming its effects on the HPA axis. Surprisingly, the highest concentration used (100 mg/L) did not alter the cortisol response, suggesting that L-theanine may have a U-shaped dose-response curve similar to that of alcohol on fish behavior (Oliveira et al., 2013; Gerlai et al., 2000) and stress response (Barreto, Volpato, 2004).
Interestingly, the effect of L-theanine in reducing the level of tissue cortisol occurred only in animals subjected to the stress protocol, suggesting that it did not reduce hormone levels under physiological homeostasis conditions. It then modulates cortisol levels in stressful situations without altering them in basal situations.
Regarding behavioral parameters, it was not possible to observe changes in locomotor capacity or exploratory patterns in the groups studied (Figure 2). Although there are several methods to induce acute stress in zebrafish, the net chasing method robustly increases cortisol levels, which reflects a high-stress condition (Abreu et al., 2014; Barcellos et al., 2011; Mocelin et al., 2015), although it may not cause behavioral changes in these animals (Tran et al., 2014). In the NTT, the anxiety behavior of the zebrafish was characterized by an increase in the time spent in the bottom area of the tank escaping from the surface. However, stressed animals do not necessarily exhibit the characteristic behavior of anxiety (Giacomini et al., 2016; Lunkes et al., 2021), as evidenced by the fact that an increase in tissue cortisol levels was not accompanied by anxiety-indicative behavior.
Behavioral effects of L-theanine in acutely stressed zebrafish. Data are expressed as mean±standard error of mean. G1 (n=16); G2 (n=18); G3 (n=17); G4 (n=18); G5 (n=18); G6 (n=18).
Although not demonstrated in this study, other studies in humans and animals have already described that L-theanine has anxiolytic effects, with no side effects such as drowsiness (Evans et al., 2021; Sakamoto et al., 2019). This effect is related to the increased activity of alpha frequency waves, as evidenced by a human-electroencephalogram (EEG), indicating relaxation of the mind without inducing drowsiness (Evans et al., 2021). Its effects on relaxation, tranquility, and well-being are related to its ability to bind to glutamate receptors and block the binding of l-glutamic acid to these receptors in cortical neurons. Hence, it is hypothesized that L-theanine may reduce the stress-induced excitation of peripheral sympathetic activity (Hackett, Steptoe, 2016). L-theanine consumption can also suppress excessive glutamatergic tone, which is implicated as a possible cofactor in increasing stress and related anxiety responses (Williams et al., 2020).
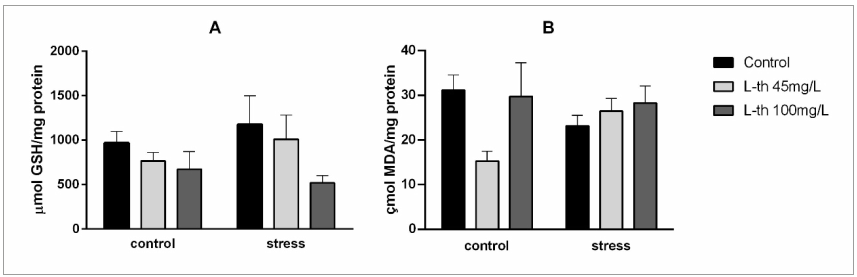
It is noteworthy that L-theanine in the tested concentrations did not exhibit stimulating effects, as it did not alter locomotor or exploratory capacity (Giles et al., 2017), which may be an advantage to other substances used to mitigate the effects of stress, such as caffeine, that can have a stimulatory hyper-effect (Bryan, 2008). Regarding the oxidative status, changes in lipoperoxidation levels and non-protein thioic compounds could not be verified (Figure 3). Although oxidative stress is associated with acute stress, the induction time used in this model may have been insufficient to alter these parameters. Lunkes et al. (2021) observed oxidative stress in fish subjected to prolonged acute stress. Furthermore, pretreatment with L-theanine did not demonstrate antioxidant activity, suggesting that this is not the mechanism underlying its anti-stress actions.
Brain oxidative status markers of zebrafish treated with L-theanine and submitted to an acute stress protocol. NPSH: Non-protein sulfhydryl compounds, n=6. TBARS: Thiobarbituric acid reactive substances, n=8. The values are expressed as mean±standard error of mean.
This study had some limitations. No other markers, such as antioxidant enzymes or damage markers in other biomolecules, such as proteins and DNA, were evaluated regarding oxidative status. In the behavioral evaluation, the NTT was not as specific for the evaluation of anxiety parameters, which may explain why we did not observe anxiogenic effects after the application of the acute stress protocol. Nevertheless, this study demonstrates L-theanine’s important hormonal modulatory activity, contributing to the elucidation of its previously described clinical effects.
We conclude that a single exposure to L-theanine can significantly attenuate acute stress by inhibiting the increase in tissue cortisol levels. This effect does not seem to be dose-dependent and does not occur under physiological conditions, characterizing hormonal modulation only under periods of stress. Thus, considering the absence of preventive therapies against stress-related pathologies, the data presented herein warrant further investigation of L-theanine as a potential anti-stress therapeutic agent.
ACKNOWLEDGEMENTS
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brasil) for the master’s scholarship granted to M. S. Mayer (Finance Code 001).
REFERENCES
- Abreu MS, Koakoski G, Ferreira D, Oliveira TA, Rosa JG, Gusso D, et al. Diazepam and fluoxetine decrease the stress response in zebrafish. PloS one. 2014; 9(7): e103232.
- Abreu MS, Giacomini ACVV, Koakoski G, Piato ALS, Barcellos LJG. Divergent effect of fluoxetine on the response to physical or chemical stressors in zebrafish. Peer J. 2017; 9(5): e3330.
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2008; 294(3): R711-9.
- Anghelescu IG, Edwards D, Seifritz E, Kasper S. Stress management and the role of Rhodiola rosea: a review. Int J Psychiatry Clin Pract. 2018; 22(4): 242-252.
- Barcellos LJ, Volpato GL, Barreto RE, Coldebella I, Ferreira D. Chemical communication of handling stress in fish. Physiol Behav. 2011; 103(3-4): 372-5.
- Barreto RE, Volpato GL. Caution for using ventilatory frequency as an indicator of stress in fish. Behav Processes. 2004; 66(1): 43-51.
- Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972; 46(2): 639-53.
- Bryan J. Psychological effects of dietary components of tea: caffeine and L-theanine. Nutr Rev. 2008; 66(2): 82-90.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52: 302-10.
- Camfield DA, Stough C, Farrimond J, Scholey AB. Acute effects of tea constituents L-theanine, caffeine, and epigallocatechin gallate on cognitive function and mood: a systematic review and meta-analysis. Nutr Rev. 2014; 72(8): 507-22.
- Demin KA, Taranov AS, Ilyin NP, Lakstygal AM, Volgin AD, de Abreu MS, et al. Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress. 2021; 24(1): 1-18.
- Evans M, McDonald AC, Xiong L, Crowley DC, Guthrie N. A Randomized, Triple-Blind, Placebo-Controlled, Crossover Study to Investigate the Efficacy of a Single Dose of AlphaWave® L-Theanine on Stress in a Healthy Adult Population. Neurol Ther. 2021; 10(2): 1061-1078.
- Fiksdal A, Hanlin L, Kuras Y, Gianferante D, Chen X, Thoma MV, et al. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology. 2019; 102: 44-52.
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000; 67(4): 773-82.
- Giacomini ACVV, Abreu MS, Giacomini LV, Siebel AM, Zimerman FF, Rambo CL, et al. Fluoxetine and diazepam acutely modulate stress induced-behavior. Behav Brain Res. 2016; 1(296): 301-310.
- Giacomini ACVV, Piassetta AS, Genario R, Bonan CD, Piato A, Barcellos LJG, et al. Tryptophan alleviates neuroendocrine and behavioral responses to stress in zebrafish. Behav Brain Res. 2020; 27(378): 112264.
- Giles GE, Mahoney CR, Brunyé TT, Taylor HA, Kanarek RB. Caffeine and theanine exert opposite effects on attention under emotional arousal. Can J Physiol Pharmacol. 2017; 95(1): 93-100.
- Glienke K, Piefke M. Stress-related cortisol responsivity modulates prospective memory. J Neuroendocrinol. 2017; 29(12): 10-111.
- Hackett RA, Steptoe A. Psychosocial Factors in Diabetes and Cardiovascular Risk. Curr Cardiol Rep. 2016; 18(10): 95.
- Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, et al. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients. 2019; 11(10): 2362.
- Kimura K, Ozeki M, Juneja LR, Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol Psychol 2007; 74: 39-45.
- Lardner AL. Neurobiological effects of the green tea constituent theanine and its potential role in the treatment of psychiatric and neurodegenerative disorders. Nutr. Neurosci. 2014; 17(4): 145-155.
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007; 90(1): 54-8.
- Lunkes LC, Paiva IM, Egger RC, Braga WF, Alvarez-Leite JI, da Cunha Barreto-Vianna AR, et al. Melatonin administration attenuates acute stress by inducing sleep state in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol. 2021; 246: 109044.
- Mocelin R, Herrmann AP, Marcon M, Rambo CL, Rohden A, Bevilaqua F, de Abreu MS, Zanatta L, Elisabetsky E, Barcellos LJ, Lara DR, Piato AL. N-acetylcysteine prevents stress-induced anxiety behavior in zebrafish. Pharmacol Biochem Behav. 2015; 139: 121-6.
- OECD. Test No. 203: Fish, Acute Toxicity Test. OECD Guidelines for the Testing of Chemicals, Section 2. 2019. OECD Publishing, Paris.
- Oliveira TA, Koakoski G, Kreutz LC, Ferreira D, da Rosa JG, de Abreu MS, et al. Alcohol impairs predation risk response and communication in zebrafish. PLoS One. 2013; 7; 8(10): e75780.
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977; 83(2): 346-56.
- Sakamoto FL, Ribeiro RMP, Bueno AA, Santos HO. Psychotropic effects of L-theanine and its clinical properties: From the management of anxiety and stress to a potential use in schizophrenia. Pharmacol Res. 2019; 147: 104395.
- Sink TD, Lochmann RT, Fecteau KA. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol Biochem. 2008; 34: 95-101.
- Sharma E, Joshi R, Gulati A. l-Theanine: An astounding sui generis integrant in tea. Food Chem. 2018; 1(242): 601-610.
- Tran S, Chatterjee D, Gerlai R. Acute net stressor increases whole-body cortisol levels without altering whole-brain monoamines in zebrafish. Behav Neurosci. 2014; 128(5): 621-624.
- Tsigos C, Kyrou I, Kassi E, Chrousos GP. Stress: Endocrine Physiology and Pathophysiology. 2020. editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.
- Türközü D, Şanlier N. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit Rev Food Sci Nutr. 2017; 57(8): 1681-1687.
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 2000; 4th ed. Eugene: University of Oregon Press.
- White DJ, de Klerk S, Woods W, Gondalia S, Noonan C, Scholey AB. Anti-Stress, Behavioural and Magnetoencephalography Effects of an L-Theanine-Based Nutrient Drink: A Randomised, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients. 2016; 8(1): 53.
- Williams JL, Everett JM, D’Cunha NM, Sergi D, Georgousopoulou EN, Keegan RJ, et al. The Effects of Green Tea Amino Acid L-Theanine Consumption on the Ability to Manage Stress and Anxiety Levels: a Systematic Review. Plant Foods Hum Nutr. 2020; 75(1): 12-23.
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
26 May 2023 -
Accepted
14 May 2024

 L-theanine modulates tissue cortisol levels in zebrafish after single stress
L-theanine modulates tissue cortisol levels in zebrafish after single stress