Abstract
Colorectal cancer (CRC) is the third leading cause of cancer death in the world, and its incidence is steadily rising in developing nations. Cell cycle aberrations due to deregulation of cyclin dependent kinases (CDKs) and cyclins are common events during colorectal carcinogenesis. Herein, we investigate the anticancer potential of two multitarget CDK inhibitors viz. roscovitine (specific inhibitor of CDK1, 2, 7, and 9) and UCN-01 (pan CDK inhibitor) against three CRC cell lines. Both the drugs exerted cytotoxicity and inhibited clonogenic potential of human CRC cell lines. These drugs induced apoptosis, downregulated cell cycle regulatory and transcriptional CDKs and cyclins’ protein expression as well as their activity. Moreover, dual combination of either of these CDK inhibitors with standard chemotherapeutic drugs was found to be synergistic in CRC cells. Thus, we demonstrate that multiple CDK inhibition offers promising therapeutic strategy against CRC.
Keywords:
CDK inhibitors; Roscovitine; UCN-01; Colorectal cancer; Cell cycle; Apoptosis; Transcription inhibition; Drug combination
INTRODUCTION
Colorectal cancer (CRC) is one of the most commonly diagnosed cancer and among the leading causes of cancer mortality in the world (Bray et al., 2024). CRC is more frequent in developed countries. CRC can arise spontaneously or due to family history or due to inflammatory conditions such as colitis. Extensive metastasis is the main cause of CRC mortality within five years of diagnosis in about half of the patients. Treatment is primarily surgery for localized disease, and chemotherapy-based regimen is used for metastatic tumors (Lee, Oh, 2016). However, drug resistance causes relapse in most of the patients.
Deregulated cell cycle progression has been shown to be closely associated with malignant transformation in gastrointestinal (GI) cancers which includes CRC (Costa, Gil Da Costa, Medeiros, 2018). In human CRC, cell cycle abnormalities are due to hyperactivation of CDKs and their cyclin partners along with loss of activity of natural CDK inhibitors (Manohar, 2022). CDK1 has been identified to be an attractive drug target for CRC due to its constitutive activation in CRC (Salh et al., 1999). In an integrated bioinformatics analysis study, CDK1 along with CDC20 was shown to be strongly correlated to poor survival in CRC patients (Li et al., 2020). CDK1 upregulation has been observed in a subset of human BRAF V600E mutant therapy-resistant colorectal cancers (Ghafouri-Fard et al., 2022). Cyclin B1 is often overexpressed in colorectal tumors (Korenaga et al., 2002). Cyclin D1 is overexpressed in CRC and is also linked to poor survival and drug/radio resistance (Maeda et al., 1997). CDK2 overexpression is more common in malignant CRC as compared to early lesions (Tong et al., 2017). Cyclin A is overexpressed in about 75% of CRCs and has been considered as a prognostic factor for CRC (Handa et al., 1999). Cyclin B1 and cyclin E overexpression is also linked to CRCs (Bonelli et al., 2014).
KRAS mutations are frequently associated with CRC. Due to the challenges in designing direct KRAS inhibitors, various inhibitors of upstream and downstream pathways of KRAS have been studied in KRAS-mutant CRCs (Ramani, Samant, Manohar, 2022; Bteich et al., 2023). Palbociclib (CDK4/6 inhibitor), the approved therapy for treatment of advanced breast cancers, shows limited efficacy in KRAS-mutant CRCs when used alone (Damato et al., 2018). It has been hypothesized that given the hyperactivation and redundancy of many CDKs in CRC, multitarget CDK inhibition offers more promising treatment strategy (Manohar, 2022). Based on the available literature, it emerges that multitarget CDK inhibitors are more likely to hit several redundant target CDKs simultaneously thereby reducing the risk of unresponsiveness and drug resistance in cancer (Canavese, Santo, Raje, 2012). Thus, in this study, we sought to determine anticancer activity of two well-known CDK inhibitors with differing CDK inhibitory spectrum, on CRC cell lines viz. roscovitine and UCN-01 alone and in combination with standard drugs in three CRC cell lines. Roscovitine belongs to the 2, 6, 9-tri-substituted purines and is an ATP-competitive inhibitor of CDK1, 2, 7 and 9 with almost similar IC50s against them (Meijer et al., 1997). R-isomer of roscovitine (also known as seliciclib or CYC202) has been shown to be more potent over racemic roscovitine. Although results from several clinical trials are not that promising, roscovitine is frequently used as an experimental ‘selective’ CDK inhibitory drug in several biological studies (Delehouzé et al., 2014). UCN-01 (7-hydroxystaurosporine) is an indolocarbazole compound that was initially developed as protein kinase C inhibitor. Later, it was discovered to inhibit several other kinases including CDKs (Mull et al., 2020). UCN-01 has been tested in several clinical trials across various types of cancers, though its activity was not promising most likely due to its pharmacokinetic profile (Ma et al., 2013). In the present study, we have also scrutinized their effects on target CDKs in order to predict the mechanism of action.
MATERIAL AND METHODS
Cell culture and reagents
Human CRC cell lines Colo-205, HCT116 and HCT-15 were obtained from ATCC (Rockville, MD, USA). Colo-205 and HCT-15 were cultured in RPMI-1640 medium and HCT116 in McCoy’s 5A, both the media containing 10% fetal bovine serum (FBS) (Hyclone, UT, USA), 2 mmol/L L-glutamine (Gibco, Grand Island, NY, USA), 1X Antibiotic-Antimycotic solution (Gibco). Cells were maintained at 37°C in a humidified 5% CO2 incubator. All the drugs were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mmol/L (10 mM) and stored at -20°C until use; diluted in culture medium before use and used within 4 h. All reagents were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise.
In vitro cytotoxicity assay
Cytotoxic activity of CDK inhibitors on CRC cell lines was assessed using CCK-8 assay (Dojindo laboratories, Japan). Briefly, cells were seeded at a density of 3000 cells/well in 96-well plates and after overnight incubation, treated with desired concentrations of the drugs. At the end of the treatment, CCK-8 was added and after 4 h incubation, absorbance was measured at 450 nm using a Spectramax Microplate Reader (Molecular Devices, CA, USA). Data was analyzed to determine the IC50 (concentration of drug that inhibited cell growth by 50%).
Clonogenic assay
Adherent CRC cell lines viz. HCT116 and HCT-15 cells were plated into six-well plates at a density of 300 cells/well and 400 cells/well respectively and incubated overnight at 37°C in a 5% CO2 incubator. Next day, cells were treated with roscovitine or UCN-01 for 24 h. Medium with drug was then replaced with medium without drug and plates were further incubated till visible colonies appeared. Further, cells were fixed with methanol: glacial acetic acid (2:1) and stained with 0.5% crystal violet. After staining, plates were rinsed twice with distilled water, dried and images were captured. Stained colonies were counted using ImageJ software (NIH ImageJ, Biocompare, South San Franscisco, CA, USA). Plating efficiency (PE) and surviving fraction (SF) after treatment were calculated as per the following formulae:
The number of colonies that arise after treatment of cells expressed in terms of PE, is called the surviving fraction.
Trypan blue viability assay
Cells were seeded at a density of 0.3 × 106 per well into six-well plates and incubated overnight at 37°C and 5% CO2. Next day, cells were treated with range of concentrations of CDK inhibitors for 24 h. At the end of the incubation period, both adherent and floating cells were collected and washed once with PBS. Cells were incubated at room temperature with 0.2% trypan blue in PBS for 5 min. Samples were kept on ice, trypan blue positive as well as total cell population were counted microscopically using a hemocytometer and number of viable cells was calculated.
Analysis of cell cycle distribution by flow cytometry
CRC cell lines were seeded in T-25 tissue culture flasks at a density of 0.5 × 106/mL and incubated overnight at 37°C in a 5% CO2 incubator. Next day, cells were treated with or without (control) CDK inhibitors. At the end of treatment period, cells were harvested and processed for flow cytometry on BD FACS Aria™ (BD Biosciences, San Jose, CA, US) (Manohar et al., 2011).
Annexin V/PI staining
Colo-205 cells were seeded at a density of 0.5 × 106 cells per T-25 flask and incubated overnight at 37°C in a humidified 5% CO2 incubator. Next day, cells were treated with CDK inhibitors at 2 × IC50 concentration for 24 h. Annexin V/PI staining was performed using kit (Promega, Madison, WI, USA) and the samples were analyzed by flow cytometry on BD FACS AriaTMwithin 30 min (BD Biosciences, San Jose, CA, US) (Manohar et al., 2019).
TdT‑mediated dUTP Nick‑End Labeling (TUNEL) assay
HCT116 cells were seeded at a density of 0.3 × 106/well onto coverslips in six-well plate and were allowed to adhere overnight at 37°C in a humidified 5% CO2 incubator. Next day, cells were treated with 2 × IC50 concentrations of CDK inhibitors for 24 h and then TUNEL assay was performed using DeadEnd Flurorometric TUNEL system (Promega, Madison, WI, USA) as per manufacturer’s instructions. Briefly, cells were fixed using 3.7% paraformaldehyde in PBS for 25 min at 4°C and subsequently washed with PBS twice (5 min each). Then permeabilization was done using 0.2% Triton X-100 in PBS for 5 min followed by washing with PBS twice (5 min each). Hundred µl of equilibration buffer was added and equilibration was carried out for 5-10 min at room temperature. Fifty µl of TdT reaction mix was then added onto the coverslips and incubated for 1 h at 37°C in a humidified chamber in dark. Cells were then treated with 2X SCC buffer for 15 min and washed subsequently with PBS twice (5 min each). The coverslips were mounted using UltraCruz mounting medium onto glass slides and images were captured using Carl Zeiss inverted fluorescence microscope Z1.
Caspase‑Glo 3/7 assay
CRC cells were seeded (10,000 cells/well) in a 96-well white bottom plate and were allowed to adhere overnight at 37°C in a humidified 5% CO2 incubator. Next day, cells were treated with two different concentrations of CDK inhibitors for 24 h (roscovitine-10, 30 µM; UCN-01-0.3, 1 µM). At the end of incubation, 100 µl of Caspase-Glo 3/7 reagent (Promega, Madison, WI, USA) was added to each well and after 1 h incubation at room temperature; luminescence was read on PolarSTAR Optima plate reader BMG Technologies (BMG Labtech GmbH, Ortenberg, Germany).
Protein expression analysis by western blotting
Cells were seeded, treated with or without CDK inhibitors, harvested at desired time points and western blotting was carried out as previously described (Manohar et al., 2011). Blots were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, IL, USA) and imaged using ChemiDoc XRS+ imaging system and image lab software (Bio-Rad Laboratories, MA, USA). Densitometric analysis of western blots was carried out using ImageJ software.
Antibodies used in this study were: PARP, caspase-3, Mcl-1, Bcl-2, cyclin B1, phospho RNA Pol II CTD Ser 2/5 (Cell Signaling Technology, USA), Bcl-Xl (BD transduction laboratories, USA), Bax, cyclin D1, CDK4, cyclin E, cyclin A, CDK2, CDK1, CDK9, CDK7, cyclin T1, cyclin H, phospho Rb Ser 780, total Rb, total Pol II, anti-rabbit-IgG-HRP and anti-mouse-IgG-HRP secondary antibodies (Santacruz Biotechnology, CA, USA), β-actin (Sigma).
In vitro cytotoxicity assay for combination studies
CRC cells were plated in 96-well plates and treated with standard chemotherapeutic drug or either of the CDK inhibitors or dual combination of a standard drug and a CDK inhibitor simultaneously for a period of 96 h. In combination, each concentration of the standard drug and that of CDK inhibitor were combined in a fixed ratio (as described in Results section and shown in Table SI). Oxaliplatin concentration range selected for HCT116 was different as it was less potent as a single drug in this cell line. Effectiveness of the combination was evaluated on the basis of combination index (CI) calculated using CompuSyn software wherein CI < 1, = 1, and > 1 indicate synergism, additive effect and antagonism respectively (Chou, 2006).
Statistical analysis
Statistical comparison was made using GraphPad PRISM (version 8.0 GraphPad Software, Inc., USA) software wherein Student’s paired t-test was employed. Data are presented as mean ± SD of at least three independent experiments. Statistical significance was evaluated by calculating p values and differences of p < 0.05 were considered as statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001).
RESULT
Roscovitine and UCN‑01 exert cytotoxicity in human CRC cells
We recently showed that the three small molecule CDK inhibitors viz. riviciclib, roscovitine and UCN-01 exert cytotoxicity in CRC cell lines viz. Colo-205, HCT116 and HCT-15 (Manohar, Joshi, 2022). In this previous study by us, roscovitine was found to be moderately potent while UCN-01 was highly potent across all three CRC cell lines with average IC50 values of 25 μM and 0.2 μM respectively after 48 h treatment (Figure S1, Figure S2). In the present study, we have used the same IC50 values for assays in order to further delineate the efficacy and mechanism of action of roscovitine and UCN-01 in CRC cells.
Multitarget CDK inhibitors inhibit clonogenicity and viability of CRC cells
To determine whether roscovitine and UCN-01 inhibit the clonogenic potential, conventional clonogenic assay was carried out using adherent CRC cell lines HCT116 and HCT-15. Plating efficiency (PE) for HCT116 was 33% with surviving fraction (SF) < 0.0001 whilst in HCT-15, PE was 21% with SF ~ 0.002 for both drugs. Hence, both the drugs significantly decreased colony forming potential of these cell lines (Figure 1B). Since this assay could not be done in semi-adherent cell line Colo-205, cell viability was tested in these cells after 24 h treatment with range of concentrations of CDK inhibitors using trypan blue dye exclusion assay (Figure 1C). UCN-01 inhibited cell viability most potently at all concentrations (0.03-1 µM) followed by roscovitine which elicited a dose-dependent response at 10-100 µM.
(A) Chemical structures of roscovitine and UCN-01, roscovitine and UCN-01 potently inhibit (B) clonogenic potential and (C) viability of CRC cell lines.
Roscovitine and UCN‑01 abrogate cell cycle progression in CRC cells
Cell cycle analysis of drug-treated CRC cells was done using flow cytometry (Figure 2). At 6 h in Colo-205, UCN-01 decreased number of cells in S phase in comparison to untreated control and increased in sub G1 which signifies cell death. No significant changes in the cell cycle distribution were seen with roscovitine in this cell line at 6 h. At the same timepoint, In HCT116, roscovitine decreased G0-G1 fraction and UCN-01 lead to reduced G0-G1 fraction and significantly increased sub G1 fraction. At 6 h, in HCT-15, roscovitine 3 × IC50 increased sub G1 fraction and UCN-01 showed decreased G0-G1 with increase in sub G1 at 6 h for both IC50 and 3 × IC50. After 18 h, increase in cell death with roscovitine and UCN-01 was significant. At a later time point of 24 h, HCT-15 was least sensitive to both the drugs and the pattern of cell cycle distribution was similar to that seen at 18 h treatment.
Effect of CDK inhibitors roscovitine and UCN-01 on the cell cycle in CRC cells. (A) Representative data of cell cycle analysis after 24 h treatment. (B) Percent population of CRC cells in sub G1 (indicating apoptosis) after drug treatment.*: p < 0.05; **: p < 0.01; ***: p < 0.001.
CDK inhibitors induce apoptosis in CRC cells
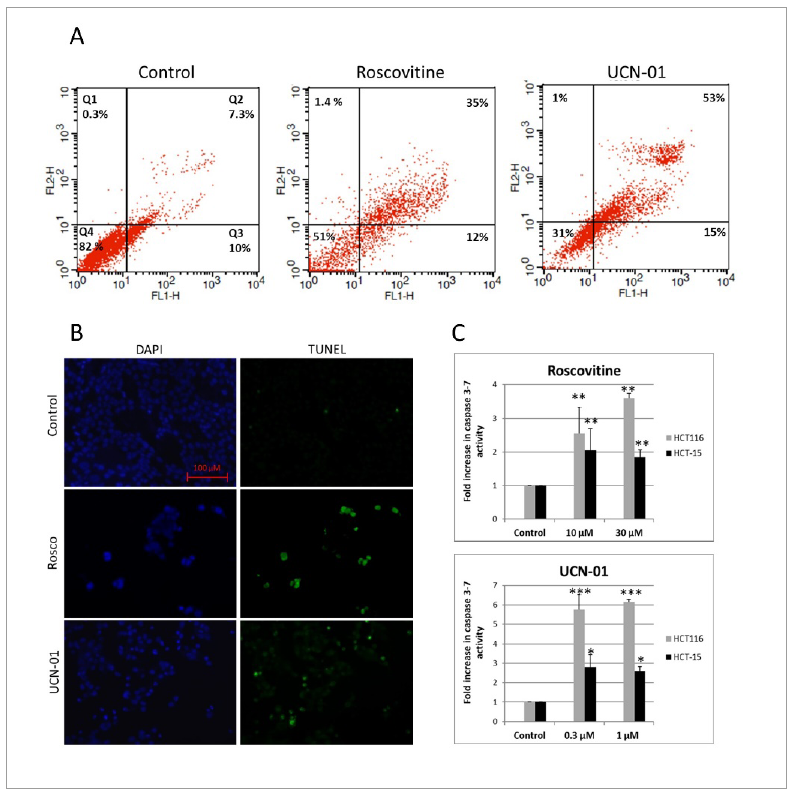
Apoptosis induction by CDK inhibitors in CRC cell lines was confirmed using annexin V/PI, TUNEL and Caspase-glo 3/7 assays. Twenty-four hours timepoint was selected which is equivalent to one complete cell cycle and concentration of 2 × IC50 was used. Significant apoptosis was induced in Colo-205 as evident from significantly increased annexin V positive-PI positive population after roscovitine or UCN-01 treatment (p < 0.01) (Figure 3A).
Apoptosis-inducing potentital of roscovitine and UCN-01 in CRC cell lines is confirmed by: (A) annexin V/PI staining in Colo-205 (B) TUNEL assay in HCT116 and (C) caspase-3/7 enzyme activity assay in HCT116 and HCT-15.
Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay was employed to confirm drug-induced apoptosis in HCT116. When compared with the control, treated cells were positively labeled by TUNEL and nuclear fragmentation in these apoptotic cells was also detectable when counterstained with DAPI (Figure 3B). Thus, it was evident that roscovitine and UCN-01 induced apoptosis in this cell line.
Further, Caspase-glo 3/7 assay was used to confirm increase in caspase-3/7 activity upon CDK inhibitor treatment-a hallmark of apoptosis. Both the CDK inhibitors significantly increased caspase-3/7 activity in both cell lines. UCN-01 showed remarkable caspase activation in HCT116 (upto 6 fold) (Figure 3C). Overall, increase in caspase-3/7 activity was significantly more in HCT116 as compared to HCT-15.
After confirming apoptosis by the above mentioned cell-based assays, effect of roscovitine and UCN-01 was tested on apoptotic markers i.e. cleaved caspase-3, cleaved PARP (substrate for caspase-3) and Bcl-2 family proteins after 24 h drug treatment. There was a significant increase in cleaved capsase-3 levels in response to treatment with both drugs in all the three cell lines (Figure 4). Cleaved PARP levels were found to be significantly increased by both the drugs in HCT116 and HCT-15. In Colo-205, there was no detectable increase in cleaved PARP upon roscovitine treatment. Mcl-1 was downregulated most significantly in HCT116 by both drugs while Bcl-2 was downregulated most potently in HCT-15 by both drugs. Bcl-Xl levels remained unchanged in all three cell lines upon drug treatment. Bax levels were unchanged in Colo-205, slightly increased in HCT116 and slightly decreased upon treatment in HCT-15. Densitometric analysis of all western blots is given in Figure S3.
Roscovitine and UCN-01 induce PARP and caspase-3 cleavage and downregulate anti-apoptotic proteins in CRC cell lines. Control: untreated, Rosco: roscovitine, x: IC50, 3x: 3 × IC50, Cl: cleaved, Casp-3: caspase-3.
Roscovitine and UCN‑01 modulate expression of cell cycle regulatory proteins
Changes in protein levels of cell cycle regulatory CDKs and cyclins in response to treatment with CDK inhibitors were analyzed by western blotting in HCT116 (Figure 5A-C). Both the drugs downregulated cyclin D1 and CDK4 protein levels. Surprisingly, cyclin E was up regulated by roscovitine at IC50 concentration at 18 h and 24 h time points. As anticipated, cyclin A expression was inhibited by roscovitine whereas CDK2 levels remained almost unchanged. Whilst cyclin B1 was downregulated after 18 h and 24 h, CDK1 remained almost unchanged in response to roscovitine treatment.
Effect of (A) roscovitine and (B) UCN-01 on expression of cell cycle-related CDKs and cyclins in HCT116. Well no. 1: untreated, 2: treated with IC50, 3: treated with 3 × IC50.
Cyclin E levels were downregulated by UCN-01 after 24 h treatment with both concentrations. UCN-01 also increased cyclin A levels after 6 h with 3 × IC50 followed by significant downregulation after 18 and 24 h treatment with both concentrations. This drug led to down regulation of CDK2, CDK1 and cyclin B1 at all three time points at both concentrations.
Overall, results indicate that CDK inhibitors down regulated cell cycle-related cyclins and CDKs with varied potency in HCT116 cell line.
Roscovitine and UCN‑01 inhibit transcription
Previously, it has been reported by us and others that CDK inhibitory drugs inhibit transcriptional CDKs and cyclins in hematological cancers (MacCallum et al., 2005; Manohar et al., 2011). In the present study, both the drugs downregulated cyclin T1, CDK9, and CDK7 protein levels after 18 h treatment in HCT116 cells. Unpredictably, cyclin H levels were found to be upregulated by roscovitine IC50 and UCN-01 3 × IC50 (Figure 6A).
Roscovitine and UCN-01 downregulate protein expression of (A) transcriptional CDKs and cyclins and (B) phospho-Rb and phospho-RNA Pol II CTD in HCT116. Control: untreated, Rosco: roscovitine, x: IC50, 3x: 3 × IC50.
Both roscovitine and UCN-01 were shown to potently inhibit phosphorylation of Rb at Ser 780 (cell cycle CDK substrate) and RNA polymerase II carboxy-terminal domain at Ser 2/5 (RNA Pol II CTD) (transcriptional CDK substrate) after 18 h treatment in HCT116 (Figure 6B).
CDK inhibitors are synergistic with standard chemotherapeutic drugs against CRC cells
Currently, standard-of-care for CRC includes chemotherapeutic drugs viz. 5-FU, irinotecan and oxaliplatin. Efficacy of dual combination of these standard drugs with CDK inhibitors in CRC cell lines was tested using CCK-8 cytotoxicity assay. Each of the standard chemotherapeutic drug was combined with a CDK inhibitor in a fixed ratio which was based on the IC50 values of the single drugs (Chou, 2006) (Table SI). Average 72 h IC50 values of standard chemotherapeutic drugs were: 5-FU: 6 μM, oxaliplatin: 0.8 μM (for HCT-15) and 5.5 μM (for HCT116). The drugs were added together for 96 h. Effectiveness of the combination was evaluated using combination index (CI) calculated by the CompuSyn software (CI < 1, = 1, and > 1 indicate synergism, additive effect and antagonism respectively). Remarkably, both CDK inhibitors showed synergism with at least one of the standard drugs in CRC cell lines as described below.
Colo‑205
UCN-01 was synergistic when added together with 5-FU (CI = 0.15-0.94) or with irinotecan (CI = 0.21-0.71). Roscovitine showed synergism when added with oxaliplatin (CI = 0.09-0.8) (Figure 7A).
Combination indices (CI) of synergistic dual combinations of roscovitine or UCN-01 with standard drugs in CRC cell lines. Values are presented as mean ± SD of three independent experiments, each in triplicates. Irino: irinotecan, Oxalo: oxaliplatin, 5-FU: 5-fluorouracil.
HCT116
Notably, UCN-01 was very strongly synergistic in combination with oxaliplatin (CI= 0.03-0.77) and so was roscovitine (CI= 0.2-0.9) (Figure 7B). UCN-01 was moderately synergistic with 5-FU (CI= 0.4-0.7).
HCT‑15
In this multidrug resistant cell line, UCN-01 was moderately synergistic with oxaliplatin (CI= 0.16-1.18) (Figure 7C).
DISCUSSION
Aberrant activation of cell cycle-related oncogenic proteins in human colorectal cancer offers prospects for anticancer drug discovery. In the present study, we compared the efficacy of two CDK inhibitors with differential CDK inhibitory spectrum in three CRC cell lines. Both the drugs exerted potent anticancer activity irrespective of their specificity for target CDKs. UCN-01 being a non-specific (pan CDK) inhibitor was more potent with almost 10 times lower IC50 than that of roscovitine which is specific for CDK1, 2, 7 and 9. Both drugs effectively restrained clonogenic capacity of CRC cells which indicates loss of tumor cell reproductive viability. We also confirmed the apoptosis-inducing potential of these inhibitors using cell cycle analysis, annexin V/PI assay, TUNEL assay and Caspase-glo 3/7 assay in CRC cell lines.
Most of the first generation CDK inhibitors were less selective, these drugs affected several CDK family members (Blagosklonny, 2004). Although these broad spectrum CDK inhibitors showed good cytotoxicity in vitro, many of them failed in clinical trials as single agents due to toxic effects because of their lack of specificity. Targeting cell cycle regulatory CDKs along with transcriptional CDKs has been shown to induce apoptosis in various types of cancer cells (Shirsath, Manohar, Joshi, 2012). Yet, the spectrum of CDKs to be targeted for optimum efficacy would highly vary for every type of cancer (Graf, Wuest, Pietzsch, 2011; Joshi et al., 2012). Based on the literature, it is hypothesized that a multitarget CDK inhibitor increases the likelihood of hitting a particular target CDK in the case of a heterogeneous disease like cancer. Several of the CDK inhibitors currently under clinical development are essentially CDK1, CDK2, CDK7 and CDK9 inhibitors, in addition to CDK4/6 inhibitors in the pipeline. Other kinases outside the CDK family are also inhibited by some of these drugs (Galons et al., 2013). It has become increasingly apparent that such agents act through mechanisms other than or in addition to cell cycle arrest viz. apoptosis and anti-angiogenesis (Krystof, Baumli, Fürst, 2012; Manohar, Joshi, 2022; Manohar, Joshi, 2023).
In the present study, roscovitine and UCN-01 abrogated expression of not only cell cycle regulatory cyclins and CDKs but also transcriptional cyclins and CDKs along with their substrates viz. phospho Rb and phospho-RNA Pol II CTD. Therefore, efficacy of roscovitine and UCN-01 against CRC cells can be clearly attributed to their CDK1, CDK2, CDK4, CDK7 and CDK9 inhibitory activity although they were found to only moderately affect CDK2 and cyclin E and cyclin A expression in the present study. These results are in line with previous work by others (Senderowicz, 2002; Whittaker et al., 2004; Crescenzi, Palumbo, Brady, 2005).
Roscovitine is an orally bioavailable small molecule inhibitor of several CDKs competing at their ATP-binding sites. Previous studies indicate that, it inhibits Rb phosphorylation and it also decreases expression of cyclin D1, A and B1 probably through loss of RNA Pol II phosphorylation (Whittaker et al., 2004). Roscovitine has been shown to have significant antitumor activity in HCT116 and LoVo human colon cancer xenografts models (79% and 45% tumor growth inhibition respectively) (Raynaud et al., 2005). Synergistic effect of roscovitine with conventional cytostatic drugs such as gemcitabine, doxorubicin and cisplatin has been reported (Crescenzi, Palumbo, Brady, 2005; MacCallum et al., 2005; Raje et al., 2005). It has been shown to induce apoptosis via short-lived anti-apoptotic protein Mcl-1 down regulation through RNA Pol II-dependent transcription inhibition in multiple myeloma cells and mantle cell lymphoma cells (MacCallum et al., 2005). In fact, transcription inhibition and induction of apoptosis via Mcl-1 downregulation has been suggested to be the key mechanism that is attributable to the anticancer activity of roscovitine in these cell types. In the present study, roscovitine-induced apoptosis of CRC cells can be certainly attributed to Mcl-1 downregulation since Mcl-1 plays a key role in CRC cell survival and is known to mediate drug resistance in CRC cells (Manohar, Joshi 2022). In fact, it has been proposed that inhibition of RNA Pol II activity by roscovitine leads to downregulation of a large no. of genes which leads to rapid onset of apoptosis in cancer cells (Delehouzé et al., 2014).
UCN-01 is a staurosporine derivative isolated from Streptomyces (Senderowicz, 2002). UCN-01 induces cell cycle arrest in a specific phase of cell cycle depending on the cell type. It was shown to lead to S and G2/M arrest in hepatocellular carcinoma cells (Wu et al., 2013) and was also shown to induce DNA damage and autophagy in osteosarcoma cells (Lien et al., 2018). Roscovitine has also been shown to induce cell cycle arrest at S phase and G2/M transition and apoptosis at higher concentrations (Crescenzi, Palumbo, Brady, 2005). In the present study, both roscovitine and UCN-01 directly led to increase in sub G1 population in all three CRC cell lines. Among the CRC cell lines, HCT-15 was found be slightly less sensitive to both the drugs (w.r.t. increase in sub G1 fraction in treated cells), this could be attributed to inherent multidrug resistant feature of this cell line. Literature suggests that transcription inhibitors lead to p53-independent apoptosis in cancer cells, hence it is unlikely that HCT-15 is less sensitive to CDK inhibitors used in this study owing to its p53 mutant status (Gartel, 2008). Previously, it has been shown that UCN-01 downregulates Bcl-Xl in CRC cell lines (LS513 and SW48) thereby inducing apoptosis (Bhonde et al., 2005). However, in the present study, we observed that Bcl-Xl levels did not change after treatment with UCN-01 in Colo-205 and HCT-15 and only Bcl-2 downregulation was observed. Moreover, Mcl-1 expression was inhibited to much lesser extent in HCT-15 as compared to Colo-205 by roscovitine and not altered at all in HCT-15 by UCN-01. This could be due to much higher basal expression of Mcl-1 in HCT-15.
We recently showed that in addition to the action against cell cycle regulatory proteins and apoptotic proteins, multitarget CDK inhibitors also exhibit off-target activity against other aberrant signaling pathways in CRC cells such as Ras/MAPK and PI3K/Akt. Further, these drugs exhibit potent anti-migratory and anti-angiogenic potential which certainly contributes to their potency in CRC cells (Manohar, Joshi 2023).
Combination therapy has been the standard-of-care in cancer treatment that aims to increase fractional cell kill using suboptimal concentrations of two or more drugs leading to improved overall response. In the in vitro studies, concentration and duration of administered drugs can be tightly controlled and the inhibition of tumor cell growth can be easily measured (Chou, 2006). Inclusion of CDK inhibitors in combination therapeutic regimens has been proposed to be a potential strategy to increase their potency against cancers including CRC (Chou 2006; Rathos et al., 2012; Rathos et al., 2013). Recently, it was shown that multiple CDK inhibition enhances cytotoxicity of 5-FU in colon cancer cells by upregulation of DR5 via p73 (Tong et al., 2023). In the present study, both roscovitine and UCN-01 were found to be synergistic with at least one of the standard drugs in CRC cell lines. Yet, these drugs exhibited limited synergism in HCT-15 perhaps owing to multidrug resistant nature of this cell line.
Overall, our findings implicate that multitarget CDK inhibitors roscovitine and UCN-01 potently abrogate cell cycle progression in CRC cells and also inhibit transcription thereby inducing significant apoptosis. Further, both the drugs are synergistic with standard drugs used for CRC treatment in in vitro combination studies. This study suggests that multitarget CDK inhibitors may serve as effective anticancer agents against CRC even in the therapy resistant setting.
ACKNOWLEDGMENTS
We would like to thank Piramal Life Sciences, Goregaon, Mumbai, India for supporting this research work and for providing necessary permissions to publish the same. We are grateful to the anonymous reviewers for improving quality of this article by providing their valuable inputs.
REFERENCES
- Bhonde MR, Hanski ML, Magrini R, Moorthy D, Müller A, Sausville EA, et al. The broad-range cyclin-dependent kinase inhibitor UCN-01 induces apoptosis in colon carcinoma cells through transcriptional suppression of the Bcl-x(L) protein. Oncogene 2005;24(1):148-56.
- Blagosklonny M.V. Flavopiridol, an inhibitor of transcription: implications, problems and solutions. Cell Cycle. 2004;3(12):1537-42.
- Bonelli P, Tuccillo FM, Borrelli A, Schiattarella A, Buonaguro FM. CDK/CCN and CDKI alterations for cancer prognosis and therapeutic predictivity. Biomed Res Int. 2014;2014: 361020.
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-63.
- Bteich F, Mohammadi M, Li T, Bhat MA, Sofianidi A, Wei N, et al. Targeting KRAS in Colorectal Cancer: A Bench to Bedside Review. Int J Mol Sci. 2023;24(15):12030.
- Canavese M, Santo L, Raje N. Cyclin dependent kinases in cancer-Potential for therapeutic intervention. Cancer Biol Ther. 2012;13(7):451-7.
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621-81.
- Costa NR, Gil Da Costa RM, Medeiros R. A viral map of gastrointestinal cancers. Life Sci. 2018;199:188-200.
- Crescenzi E, Palumbo G, Brady HJ. Roscovitine Modulates DNA Repair and Senescence: Implications for combination chemotherapy. Clin Cancer Res. 2005;11(22):8158-71.
- Damato A, Astarita C, Sun A, Pesce A, Pinto C, Giordano A. Sequential inhibition of DNA methyltransferase (DNMT) and CDK 4/6 in a panel of colon cancer cells. J Clin Oncol. 2018;36(15):e15704.
- Delehouzé C, Godl K, Loaëc N. Bruyère C, Desban N, Oumata N, et al. CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells. Oncogene 2014;33(50):5675-87.
- Galons H, Oumata N, Gloulou O, Meijer L. Cyclin-dependent kinase inhibitors closer to market launch? Expert Opin. Ther Patents 2013;23(8):945-63.
- Gartel AL. Transcriptional inhibitors, p53 and apoptosis. Biochim Biophys Acta 2008;1786(2):83-6.
- Ghafouri-Fard S, Khoshbakht T, Hussen BM, Dong P, Gassler N, Taheri M, et al. A review on the role of cyclin dependent kinases in cancers. Cancer Cell Int. 2022;22(1):325.
- Graf F, Wuest F, Pietzsch J. Cyclin-Dependent Kinases (Cdk) as Targets for Cancer Therapy and Imaging. Gali-Muhtasib H (ed.) Advances in Cancer Therapy, Rijeka: InTech; 2011,265-88 pp.
- Handa K, Yamakawa M, Takeda H, Kimura S, Takahashi T. Expression of the cell cycle markers in colorectal carcinoma: Superiority of cyclin A as an indicator of poor prognosis. Int J Cancer. 1999;84(3):225-33.
- Joshi KS, Padganokar A, Rathos M, Wagh V, Manohar S, Bhatia D, et al. P1446A-05: a new oral cyclin-dependent kinase inhibitor with potent preclinical antitumor activity. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012;72(8 Suppl): Abstract no. 3054.
- Korenaga D, Takesue F, Yasuda M, Honda M, Nozoe T, Inutsuka S. The relationship between cyclin B1 overexpression and lymph node metastasis in human colorectal cancer. Surgery 2002;131:S114-S120.
- Krystof V, Baumli S, Fürst R. Perspective of Cyclin-dependent kinase 9 (CDK9) as a Drug Target. Current Pharm Des. 2012;18(20):2883-90.
- Lee SY, Oh SC. Advances of targeted therapy in treatment of unresectable metastatic colorectal cancer. Biomed Res Int . 2016;2016:7590245.
- Li J, Wang Y, Wang X, Yang Q. CDK1 and CDC20 overexpression in patients with colorectal cancer are associated with poor prognosis: evidence from integrated bioinformatics analysis. World J Surg Oncol. 2020;18(1):50.
- Lien WC, Chen TY, Sheu SY, Lin TC, Kang FC, Yu CH, et al. 7-hydroxy-staurosporine, UCN-01, induces DNA damage response, and autophagy in human osteosarcoma U2-OS cells. J Cell Biochem. 2018;119(6):4729-41.
- Ma CX, Ellis MJ, Petroni GR, Guo Z, Cai SR, Ryan CE, et al. A phase II study of UCN-01 in combination with irinotecan in patients with metastatic triple negative breast cancer. Breast Cancer Res Treat. 2013;137(2):483-92.
- MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA Polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65(12):5399-407.
- Maeda K, Chung Y-S, Kang S-M, Ogawa M, Onoda N, Nakata B, et al. Overexpression of cyclin D1 and p53 associated with disease recurrence in colorectal adenocarcinoma. Int J Cancer . 1997;74(3):310-5.
- Manohar S, Shah P, Biswas S, Mukadam A, Joshi M, Viswanathan G. Combining fluorescent cell barcoding and flow cytometry-based phospho-ERK1/2 detection at short time scales in adherent cells. Cytometry A. 2019;95:192-200.
- Manohar SM, Joshi KS. Molecular pharmacology of multitarget cyclin-dependent kinase inhibitors in human colorectal carcinoma cells. Expert Opin Ther Targets. 2023;27(3):1-11.
- Manohar SM, Joshi KS. Promising anticancer activity of multitarget cyclin dependent kinase inhibitors against human colorectal carcinoma cells. Curr Mol Pharmacol. 2022;15(7):1024-33.
- Manohar SM, Rathos MJ, Sonawane V, Rao SV, Joshi KS. Cyclin-dependent kinase inhibitor, P276-00 induces apoptosis in multiple myeloma cells by inhibition of Cdk9-T1 and RNA polymerase II-dependent transcription. Leuk Res. 2011;35(6):821-30.
- Manohar SM. Cyclin-dependent kinases as potential targets for colorectal cancer: past, present and future. Future Med Chem. 2022;14(14):1087-105.
- Meijer L, Borgne A, Mulner O, Chong JPJ, Blow J, Inagaki N, Delcros J-G, Moulinoux J-P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527-536.
- Mull BB, Livingston JA, Patel N, Bui T, Hunt KK, Keyomarsi K. Specific, reversible G1 arrest by UCN-01 in vivo provides cytostatic protection of normal cells against cytotoxic chemotherapy in breast cancer. Br J Cancer 2020; 122(6): 812-822.
- Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H, et al. Seliciclib (CYC-02 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma. Blood 2005;106(3):1042-7.
- Ramani S, Samant S, Manohar SM. The story of EGFR: from signaling pathways to a potent anticancer target. Future Med Chem . 2022;14(17):1267-88.
- Rathos MJ, Joshi K, Khanwalkar H, Manohar SM, Joshi KS. Molecular evidence for increased antitumor activity of gemcita-bine in combination with a cyclin-dependent kinase inhibitor, P276-00 in pancreatic cancers. J Transl Med. 2012;10:161.
- Rathos MJ, Khanwalkar H, Joshi K, Manohar SM, Joshi KS. Potentiation of in vitro and in vivo antitumor efficacy of doxo-rubicin by cyclin-dependent kinase inhibitor P276-00 in human non-small cell lung cancer cells. BMC Cancer. 2013;13:29.
- Raynaud FI, Whittaker SR, Fischer PM, McClue S, Walton MI, Barrie SE, et al. In vitro and in vivo pharmacokinetic-pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin Cancer Res . 2005;11(13):4875-87.
- Salh B, Bergman D, Marotta A, Pelech SL. Differential cyclin-dependent kinase expression and activation in human colon cancer. Anticancer Res. 1999;19(1B):741-8.
- Senderowicz AM. The cell cycle as a target for cancer therapy: basic and clinical findings with the small molecule inhibitors Flavopiridol and UCN-01. Oncologist 2002;7:12-9.
- Shirsath NP, Manohar SM, Joshi KS. P276-00, a cyclin-dependent kinase inhibitor, modulates cell cycle and induces apoptosis in vitro and in vivo in mantle cell lymphoma cell lines. Mol Cancer 2012;11:77.
- Tong J, Tan X, Hao S, Ermine K, Lu X, Liu Z, et al. Inhibition of multiple CDKs potentiates colon cancer chemotherapy via p73-mediated DR5 induction. Oncogene 2023;42(12):869-80.
- Tong J, Wang P, Tan S, Chen D, Nikolovska-Coleska Z, Zou F, et al. Mcl-1 Degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res . 2017;77(9): 2512-21.
- Whittaker SR, Walton MI, Garrett MD, Workman P. The cyclin-dependent kinase inhibitor CYC202 (R-roscovitine) inhibits RB phosphorylation, causes loss of cyclin D1 and activates the mitogen activated protein kinase pathway. Cancer Res . 2004;64(1):262-72.
- Wu G, Xu L, Lin N, Feitelson MA. UCN-01 induces S and G2/M cell cycle arrest through the p53/p21waf1or CHK2/CDC25C pathways and can suppress invasion in human hepatoma cell lines. BMC Cancer 2013;13:167.
SUPPLEMENTARY INFORMATION
Densitometric analysis of western blots. N=3, bars indicate mean ± SD of three independent experiments.
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
21 May 2024 -
Accepted
18 July 2024

 Multitarget CDK inhibitors roscovitine and UCN‑01 induce apoptosis in colorectal cancer cells by inhibiting cell cycle progression and transcription
Multitarget CDK inhibitors roscovitine and UCN‑01 induce apoptosis in colorectal cancer cells by inhibiting cell cycle progression and transcription