Abstract
Since its inception, biodiversity has largely been understood as species diversity and assessed as such. Interactions among species or functional groups are gradually becoming part of an expanded concept of biodiversity. As a case study of the development of a research program in biodiversity, we summarize our multi-decade studies on interactions of Asteraceae and flowerhead-feeding insects in Brazil. Initially, host species were treated as independent replicates in order to assess the local and turnover components of their herbivore diversity. Research then expanded into sampling entire interactive communities of host plants and their associated herbivores in different localities and regions, enabling new research lines to be pursued. Interaction diversity could be assessed and factored into spatial and among-host components, suggesting a new field of interaction geography. Second, host specialization, a key component of interaction diversity, was reframed considering simultaneously relatedness and local availability of plant hosts. Third, with the influence of complex network theory, community-wide species interactions were probed for topological patterns. Having identified the modular structure of these plant-herbivore systems, later we demonstrated that they fit a compound hierarchical topology, in which interactions are nested within large-scale modules. In a brief survey of research funded by Fapesp, especially within the Biota-Fapesp program, we highlight several lines of internationally recognized research on interaction diversity, notably on plant-frugivore and plant-pollinator interactions, together with new theoretical models. The interplay of field studies with new theoretical and analytical approaches has established interaction diversity as an essential component for monitoring, conserving and restoring biodiversity in its broader sense.
Keywords
plant-animal interactions; Asteraceae; interaction networks
Resumo
Desde seu início, a biodiversidade geralmente tem sido entendida e avaliada principalmente como diversidade de espécies. Interações entre espécies ou grupos funcionais vêm sendo incorporadas em um conceito expandido de biodiversidade. Como um estudo de caso da evolução de um programa de pesquisa em biodiversidade, resumimos aqui nossos estudos das interações de Asteráceas com insetos endófagos em capítulos no Brasil, desenvolvidos por várias décadas. Inicialmente a diversidade de herbívoros foi estimada em diferentes espécies hospedeiras, tratando-as como réplicas independentes para estimar os componentes locais e de substituição da diversidade dos insetos associados. Posteriormente, passamos a amostrar comunidades interativas de plantas e insetos associados em diferentes localidades e regiões, o que abriu novas linhas de investigação. A diversidade de interações, agora fatorada em componentes espaciais e inter-hospedeiras, sugere um novo campo, a geografia de interações. Em segundo lugar, um componente essencial da diversidade de interações, a especialização trófica, foi redefinida como função da contiguidade filogenética bem como da disponibilidade local de plantas hospedeiras. Terceiro, sob influência da teoria de redes complexas, foram investigados padrões topológicos de comunidades interativas. Identificamos a estrutura modular dessas comunidades de plantas e herbívoros; posteriormente, demonstramos a topologia hierárquica dessas interações, composta por módulos internamente aninhados. Numa revisão sucinta de pesquisas sustentadas pela Fapesp, especialmente no programa Biota-Fapesp, destacamos diversas linhas de pesquisa sobre diversidade de interações que alcançaram reconhecimento internacional, tais como interações de plantas e frugívoros ou polinizadores, além de novos modelos teóricos. A conjugação de estudos de campo com novas abordagens teóricas e analíticas firmou a diversidade de interações como um componente essencial para monitorar, conservar e restaurar a biodiversidade em seu sentido mais amplo.
Palavras-chave
interações planta-animal; Asteraceae; redes de interações
Introduction
In this paper, we review a set of Fapesp-funded studies that centered on a particular aspect of biodiversity within the framework of the Biota-Fapesp program: the diversity of interactions, or interaction biodiversity for short. Although this represents a relatively narrow segment of studies within the Biota-Fapesp Program, its importance was noted early on and has increased steadily in the last decades.
We will explore this as follows. First, we briefly review studies in the decades that preceded the Biota-Fapesp Program (BFP). Second, we comment on the research and theoretical context at the time the BFP was launched. This serves to situate the research program that we undertook, which to some extent embodies the initial thrust into interaction diversity within the BFP. Next, we present the initial questions and study design, focusing on the choice of study system and sites, along with other practical issues. Then, we summarize results, showing how they unfolded new questions that led to further research. Finally, we point at studies within the BFP that targeted other ecological systems as well as theoretical issues. We conclude speculating on future directions in interaction diversity research.
In a series of projects in which we investigated the interactions and diversity of flowerhead-feeding insects associated with Asteraceous host plants in Brazil, the geographical and thematic scope expanded in what can be regarded as a research program (Lakatos 1970LAKATOS, I. 1970. Falsification and the methodology of scientific research programmes. In Criticism and the Growth of Knowledge (I. Lakatos and A. Musgrave, eds). Cambridge University Press, Cambridge, p. 91–196.). Rather than a standard synthesis of projects and their results, we chose to present an account of this program, the circumstances and choices which led to certain research objectives, and how these evolved over more than two decades. We hope this format will be of interest to ecologists and historians of science concerned with the development of the BFP and of ecological research in Brazil. Even more, we hope that it will be useful especially to young researchers who face such choices and decisions in setting up their own research.
Research Funded by Fapesp, 1962–1991
In these three decades, very little research concerned species diversity. The Fapesp database on grants during this early period only includes project titles and disciplines. Of the 39.4 thousand total records, we scanned actual research grants, plus doctoral and postdoctoral scholarships (overseas scholarships were excluded because there was no information whether their research concerned an ecological system in Brazil).
Of the 409 total records in the field of Ecology, 139 were actual research grants, none of which included interactionor diversity in their titles. Local diversity was assessed in projects on freshwater plankton communities (9); plant communities, especially in the cerrado biome (6), and marine rocky shore communities (5). These studies were largely descriptive. One project, which explicitly addressed insect-plant diversity at different scales, is reported below (Results, section 1).
In 1988, an international symposium on plant-animal interactions, held in Campinas was co-funded by Fapesp, CNPq and the U.S. National Science Foundation. The symposium, attended by more than 300 participants, highlighted evolutionary and ecological aspects of various ecological systems, including their diversity. The ensuing book (Price et al. 1991PRICE, P.W., LEWINSOHN, T.M., FERNANDES, G.W. & BENSON, W.W. (eds.) 1991. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Wiley/Interscience, New York.) received highly positive reviews (see e.g. Berenbaum 1991BERENBAUM, M. 1991. Meat and veg (review of P.W. Price et al. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions). Nature 351:617–618., Thompson 1991THOMPSON, J.N. 1991. An exuberance of life (review of Plant-Animal Interactions; Peter W. Price, Thomas M. Lewinsohn, G. Wilson Fernandes & Woodruff W. Benson, eds). Science 252:1435–1435.) and was instrumental in raising international awareness of Brazilian ecological science, with species interactions figuring conspicuously among the main research themes pursued from then onwards.
The Scientific Context
1. The emergence of biodiversity
Biological diversity, first employed by Lovejoy (1980)LOVEJOY, T.E. 1980. Foreword. In Conservation Biology (M.E. Soule and B.A. Wilcox, eds). Sinauer, Sunderland, Mass. p. ix–x., extended the concept of species diversity to encompass other modes of biotic diversity. The formulation by Norse et al. (1986)NORSE, E.A., ROSENBAUM, K.L., WILCOVE, D.S. & WILCOX, B.A. 1986. Conserving Biological Diversity in our National Forests. The Wilderness Society, Washington. was adopted in the Convention of Biological Diversity (CBD 1992)CBD (UNITED NATIONS ENVIRONMENT PROGRAMME) 1992. Convention on Biological Diversity. United Nations Environment Programme, Nairobi.: “ ‘Biological diversity’ means the variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems.” The shortened form biodiversity was widely propagated after Wilson (1988)WILSON, E. O. (ed.) 1988. Biodiversity. National Academy of Sciences/Smithsonian Institution.; within a few years, it became a prime keyword in publications, including books and new journals. Nevertheless, there is no strong consensus on the concept of biodiversity, either theoretical or operational. In practice, most studies and data on biodiversity focus on species diversity; in second place comes genetic (largely intraspecific) diversity. As to diversity “of ecosystems”, it is the most equivocal of the categories in the CBD’s definition. Harper & Hawksworth (1995)HARPER, J.L. & HAWKSWORTH, D.L. 1995. Preface. In Biodiversity: measurement and estimation, (D.L. Hawksworth, ed), Chapman and Hall, London, p. 5–12. criticize “ecosystem diversity” for conflating two discrepant concepts; instead, they advocate “community diversity” or “ecological diversity” to characterize a further level of biodiversity.
Initial global assessments (Groombridge 1992GROOMBRIDGE, B. (ed) 1992. Global Biodiversity – Status of the Earth’s Living Resources. World Conservation Monitoring Centre, London., Heywood 1995HEYWOOD, V.H. (ed) 1995. Global Biodiversity Assessment. United Nations Environment Programme. Cambridge University Press, Cambridge.) had outlined rough general patterns of biodiversity, but also exposed huge knowledge gaps that limited their application. These were especially critical in the so-called megadiverse countries, largely in tropical regions, where many species had never been collected, let alone described. Thus, after the CBD was put in place, a major aim of many large-scale enterprises, including the Biota-Fapesp program, was to thoroughly sample ecological communities in undercollected regions, before they disappeared (NRC 1980NRC (National Research Council USA) 1980. Research Priorities in Tropical Biology. National Academies Press, Washington, DC., Raven & Wilson 1992RAVEN, P.H. & WILSON, E.O. 1992. A fifty-year plan for biodiversity surveys. Science 258:1099–1100.). If this is a concern even for well-studied taxa such as birds (Lees & Pimm 2015LEES, A.C. & PIMM, S.L. 2015. Species, extinct before we know them? Curr. Biol. 25:R177–R180.), it is a huge task for much larger and much less-described taxa, such as many insect groups (Berenbaum 2017BERENBAUM, M. 2017. Insect biodiversity–millions and millions. In Insect Biodiversity: Science and Society (R.G. Foottit & P.H. Adler, eds). Vol.1, 2nd edition. Wiley, New York, p. 783–792.).
Biodiversity emerged without a central or unifying theory. Theories pertaining to community ecology, biogeography, population genetics, evolution or paleobiology can engender hypotheses or models applicable to distinct spatiotemporal domains of biodiversity. In the 1990s, the Theory of Island Biogeography (MacArthur & Wilson 1967MACARTHUR, R.H. & WILSON, E.O. 1967. The Theory of Island Biogeography. Princeton University Press, Princeton, NJ.) was still the most influential forerunner of a general theory of biodiversity. This audacious model that predicted species diversity from few variables and premises stimulated ecologists to seek powerful explanatory and general models, rather than describing and classifying communities. For instance, conservation units modeled as islands within human-modified landscapes subtended propositions of optimal reserve design (Wilson & Willis 1975WILSON, E.O. & WILLIS, E.O. 1975. Applied biogeography. In Ecology and Evolution of Communities, (M.L. Cody & J.M. Diamond, eds). Harvard Univ. Press, Cambridge, MA, p. 522–34.).
2. Including interactions in biodiversity
The vast majority of insect herbivores feed on a small set of related host plants. This pattern and the Theory of Island Biogeography inspired Janzen (1968JANZEN, D.H. 1968. Host plants as islands in evolutionary and contemporary time. Am. Nat. 102:592–595., 1973JANZEN, D.H. 1973. Host plants as islands. II. Competition in evolutionary and contemporary time. Am. Nat. 107:786–790.) and others to consider host plants as ecological or evolutionary “islands”, asking which variables might determine differences in the number of species that feed on various hosts, by analogy with the variables that set species diversity on geographical or ecological islands. Even before, Southwood (1961)SOUTHWOOD, T. 1961. The number of species of insect associated with various trees. J. Anim. Ecol. 30:1–8. had raised a similar question regarding herbivores associated with British trees.
These pioneering papers prompted a flurry of studies of insect diversity on native, introduced or cultivated plant species (see Strong et al. 1984STRONG, D.R., JR., LAWTON, J.H. & SOUTHWOOD, T.R. 1984. Insects on Plants: Community Patterns and Mechanisms. Blackwell, Oxford., Lewinsohn et al. 2005LEWINSOHN, T.M., NOVOTNY, V. & BASSET, Y. 2005. Insects on plants: Diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Evol. Syst. 36:597–620.). Geographical distribution, antiquity in the region and taxon size of the host plants were the most common predictors of herbivore diversity. These initial studies sought species lists culled from the literature, either records of insects that feed on a major crop in different geographical regions (such as the pests of cacao, Strong 1974STRONG, D.R., JR. 1974. Rapid asymptotic species accumulation in phytophagous insect communities: the pests of cacao. Science 185:1064–1066.), or monographs of insect taxa that listed their known hosts (e.g. British agromyzid flies that mine leaves of Umbelliferae; Lawton & Price 1979LAWTON, J.H. & PRICE, P.W. 1979. Species richness of parasites on hosts: agromyzid flies on the British Umbelliferae. J. Anim. Ecol. 48:619–637.). In the first case, these are bottom-up diversity studies focused on a particular set of plants – called source webs according to Cohen (1977)COHEN, J.E. 1977. Food webs and the dimensionality of trophic niche space. Proc. Natl. Acad. Sci. USA 74:4533–4536.. In the second case, studies focus on a given herbivore taxon or guild (e.g. leaf miners which can include different insect orders) and their hosts, or sink webs (Cohen 1977COHEN, J.E. 1977. Food webs and the dimensionality of trophic niche space. Proc. Natl. Acad. Sci. USA 74:4533–4536.).
Almost all initial analyses, which relied on data-mining in the literature, were produced for large areas: countries, continents or the entire known geographical distribution of host plants. These held no information on assemblages of insects on local host populations. Far fewer studies did investigate local insect assemblages on host plants, mostly asking whether their richness or composition were limited by interspecific competition (e.g. Strong 1977STRONG, D.R., JR. 1977. Insect species richness: hispine beetles of Heliconia latispatha. Ecology 58:573–582., Benson 1978BENSON, W.W. 1978. Resource partitioning in passion vine butterflies. Evolution 32:493–518., Lawton 1984LAWTON, J.H. 1984. Non-competitive populations, non-convergent communities, and vacant niches: the herbivores of bracken. In Ecological Communities: Conceptual Issues and the Evidence (D.R. Strong, D. Simberloff, L.G. Abele & A.B. Thistle, eds), Princeton University Press, Princeton, NJ, p. 67–101.).
A key question emerged from these initial results: how is the diversity of herbivorous insect assemblages on local plant populations related to the regional, or total diversity, of insects associated with those host species? In other words, are they subject to the same factors and do they respond similarly to them at these different scales? If competition limits the local coexistence of closely-related species, their local diversity should be uncoupled from regional diversity. This could only be resolved obtaining commensurate data for insects associated with each host plant in several local populations across its geographic range. A pioneering study assessed the local and regional diversity of gall-making cynipid wasps on different species of North-American oaks (Cornell 1985CORNELL, H.V. 1985. Local and regional richness of cynipine gall wasps on California oaks. Ecology 66:1247–1260.). The results showed that local and regional diversities of cynipids on different host species were highly correlated with their hosts’ geographical range, with no evidence of competitive limitation of local assemblages. However, would this conclusion hold for interactions involving other taxa or feeding guilds? More importantly, would the same pattern be found in tropical herbivorous insects and their host plants, given the much larger diversity of insects and plants, and the rarity of most species, in the tropics?
The foregoing questions can be considered the beginnings of interaction diversity, an expression first used by Thompson (1996THOMPSON, J.N. 1996. Evolutionary ecology and the conservation of biodiversity. Trends Ecol. Evol. 11:300–303., 1997THOMPSON, J.N. 1997. Conserving interaction biodiversity. In: The Ecological Basis of Conservation: Heterogeneity, Ecosystems, and Biodiversity (S.T.A. Pickett, R.S. Ostfeld, M. Shachak & G.E. Likens, eds). Chapman & Hall, New York, p. 285–293.) to highlight the significance of interactions to biodiversity, especially in the tropics. These questions also motivated the research program whose development we recount in the following sections.
Designing Research in Interaction Diversity
1. Questions evolve
The initial aim of this research program was to untangle the diversity of insect herbivore assemblages associated with various host plants. Here, host plants corresponded to resources which, with their herbivore assemblages, form source webs (Cohen 1977COHEN, J.E. 1977. Food webs and the dimensionality of trophic niche space. Proc. Natl. Acad. Sci. USA 74:4533–4536.). Given the high diversity of most insect taxa in the tropics, would this be reflected as high local (alpha) diversity on host populations? Or, given the local rarity of most tropical insects (e.g., Price et al. 1995PRICE, P.W., DINIZ, I.R., MORAIS, H.C. & MARQUES, E.S.A. 1995. The abundance of insect herbivore species in the tropics: the high local richness of rare species. Biotropica 27:468–478.), would there be high turnover of herbivorous species among local populations of a host species, entailing a high beta diversity in the herbivore assemblages? Of course, while the high total, or regional (gamma) diversity of herbivores could be due solely to high alpha or high beta diversity, they are not mutually exclusive and could both contribute to a high gamma diversity. In formal terms, we followed Whittaker (1977)WHITTAKER, R.H. 1977. Evolution of species diversity in land communities. Evol. Biol. 10:1–67. and considered gamma diversity as the product of average alpha diversity and beta diversity; thus, regional diversity is local diversity multiplied by turnover among localities.
To answer these questions, a number of different plant species would have to be sampled for associated herbivores across a region. An important point is that, at first, insect assemblages were analyzed separately for each host, so that each plant species represented a replicate for the decomposition of its herbivore diversity into alpha and beta components, as in Cornell (1985)CORNELL, H.V. 1985. Local and regional richness of cynipine gall wasps on California oaks. Ecology 66:1247–1260.. However, we were aware that hosts and their interactive assemblages are not truly independent replicates, since hosts are phylogenetically linked to various degrees, and they share herbivore species as well.
In a further stage we came to envisage sets of local host species with their herbivores as distinct ecological entities. As our framework broadened, so did our questions: how did the diversity of assemblages of herbivores scale with the local diversity of their host plants? Did they accumulate more across hosts, over space, or over both? In other words, what is the beta diversity of herbivores over space and across hosts (Novotny 2009NOVOTNY, V. 2009. Beta diversity of plant-insect food webs in tropical forests: a conceptual framework. Insect Conserv. Diver. 2:5–9.)? The answer to these questions would depend critically on the overlap of herbivore species between plants and, conversely, on the overlap of host species between different herbivores. For that, it became necessary to effectively sample host plant communities; thus, the entities investigated expanded from sets of source webs to community webs (Cohen 1977COHEN, J.E. 1977. Food webs and the dimensionality of trophic niche space. Proc. Natl. Acad. Sci. USA 74:4533–4536.). This framework also brought into play the extensive theory and empirical studies on host specialization (Bernays & Chapman 1994BERNAYS, E.A. & CHAPMAN, R.F. 1994. Host-plant Selection by Phytophagous Insects. Chapman & Hall, New York.), a key element in evolutionary and ecological processes that determine interaction diversity. Host specialization can be invariant over a herbivore’s range, or it can exhibit local specialization, shifting hosts among localities (Fox & Morrow 1981FOX, L.R. & MORROW, P.A. 1981. Specialization: species property or local phenomenon? Science 211:887–893.), adding a further variable to the diversity of interactions in geographical space.
In the late 1990s, the theory of complex networks, which combined graph theory, statistical mechanics and increased computing power, was applied at once in many areas of science and technology (Strogatz 2001STROGATZ, S. 2001. Exploring complex networks. Nature 410:268–276., Newman 2003NEWMAN, M. 2003. The structure and function of complex networks. Siam Rev. 45:167–256.). In a complex network, the distribution of links connecting its units is neither constant nor random. Food webs are instances of complex networks because consumer species rarely use their resources indiscriminately. Although even the more complete empirical webs are much smaller than other kinds of real-word webs, their statistical properties conform to complex webs of that size (Dunne et al. 2002DUNNE, J.A., WILLIAMS, R.J. & MARTINEZ, N.D. 2002. Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. USA 99:12917–12922.).
Two other non-random network patterns that called the attention of ecologists were nestedness and modularity. Nested patterns were found in biogeography and landscape ecology (Atmar & Patterson 1993ATMAR, W. & PATTERSON, B.D. 1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96:373–382.) and later extended to species interactions. Regarding ecological networks, mutualisms at first were mostly probed for a nested pattern of interactions among plants and animal agents (Bascompte et al. 2003BASCOMPTE, J., JORDANO, P., MELIÁN, C.J. & OLESEN, J.M. 2003. The nested assembly of plant–animal mutualistic networks. Proc. Natl. Acad. Sci. USA 100: 9383–9387.). As noted below, our group was among the first to demonstrate modularity in plant-herbivore networks (Prado & Lewinsohn 2004PRADO, P.I. & LEWINSOHN, T.M. 2004. Compartments in insect–plant associations and their consequences for community structure. J. Anim. Ecol. 73:1168–1178.). With these new findings and the application of complex network theory to ecological systems, the architecture or the topology of interactions became a new investigation thread in the latter phase of our research program, introducing an additional layer of variables and potential determinants to the questions that were being addressed earlier on.
2. Choosing an ecological system
The choice of a study system for field studies in community ecology is a critical step in research design. First, the system must be adequate for performing experiments or obtaining field data that can answer the intended questions. Second, the question of why that taxonomic group – or that interactive system – was chosen over others, requires consideration (Lawton 1992LAWTON, J.H. 1992. There are not 10 million kinds of population dynamics. Oikos 63:337–338.); hopefully, the study’s results will apply beyond the idiosyncratic limits of that system. Third, logistic constraints can make or break a project. Not all can be foreseen, but planning ahead, including conceivable setbacks or misfortunes, will invariably be worth the effort.
A plant-based ecological network is most often circumscribed to a taxon and/or a growth form. The choice of Asteraceae for this research program involved many criteria that attend to the above issues, some of which are summarized here.
Asteraceae is the second largest family of flowering plants, with almost 25,000 described species (Christenhusz & Byng 2016CHRISTENHUSZ, M.J. & BYNG, J.W. 2016. The number of known plant species in the world and its annual increase. Phytotaxa 261: 01–217.). In Brazil, more than 2,200 species are currently recognized, of which at least 1,300 are endemic (Roque et al. 2020ROQUE, N. et al. 2020. Asteraceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Available at: <https://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB55>. Accessed on: 07 March 2022
https://floradobrasil.jbrj.gov.br/reflor...
). It is found in all continents and is studied by an impressive array of taxonomists, also in Brazil (Roque et al. 2017ROQUE, N., TELES, A.M. & NAKAJIMA, J.N. (eds.) 2017. A Família Asteraceae no Brasil: Classificação e Diversidade. EDUFBA, Salvador. Available at: https://doi.org/10.7476/9788523219994.
https://doi.org/10.7476/9788523219994...
); its phylogeny, by now, is also better covered than most other major families (Funk et al. 2009FUNK, V.A., SUSANNA, A., STUESSY, T. & BAYER, R. (eds) 2009. Systematics, Evolution and Biogeography of Compositae. IAPT, Vienna, Mandel et al. 2019MANDEL, J.R., DIKOW, R.B., SINISCALCHI, C.M., THAPA, R., WATSON, L.E. & FUNK, V.A. 2019. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 116:14083–14088.). A key advantage of this plant family was that the Botany Department of the University of Campinas had two experienced specialists on Asteraceae, Hermógenes Freitas Leitão Filho and João Semir (both deceased). This ensured invaluable guidance in selecting sampling regions and sites, training to distinguish species in the field, and swift identification of most sampled host populations.
The majority of Asteraceae are herbs, shrubs, or vines; most tree species are also fairly small. This allows sampling without any special climbing or collecting apparatus, at a much faster pace than surveying or collecting forest trees. In previous work, entire plants were surveyed and dissected for herbivores. The choice to concentrate solely on flowerhead feeders was made for three main reasons: first, because of the small size of flower heads (in most Brazilian species), many samples could be gathered and carried efficiently; second, this ensured that all herbivores were reared from reproductive individuals that could be identified; third, herbivores that feed on flower heads are more diversified than on other plant organs, for reasons such as structural complexity and nutritional quality.
The widespread distribution of Asteraceae, especially in open habitats, allowed comparisons of host populations and species at distances ranging from a few to several thousand kilometers, in different geographical regions and in distinct ecological communities, from coastal restingas to highland meadows at elevations up to 1,500 m. Sampled plants ranged from narrow endemics to cosmopolitan species, including some that are serious economic pests in other continents. Given the worldwide distribution and importance of Asteraceae, cross-continental comparisons with other studies could also be envisaged.
Regarding the herbivores, preliminary studies and the literature indicated that certain groups in several insect orders and families had diversified on Asteraceous hosts, especially within Diptera, Lepidoptera and Coleoptera. Here, again, an early decision was to concentrate on endophagous feeders whose immatures develop within flower heads. External feeding folivores, especially when sampled with mass-collecting devices (such as foliage sweeping, vacuuming or fumigation) include an undefined number of non-feeding stragglers. Not only do these insects have to be collected from identified plant individuals, but their association should be confirmed by feeding trials in situ or in the lab (Novotny et al. 2002NOVOTNY, V., BASSET, Y., MILLER, S., DROZD, P. & CIZEK, L. 2002. Host specialization of leaf-chewing insects in a New Guinea rainforest. J. Anim. Ecol. 71: 400–412.). By including only internal feeders reared from field samples, we ensured that the interaction data obtained were robust and consistent. Although, by this criterion, a part of known Asteraceous associated insects were excluded (such as certain genera of Hemiptera), the bulk of flower head feeding diversity was represented in the data and results. The assistance of experienced specialists in Brazil ensured that insects were reliably sorted to morphospecies and, as far as possible, identified as well. Further identification and description of several new species were attained through collaboration and visits to overseas specialists.
3. Practical issues
A common difficulty in planning tropical field-based studies regards the optimization of sampling. Though we do not know any proper survey on this issue, many projects seemingly suffer from an imbalance between collecting and post-processing. Since field work is costly to organize and to garner adequate human and financial support, one tends to collect as many samples as possible and extend sampling to the limit of field time. The full processing routine in these surveys involves the transcription of field information, rearing, sorting, identifying, counting or measuring, and databasing sampled biological material. Information or biological material may be lost if all samples cannot be adequately stored, identified and handled in the field and lab. Incomplete processing means wasted resources and reduces the total sampling volume or extent; moreover, it may imbalance or even compromise the study design.
To avoid losses during field trips, small practical improvements may confer large gains. Plant vouchers were pressed at once in the field and later dried in a portable oven. In long field trips, adult insects start emerging almost immediately. By carrying a small CO2 gas cylinder on trips, we were able to stun and extract adults from the collecting/rearing containers without damaging them or affecting other immatures in the collected flower heads. Emerged adults were also fixed or mounted at once. The time required to tend and check samples for adults, extract and mount them, and care for plant vouchers, set a practical limit to the distance and duration of field trips.
Results and Unfolding Questions
1. Local and regional diversity of herbivores and their hosts
Initial research on flowerhead feeders of Brazilian Asteraceae posed the following questions: whether (i) local herbivore diversity was proportional to regional diversity on a host plant, (ii) both local and regional diversity were determined by that host’s geographical range as found in Cornell (1985)CORNELL, H.V. 1985. Local and regional richness of cynipine gall wasps on California oaks. Ecology 66:1247–1260., (iii) herbivore species turnover among localities contributed significantly to regional diversity. Extensive surveys with standardized samples in Southeast Brazil ranged from coastal dune and “restinga” habitats, to upland and montane natural fields in the Espinhaço mountain range in Minas Gerais. In this initial study, 266 standardized flowerhead samples of 70 species of Asteraceae were obtained, of which 28 were sampled in two or more localities (Lewinsohn 1991LEWINSOHN, T.M. 1991. Insects in flower heads of Asteraceae in southeast Brazil: a tropical case study on species richness. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions (P.W. Price, T.M. Lewinsohn, G.W. Fernandes & W.W. Benson, eds.) Wiley/Interscience, New York, p. 525–560.). Here, as in the following studies, the main herbivore groups were Diptera (mainly Tephritidae, Agromyzidae and Cecidomyiidae), and Lepidoptera (mainly Tortricidae, Pterophoridae and Pyralidae). In the Coleoptera, only some species of Anthonomus and Apion were reared out and confirmed as true endophagous feeders.
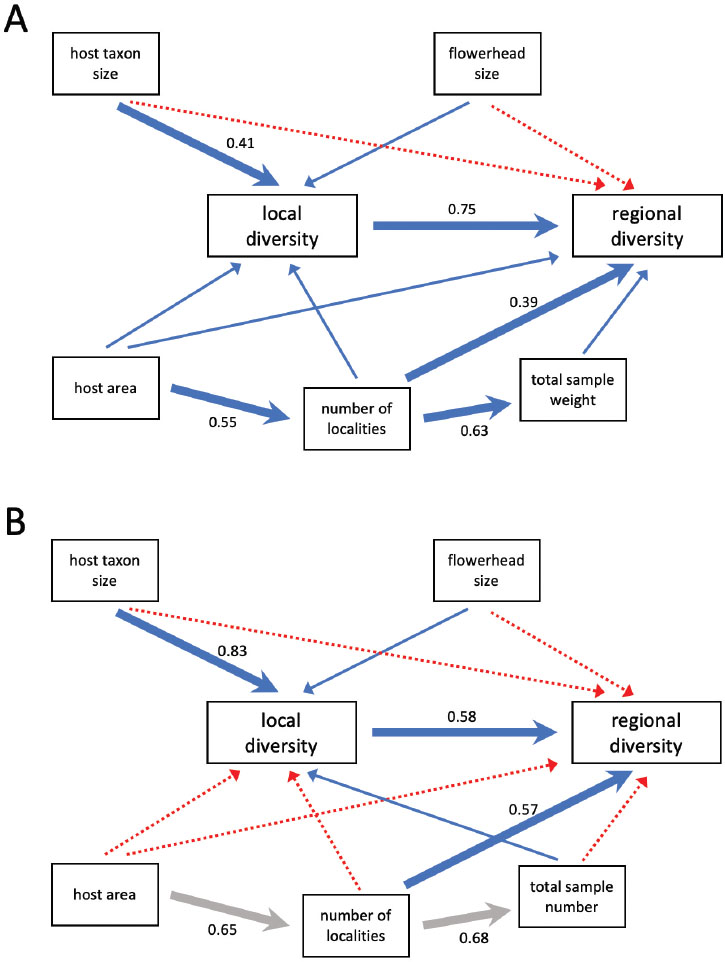
Figure 1A shows the key results of this project. Initially, simple regressions indicated that local (alpha) and regional (gamma) diversity were strongly correlated, and that host geographical range was a significant determinant of regional, but not local, diversity. However, the path analysis summarized in Figure 1A paints a different picture. Once the relationship of host geographical range with regional diversity is decomposed, the effect is clearly indirect, by way of the number of sampled localities, and no direct effect of area on regional diversity is detected. Since plant species with wider areas were proportionally sampled in more localities, this is no sampling artifact, and shows that beta diversity contributes significantly to regional diversity of the associated herbivores. Results also showed that the size of flower heads had no influence on herbivore diversity. Since Asteraceae plants produce dozens to tens of thousands of flower heads, there is no reason to expect that herbivores would be constrained by interspecific competition within individual flower heads. On the other hand, hosts belonging to larger taxa had more diverse local herbivore assemblages, but their regional diversity was only influenced through local diversity in the specified path model (for alternative path models, see Lewinsohn 1991).
Path analyses for local and regional diversity of flowerhead feeders of Asteraceae. (A) several subfamilies, especially, Asteroideae and Mutisioideae, in Brazil; modified from Lewinsohn (1991LEWINSOHN, T.M. 1991. Insects in flower heads of Asteraceae in southeast Brazil: a tropical case study on species richness. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions (P.W. Price, T.M. Lewinsohn, G.W. Fernandes & W.W. Benson, eds.) Wiley/Interscience, New York, p. 525–560., Figure 23.6). Flowerhead size: dry weight. Host taxon size: number of species in host tribe. Host area: ordinal variable, three classes. Alpha diversity: insect richness, standardized by rarefaction on twenty host individuals. Positive paths blue, continuous arrows; negative paths red, stippled. Only main path coefficients are shown. Analysis for 44 plant species. (B) Carduoideae in Europe, modified from Zwölfer (1987ZWÖLFER, H. 1987. Species richness, species packing, and evolution in insect-plant systems. Ecol. Stud. 61:301–319. Springer, Berlin., Figure 4, grey arrows from Figure 3). Flowerhead size: diameter. Host taxon size: log number of species in host genus. Host area: number of countries in Europe where the host species is found. Alpha diversity: insect richness in 100 flowerheads. Two other causal variables, habitat and host life type, had no significant effects and were excluded for simplicity; thin arrows are non-significant. Analysis for 37 plant species.
Figure 1B shows the same path model with results obtained by Zwölfer (1987)ZWÖLFER, H. 1987. Species richness, species packing, and evolution in insect-plant systems. Ecol. Stud. 61:301–319. Springer, Berlin. for flowerhead feeding insects on European Asteraceae. This study focused on the subfamily Carduoideae (thistles), whereas our study included other subfamilies. Also, variables were measured in somewhat different ways, as shown in the figure caption. Despite those differences, the resulting path models are strikingly similar. In both studies, beta diversity, or herbivore turnover among localities, together with local herbivore diversity, are major determinants of regional diversity. However, widespread hosts do not have more diversified local assemblages than hosts with smaller geographical ranges, contrary to the results of Cornell (1985)CORNELL, H.V. 1985. Local and regional richness of cynipine gall wasps on California oaks. Ecology 66:1247–1260. with gall-making wasps on North American oaks.
A further notable convergence is that, in both studies, the taxon size of each host does influence herbivore diversity. Hosts belonging to larger taxa tend to have more diverse local assemblages and, indirectly only, regional assemblages as well. A possible explanation for this effect is that larger host taxa support more species in local communities, which would then include more potential hosts for herbivores. However, in our initial study, plant species were treated as replicate units (as in Cornell 1985CORNELL, H.V. 1985. Local and regional richness of cynipine gall wasps on California oaks. Ecology 66:1247–1260. and Zwölfer 1987ZWÖLFER, H. 1987. Species richness, species packing, and evolution in insect-plant systems. Ecol. Stud. 61:301–319. Springer, Berlin.) as if they, together with their local insect assemblages, were essentially autonomous. The new questions raised by these results could only be addressed by investigating entire local plant-herbivore communities, rather than treating each host and its associates as an independent source web.
2. Interaction geography
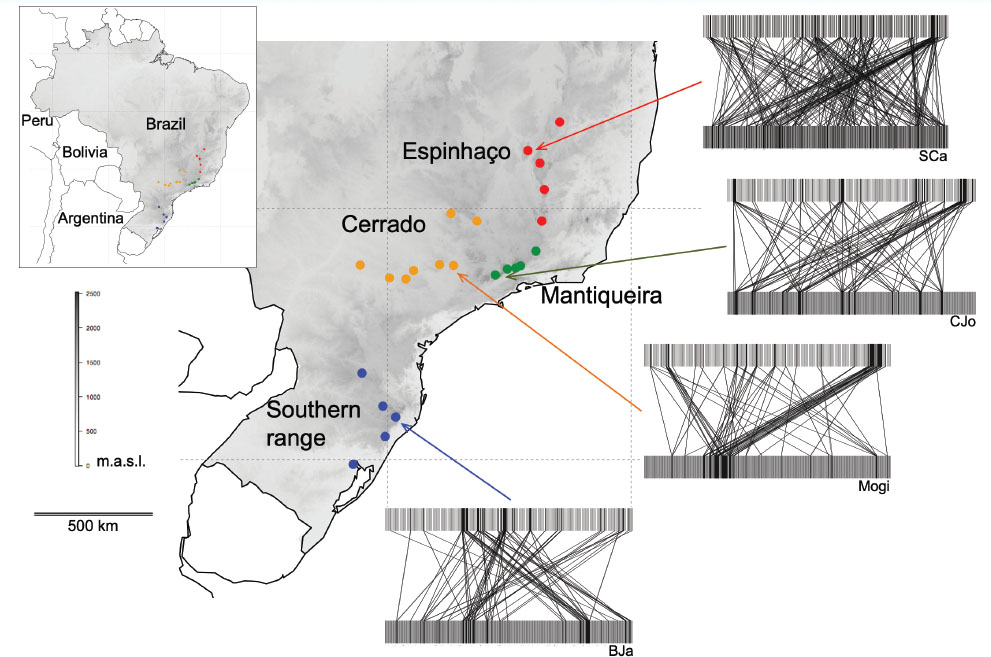
The sampling program for interactive communities of Asteraceae and their flowerhead feeders was carried through in a series of projects over a decade, from 1994 onwards. Together, these projects covered four main geographical regions, in each of which five or more localities were sampled (Figure 2). Three of these regions are mountain ranges, where Asteraceae are especially diversified. Local communities of Asteraceae can exceed 180 species in the South (e.g. the southernmost site in Figure 2), or 250 species in the Espinhaço range. The main study localities were sampled three to five times at different times of the year in order to include hosts with different seasonal phenologies. With support from many specialists in Brazil and overseas (see Acknowledgements), associated insects could be reliably sorted to species in 14 families. Our main database comprises 3,310 interactions among 485 Asteraceae and 284 herbivore species.
Regions and main localities sampled for Asteraceae and flowerhead-feeding insects in South and Southeast Brazil, between 1994 and 2002. Representative assemblages and interactions (recorded through rearing immatures from flowerhead samples) are shown as binary bipartite networks with plant species on the bottom and herbivores on top. County abbreviations: BJa (Bom Jardim da Serra, Santa Catarina); Mogi (Mogi Mirim, São Paulo); CJo (Campos do Jordão, São Paulo); SCa (Serra do Cabral, Minas Gerais).
These data multiplied the dimensions of interaction diversity to explore. By rearing insects from each host population, we produced maps of the actual pairwise links between each host and herbivore species. Moreover, these links could be quantified, either as the numbers of insects reared from each host, or by their frequency in plant samples (Figure 3). Previous studies, from Murdoch et al. (1972)MURDOCH, W., EVANS, F. & PETERSON, C. 1972. Diversity and pattern in plants and insects. Ecology 53:819–829. onwards, had investigated the correlation of plant and insect diversity; however, by mass-collecting insects from the vegetation, they were limited to correlating the total diversities of plants and insects across samples or localities. Data on individual links allowed, first, to measure diversity of hosts per herbivore and, conversely, of herbivores per host – the reciprocal specialization of plants and insects. Second, the total (i.e. regional) diversity of the interaction links could be decomposed into local (alpha) and differentiation (beta) diversity, analogously to the spatial factoring of species diversities (Novotny 2009NOVOTNY, V. 2009. Beta diversity of plant-insect food webs in tropical forests: a conceptual framework. Insect Conserv. Diver. 2:5–9.). As Figure 3 illustrates for two localities in the same region, besides changes in species composition there are switches in interactions. This sets the basis for a new domain of biogeography, interaction geography, which integrates information on the spatiotemporal distribution of sets of interacting species, with the distribution of the interactions themselves, as illustrated by the network diagrams in Figure 2.
Bipartite networks for the tribe Eupatorieae (Asteraceae) and their flowerhead-feeding insects in two localities in the Mantiqueira mountain range: (A) Itatiaia, (B) Campos do Jordão (see Figure 2). Rectangles on top are insect species, ellipses on the bottom are host species. The widths of the rectangles and ellipses represent the relative abundance in each level; basal widths of the wedges represent the relative frequency of each pairwise interaction. In insects, colors represent three feeding guilds (strict endophages with complete development within one flowerhead, green – Diptera, red – Lepidoptera; blue – facultative endophages). Different colours of plants represent subtribes of Eupatorieae. See Almeida (2001)ALMEIDA, A.M. 2001. Biogeografia de interações entre Eupatorieae (Asteraceae) e insetos endófagos de capítulos na Serra da Mantiqueira. PhD Thesis, Universidade Estadual de Campinas, Campinas. Available at https://repositorio.unicamp.br/Busca/Download?codigoArquivo=484206
https://repositorio.unicamp.br/Busca/Dow... for details.
Among the main findings of these studies, large-scale beta diversity of hosts and herbivores was strikingly different. Beta diversity of Asteraceae among regions was much higher than that of herbivores, due to a much higher level of regional endemism of the plants. This also meant that extreme host specialization is rare among their associated herbivores. As a functional group, internally feeding and developing insects are more specialized than externally feeding herbivores (Gaston et al. 1992GASTON, K.J., REAVEY, D. & VALLADARES, G.R. 1992. Intimacy and fidelity: internal and external feeding by the British microlepidoptera. Ecol. Entomol. 17:86–88.). Nonetheless, in this interactive system, monophagous herbivores, especially those associated with endemic plants, were highly exceptional. The higher beta diversity of plants than of their herbivores implies that insects need to shift among host species in different regions and localities. Thus, differences among herbivores in host ranges and host shifts became key elements to advance our understanding of the organization of this interactive system.
3. Specialization
As the importance of specialization for interaction diversity became clearer, we realized that specialization was usually assessed solely as individual species’ actual diets, leaving out the community context. This caused two shortcomings for understanding interaction diversity: first, the connection between host range variation and insect diversity could not be ascertained, which is essential to estimate the components of diversity in interacting species. Second, comparisons of herbivore specialization across communities were compromised by the lack of reference to the plants available in each community and their phylogenetic or taxonomic breadth. These are serious problems for detecting macroecological patterns or comparing specialization in different communities along an environmental or disturbance gradient.
The notion that herbivores feed on closely related plant species, and that host range should consider the relatedness of hosts, instead of only counting the number of known host species, has been present in the literature for a long time. However, this was addressed by using arbitrary specialization classes, with no regard either for evolutionary relatedness or for the set of plants available to a given herbivore across its range. As phylogenies became available for a wide range of plant groups (APG IV 2016APG IV. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 181:1–20.), including the Asteraceae (Funk et al. 2009FUNK, V.A., SUSANNA, A., STUESSY, T. & BAYER, R. (eds) 2009. Systematics, Evolution and Biogeography of Compositae. IAPT, Vienna), we devised a framework for specialization that addressed these shortcomings (Jorge et al. 2014JORGE, L.R., PRADO, P.I., ALMEIDA-NETO, M. & LEWINSOHN, T.M. 2014. An integrated framework to improve the concept of resource specialisation. Ecol. Lett. 17:1341–1350., 2017JORGE, L.R., NOVOTNY, V., SEGAR, S.T., WEIBLEN, G.D., MILLER, S.E., BASSET, Y. & LEWINSOHN, T.M. 2017. Phylogenetic trophic specialization: a robust comparison of herbivorous guilds. Oecologia 185: 551–559.). In our new metric, DSI*, specialization is a continuous trait that depends on the phylogenetic proximity of the plants used by a given herbivore, compared to the set of plants available in a given community (Figure 4A, B). When we applied this framework to our data sets on interactions, we found that most flowerhead feeders are specialized on a subgroup of closely related species within the Asteraceae. Very few species feed on a disparate set of plant hosts; these are true generalists, by contrast with the more common non-selective species that use hosts according to their local abundance, but which would also be considered generalists according to conventional criteria (Figure 4C).
A schematic representation of herbivore specialization, incorporating relatedness and availability of host plants. In this framework, herbivores are classified into three categories: specialists feed on plants that are more closely related than expected if herbivores used plants according to their abundance; generalists are the opposite, feeding on plants that are distantly related; non-selective herbivores use host plants proportionately to their availability. (A) Herbivores feed on plants with different levels of relatedness. (B) Herbivore diets can be proportional to availability, or deviate from it, selecting either similar resources (specialists) or dissimilar resources (generalists). (C) Host specialization of species in the four most important herbivore families in our previously described dataset, assessed with the metric DSI* (Jorge et al. 2014JORGE, L.R., PRADO, P.I., ALMEIDA-NETO, M. & LEWINSOHN, T.M. 2014. An integrated framework to improve the concept of resource specialisation. Ecol. Lett. 17:1341–1350., 2017JORGE, L.R., NOVOTNY, V., SEGAR, S.T., WEIBLEN, G.D., MILLER, S.E., BASSET, Y. & LEWINSOHN, T.M. 2017. Phylogenetic trophic specialization: a robust comparison of herbivorous guilds. Oecologia 185: 551–559.). Species are colored according to the specialization categories in (B). Adapted from Jorge et al. (2014)JORGE, L.R., PRADO, P.I., ALMEIDA-NETO, M. & LEWINSOHN, T.M. 2014. An integrated framework to improve the concept of resource specialisation. Ecol. Lett. 17:1341–1350..
4. Network patterns and topologies
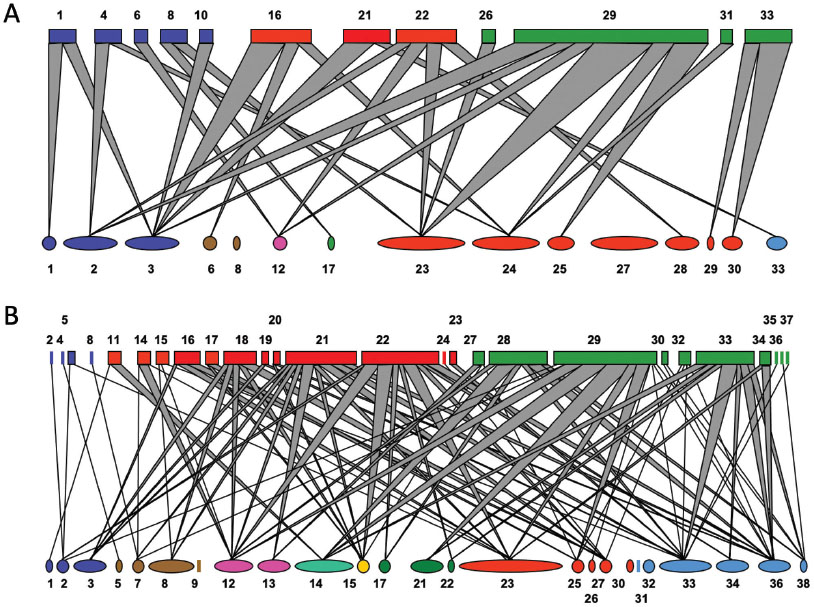
In parallel to the development of concepts and methods to tease apart the role of specialization in interaction diversity, complex network analysis expanded the questions and hypotheses to be addressed with interaction data. Ecological systems defined by a particular mode of interaction – in our case, herbivory – are rendered as bipartite networks, where two groups of organisms interact with each other, with no direct interaction within each group. Such networks are usually represented in two ways: as an adjacency matrix, where rows and columns are, respectively, plant and herbivore species, and filled cells represent links among interacting pairs (Figure 5A); or as a network diagram, where plant and herbivore species are represented by symbols that are connected when they interact with each other (Figure 5B). In quantitative networks, cells or links can be weighted by their number or frequency.
An interaction network of Asteraceae (tribe Vernonieae; 81 species) and insects (Diptera, Tephritidae; 35 species) in the Espinhaço Range, Minas Gerais. Insect-plant links (total = 163, connectance = 0.058) are depicted in two ways. (A) An adjacency matrix with plants in rows, insects in columns. Rows and columns are arranged first by detecting modules (shown in different colors) and then sorting species in each module for nestedness (details in Pinheiro et al. 2019PINHEIRO, R.B.P., FELIX, G.M.F., DORMANN, C.F. & MELLO, M.A.R. 2019. A new model explaining the origin of different topologies in interaction networks. Ecology 100:1–30.). (B) a bipartite network with plants on the left, insects on the right. Modules are identified by the same colors. Data from Prado & Lewinsohn (2004)PRADO, P.I. & LEWINSOHN, T.M. 2004. Compartments in insect–plant associations and their consequences for community structure. J. Anim. Ecol. 73:1168–1178., original figure by Rafael Pinheiro.
While most studies at the time were concerned with detecting nested interaction patterns, we demonstrated the modularity in networks of Asteraceae and their flowerhead feeders and surmised their importance in similar resource-consumer systems (Prado & Lewinsohn 2004PRADO, P.I. & LEWINSOHN, T.M. 2004. Compartments in insect–plant associations and their consequences for community structure. J. Anim. Ecol. 73:1168–1178.). In this study, we showed that most flowerhead endophages form cohesive modules together with clusters of related plant species (Figure 5), but this pattern could not be ascribed to niche partitioning among the herbivores. In a later theoretical paper we summarised the possible structures that could be observed in networks, highlighting how different visualizations of networks could provide complementary insights into these structures (Lewinsohn et al. 2006LEWINSOHN, T.M., PRADO, P.I., JORDANO, P., BASCOMPTE, J., & OLESEN, J.M. 2006. Structure in plant-animal interaction assemblages. Oikos 113:174–184.). We also proposed that simple network topologies, modularity and nestedness, could be combined in a compound hierarchical topology in which larger-scale modules are internally nested. Figure 5A shows this compound topology after reanalyzing the data of Prado & Lewinsohn (2004)PRADO, P.I. & LEWINSOHN, T.M. 2004. Compartments in insect–plant associations and their consequences for community structure. J. Anim. Ecol. 73:1168–1178. with an appropriate procedure. A compound hierarchical pattern can be produced by distinct ecological and evolutionary processes operating on different spatial and temporal scales (Pinheiro et al. 2019PINHEIRO, R.B.P., FELIX, G.M.F., DORMANN, C.F. & MELLO, M.A.R. 2019. A new model explaining the origin of different topologies in interaction networks. Ecology 100:1–30.) and has been found in other types of interaction as well (Felix et al. 2022FELIX, G.M., PINHEIRO, R.B.P., POULIN, R., KRASNOV, B.R. & MELLO, M.A.R. 2022. The compound topology of host–parasite networks is explained by the integrative hypothesis of specialization. Oikos 2022: e08462.).
5. Theoretical advances
Research on interaction diversity patterns and dynamics required suitable analytical methods. Recurrently, new empirical studies and resulting data demanded the development of new theoretical models and statistical procedures to evaluate variables and hypotheses. Parallel to field studies, we participated in developing and testing these new procedures, some of which were noted in the preceding sections. Some of the theoretical and statistical advances achieved within our research program are highlighted here.
Lewinsohn (1991)LEWINSOHN, T.M. 1991. Insects in flower heads of Asteraceae in southeast Brazil: a tropical case study on species richness. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions (P.W. Price, T.M. Lewinsohn, G.W. Fernandes & W.W. Benson, eds.) Wiley/Interscience, New York, p. 525–560. pioneered the use of individual plants as units in rarefaction analyses, to produce standardized estimates of herbivore richness. Sample-based rarefaction procedures were later incorporated into the statistical literature and have become part of the normal arsenal of diversity analyses (Colwell et al. 2012COLWELL, R.K., CHAO, A., GOTELLI, N., LIN, S.-Y., MAO, C., CHAZDON, R.L. & LONGINO, J. 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 5:3–21.).
A general framework, extending the biogeographic patterns of Leibold & Mikkelson (2002)LEIBOLD, M.A. & MIKKELSON, G.M. 2002. Coherence, species turnover, and boundary clumping: elements of meta-community structure. Oikos 97:237–250. to encompass the entire gamut of network structures in bipartite networks was expounded in Lewinsohn et al. (2006)LEWINSOHN, T.M., PRADO, P.I., JORDANO, P., BASCOMPTE, J., & OLESEN, J.M. 2006. Structure in plant-animal interaction assemblages. Oikos 113:174–184.; this is widely cited both for the general framework and for noting the complementarity of matrix, network, and multivariate analytical methods to address interaction patterns.
Particular aspects of network structure were explored in subsequent papers. Almeida-Neto et al. (2007)ALMEIDA-NETO, M., GUIMARÃES, P.R., JR, & LEWINSOHN, T.M. 2007. On nestedness analysis: rethinking matrix temperature and anti-nestedness. Oikos 116:716–722. criticized the concept of “anti-nestedness” and its false analogy with entropy or Atmar & Patterson’s (1993ATMAR, W. & PATTERSON, B.D. 1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96:373–382.) temperature. We then developed an index to measure nestedness in networks based on a concept accepted by most authors (Almeida-Neto et al. 2008ALMEIDA-NETO, M., GUIMARÃES, P.R., JR, LOYOLA, R.D. & ULRICH, W. 2008. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239.). This index, NODF, has become the standard measure of nestedness, with nearly 1,000 citations in the Web of Science base (on 25 March 2022). It was further extended for measuring nestedness in quantitative and diversity-based data (Almeida-Neto & Ulrich 2011ALMEIDA-NETO, M. & ULRICH, W. 2011. A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Modell. Softw. 26:173–178., Melo et al. 2014MELO, A.S., CIANCIARUSO, M.V. & ALMEIDA-NETO, M. 2014. treeNODF: nestedness to phylogenetic, functional and other tree-based diversity metrics. Methods Ecol. Evol. 5:563–572., Pinheiro et al. 2019PINHEIRO, R.B.P., FELIX, G.M.F., DORMANN, C.F. & MELLO, M.A.R. 2019. A new model explaining the origin of different topologies in interaction networks. Ecology 100:1–30.). An analytic solution, again for measuring nestedness in bipartite networks, was developed in Araujo et al. (2010)ARAUJO, A.I.L., CORSO, G., ALMEIDA, A.M. & LEWINSOHN, T.M. 2010. An analytic approach to the measurement of nestedness in bipartite networks. Physica A 389:1405–1411., and qualitative (binary) and quantitative versions of the same networks were compared in Corso et al. (2015)CORSO, G., CRUZ, C., PINTO, M.P., ALMEIDA, A.M. & LEWINSOHN, T.M. 2015. Binary versus weighted interaction networks. Ecol. Complex. 23:68–72..
Host specialization and its converse, faunal overlap among host plants, is a key factor in establishing the structure and dimension of interactive communities. Lewinsohn & Roslin (2008)LEWINSOHN, T.M. & ROSLIN, T. 2008. Four ways towards tropical herbivore megadiversity. Ecol. Lett.11:398–416., propounded specialization as one of four alternative explanations for the high diversity of herbivores observed in the tropics. Jorge et al. (2014)JORGE, L.R., PRADO, P.I., ALMEIDA-NETO, M. & LEWINSOHN, T.M. 2014. An integrated framework to improve the concept of resource specialisation. Ecol. Lett. 17:1341–1350. developed a novel, more inclusive metric for specialization. In Corso et al. (2020)CORSO, G., FERREIRA, G.M.F. & LEWINSOHN, T.M. 2020. Mutual information as a general measure of structure in interaction networks. Entropy 22:1–17., specialization is again a key component, evaluated as the mutual information component of a two-way entropy model, irrespective of interaction topologies.
Research on Species Interaction Diversity in the BFP
We used the Fapesp virtual library (Fapesp-CDI 2022FAPESP-CDI. 2022. Fapesp Virtual Library: The referential information source for Research Supported by FAPESP. https://bv.fapesp.br/43922. Accessed on 25 March 2022.
https://bv.fapesp.br/43922...
) to survey research on species interactions developed from 1998 to 2022. The entire database was searched for the keywords “interação”, “interações”, “diversidade” and “polinização” (interaction, interactions, diversity, pollination) in research grants and doctoral and postdoctoral scholarships. We sought studies of interactions between species groups or taxa, at the community or regional level; many other studies addressed interactions of a particular species with one or a few associates. In all, 77 projects, including 35 research grants and 42 doctoral and postdoctoral scholarships, concerned the diversity of ecological interactions in that period. Here, we briefly summarize the main research lines with some examples of their resulting publications.
Plant-pollinator interactions were the most favored theme in grants and scholarships. Studies varied from detailed studies of figs and fig-wasps, to more general projects involving well-resolved plant-pollinator webs. These produced important advances in the understanding of the interaction of various pollinator groups, especially hummingbirds, bees and hawkmoths in the Atlantic Forest and Cerrado. With highly-resolved interaction matrices, they demonstrated that hummingbird morphology and habitat occupancy were more important in determining interactions in tropical species-rich webs than species abundance, the main determinant of temperate pollination webs (Vizentin-Bugoni et al. 2016VIZENTIN-BUGONI, J., MARUYAMA, P.K., DEBASTIANI , V.J., DUARTE, L.S., DALSGAARD, B. & SAZIMA, M. 2016. Influences of sampling effort on detected patterns and structuring processes of a Neotropical plant-hummingbird network. J. Anim. Ecol. 85:262–272., Bergamo et al. 2017BERGAMO, P.J., WOLOWSKI, M., MARUYAMA, P.K., VIZENTIN-BUGONI, J., CARVALHEIRO, L.G. & SAZIMA, M. 2017. The potential indirect effects among plants via shared hummingbird pollinators are structured by phenotypic similarity. Ecology 98:1849–1858.). These studies also contributed to broader syntheses of tropical pollination networks and their implications for conservation (Maruyama et al. 2018MARUYAMA, P.K., SONNE, J., VIZENTIN-BUGONI, J., MARTÍN GONZÁLEZ, A.M., ZANATA, T.B., ABRAHAMCZYK, S., ALARCÓN, R., ARAUJO, A.C., ARAÚJO, F.P., BAQUERO, A.C., CHÁVEZ GONZÁLEZ, E., COELHO, A.G., COTTON, P.A., DEHLING, D.M., FISCHER, E., KOHLER, G., LARA, C., LAS CASAS, F.M.G., MACHADO, A.O., MACHADO, C.G., MAGLIANESI, M.A., MALUCELLI, T.S., MARÍN GÓMEZ, O.H., OLIVEIRA, P.E., ORNELAS, J.F., ORTIZ-PULIDO, R., RAMÍREZ BURBANO, M.B., ROCCA, M.A., RODRIGUES, L.C., ROSERO-LASPRILLA, L., RUI, A.M., SANDEL, B., SVENNING, J.-C., TINOCO, B.A., VARASSIN, I.G., WATTS, S., RAHBEK, C., SAZIMA, M., SCHLEUNING, M. & DALSGAARD, B. 2018. Functional diversity mediates macroecological variation in plant–hummingbird interaction networks. Glob. Ecol. Biogeogr. 27:1186–1199., Vizentin-Bugoni 2018VIZENTIN-BUGONI, J., MARUYAMA, P.K., SOUZA, C.S.D., OLLERTON, J., RECH, A.R. & SAZIMA, M. 2018. Plant-pollinator networks in the tropics: a review. In Ecological Networks in the Tropics (W. Dáttilo & V. Rico-Gray, eds), Springer, Cham, Switzerland, p. 73–91.).
Another major research line addressed frugivory and seed dispersal by vertebrate species, especially by birds and mammals. These were mainly explored in the southeastern Atlantic Forest (Figure 6), but some projects also included the Cerrado and Amazon Forest biomes. Several studies investigated seed dispersal in fragmented or defaunated landscapes (Emer et al. 2018EMER, C., GALETTI, M., PIZO, M.A., GUIMARÃES, P.R., JR, MORAES, S., PIRATELLI, A. & JORDANO, P. 2018. Seed-dispersal interactions in fragmented landscapes – a metanetwork approach. Ecol. Lett. 21:484–493., 2019EMER, C., GALETTI, M., PIZO, M.A., JORDANO, P. AND VERDÚ, M. 2019. Defaunation precipitates the extinction of evolutionarily distinct interactions in the Anthropocene. Sci. Adv. 5:eaav6699.) and assessed its role in ecosystem restoration and conservation (Vidal et al. 2014VIDAL, M.M., HASUI, E., PIZO, M.A., TAMASHIRO, J.Y., SILVA, W.R. & GUIMARÃES P.R., JR. 2014. Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95:3440–3447.). Topologies of plant-dispersal networks were analyzed at local and regional scales (Donatti et al. 2011DONATTI, C.I., GUIMARÃES, JR., GALETTI, M., PIZO, M.A., MARQUITTI, F.M.D. & DIRZO, R. 2011. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol. Lett. 14:773–781., Emer et al. 2020EMER, C., JORDANO, P., PIZO, M.A., RIBEIRO, M.C., DA SILVA, F.R., GALETTI, M. 2020. Seed dispersal networks in tropical forest fragments: Area effects, remnant species, and interaction diversity. Biotropica 52:81– 89.).
A frugivory network in the Intervales State Park within the Atlantic Forest. Green circles are plant species (184); orange squares are bird species (81). Symbol size is proportional to number of links. The most connected species are highlighted in yellow, respectively Myrsine coriacea(Primulaceae, 59 links), and Chiroxiphia caudata (Pipridae, 35 links). Data from Silva et al. (2002)SILVA, W.R., DE MARCO, P., HASUI, E., & GOMES, V.S.M. 2002. Patterns of fruit-frugivores interactions in two Atlantic Forest bird communities of South-eastern Brazil: implications for conservation. In: Seed dispersal and frugivory: ecology, evolution and conservation (eds. Levey, D.J, Silva, W.R, and Galetti, M.). CAB International, Wallingford, p.423-435., original figure by Carine Emer.
Interactive networks of herbivorous insects and their host plants were addressed in a number of projects summarized in the present paper. Other research lines with arthropods concerned predator-prey interactions, including their potential for biological control (Barros et al. 2021BARROS, A.R.A, AZEVEDO, E.B., SILVA, E.S., CASTILHO, R.C. & DE MORAES, G.J. 2021. Diversity of edaphic Gamasina mites (Acari: Mesostigmata) in different ecosystems of the Caatinga biome in northeast Brazil. Syst. Appl. Acarol. 26:1301–1313.), and studies of defensive mutualisms involving ants, plants – with or without extrafloral nectaries – and herbivores (Rico-Gray & Oliveira 2007RICO-GRAY, V. & OLIVEIRA, P.S. 2007. The Ecology and Evolution of Ant-plant Interactions. University of Chicago Press, Chicago., Sendoya et al. 2016SENDOYA, S.F., BLUETHGEN, N., TAMASHIRO, J.Y., FERNANDEZ, F. & OLIVEIRA, P.S. 2016. Foliage-dwelling ants in a neotropical savanna: effects of plant and insect exudates on ant communities. Arthropod-Plant Interact. 10:183–195.).
Diversity of parasite-host interactions were studied for instance in helminths and reptiles (Bezerra et al. 2016BEZERRA, C.H., PINHEIRO, L.T., DE MELO, G.C., ZANCHI-SILVA, D., QUEIROZ, M.S., ANJOS, L.A., HARRIS, D.J. & BORGES-NOJOSA, D.M. 2016 Assessing the influence of geographic distance in parasite communities of an exotic lizard. Acta Parasitol. 61:136–143.) and in myxozoan parasites of freshwater fishes, many of them newly discovered (e.g. Vieira et al. 2021VIEIRA, D.H.M.D., NARCISO, R.B., DE AZEVEDO, R.K., & DA SILVA, R.J. 2021. Description of two novel Henneguya (Cnidaria: Myxosporea) infecting curimatid fish, using morphological, histological, and molecular analyses Acta Parasitol. 67:233–243.).
Theoretical explorations of patterns and processes in interaction diversity have also been fruitful. They include, for instance, development and evaluation of models of interactions in communities (Pires et al. 2011PIRES, M.M., PRADO, P.I., & GUIMARÃES, P.R., JR. 2011. Do food web models reproduce the structure of mutualistic networks? PLoS ONE 6:e27280., Lima et al. 2020LIMA, R.A.F., CONDE, P.A., BANKS-LEITE, C., CAMPOS, R.C., HERNANDEZ, M.I.M., RODRIGUES, R.R. & PRADO, P.I. 2020. Disentangling the effects of sampling scale and size on the shape of species abundance distributions. PloS One 15:e0238854.), evolution of mutualistic interactions (Guimarães et al. 2017GUIMARÃES, P.R., JR, PIRES, M.M., JORDANO, P., BASCOMPTE, J. & THOMPSON, J.N. 2017. Indirect effects drive coevolution in mutualistic networks. Nature 550:511–514., Assis et al. 2020ASSIS, A.P.A., THOMPSON, J.N., SANTANA, P.C., JORDANO, P., BASCOMPTE, J. & GUIMARÃES JR., P.R. 2020. Genetic correlations and ecological networks shape coevolving mutualisms. Ecol. Lett. 23:1789–1799., Burin et al. 2021BURIN, G., GUIMARÃES JR, P.R., QUENTAL, T.B. 2021. Macroevolutionary stability predicts interaction patterns of species in seed dispersal networks. Science 372:733–737.) and reconstruction of past interactions (Pires et al. 2018PIRES, M.M., GUIMARÃES, P.R., JR., GALETTI, M. & JORDANO, P. 2018. Pleistocene megafaunal extinctions and the functional loss of long‐distance seed‐dispersal services. Ecography 41:153–163.).
Also worth noting is the Advanced School on Networks in Ecology, held in 2011 and entirely funded by Fapesp. This intensive workshop combined lecturers and young researchers from Brazil and overseas, in seminars and hands-on projects focused on two themes, interaction and spatial ecological networks. Many of the participants and several studies and publications referred here were fostered in this Advanced School, in various partnerships that are active to this day.
Conclusions and Future Research
1. Interaction diversity is essential to biodiversity
Nowadays, the diversity of interactions among species is considered an essential component of biodiversity, quite as important as the diversity of species themselves. This was foreshadowed by Daniel Janzen before the term biodiversity appeared, in an often-quoted passage: “Examples of the local or total extinction of Central American species are recounted ad nauseam […] What escapes the eye, however, is a much more insidious kind of extinction: the extinction of ecological interactions”. Furthermore: “The extinction of an interaction system occurs in many ways, but there is one consistent characteristic: the animals and plants involved no longer interact in the manner that originally led to selection for the production and maintenance of the traits they now display” (Janzen 1974JANZEN, D.H. 1974. The deflowering of Central America. Nat. Hist. 83:49–53. p. 49). Thompson (1999)THOMPSON, J.N. 1999. The evolution of species interactions. Science 284:2116–2118. put it even more forcefully: “The history of evolution and biodiversity is fundamentally a history of the evolution of species interactions. Species in pure isolation simply do not make sense.”
Interaction diversity connects two essential facets of biodiversity: on the one hand, the inventory of taxa and species and their distribution and time and space; on the other, the functions, processes and services that living systems perform and maintain. Elucidating this connection is a central goal for 21st century ecology; yet, exploring it, especially in field studies, is a formidable task (Thompson et al. 2012THOMPSON, R., BROSE, U., DUNNE, J., HALL, R., HLADYZ, S., KITCHING, R., MARTINEZ, N., RANTALA, H., ROMANUK, T., STOUFFER, D. & TYLIANAKIS, J. 2012. Food webs: reconciling the structure and function of biodiversity. Trends Ecol. Evol. 27:689–697.). Singling out and focusing on one functional interface between two well-defined sets of species leads to a large gain in tractability, enabling more kinds of biological systems to be explored across different geographical regions and distinct ecological conditions. As recounted here, herbivore-plant interactions, as well as any other mode of structured biotic interaction, can be explored for a gamut of questions ranging from quite straightforward – how are their diversities correlated at different scales?, to more complex ones – to what extent are dynamical properties, such as resilience, governed by network topologies?
In most tropical ecosystems, extremely high diversity presents huge challenges. With relatively few abundant and widespread species, and many rare and/or spatially restricted species, these challenges multiply as we consider their interactions. Links among more abundant species may govern ecosystem processes, an implicit assumption in much of ecosystem ecology (Pickett et al. 2007PICKETT, S.T.A., KOLASA, J. & JONES, C.G. 2007. Ecological Understanding: the Nature ofTheory and the Theory of Nature. 2nd Edition. Elsevier / Academic Press, Amsterdam and Boston.). However, the multitude of interactions that involve rarer species may be equally essential to system processes, for instance by increasing resilience to species loss, providing key resources in periods of scarcity, or linking distinct modules of an interaction matrix (see Figure 4).
In even more general terms, interaction diversity should be taken as a central component of biocomplexity. Among the three defining dimensions of biocomplexity in the framework of Cadenasso et al. (2006)CADENASSO, M.L., PICKETT, S.T.A. & GROVE, J.M. 2006. Dimensions of ecosystem complexity: Heterogeneity, connectivity, and history. Ecol. Complex. 3:1–12., organizational connectivity clearly should encompass interaction diversity. This completes a progression, from single instances of pairwise interactions, by way of individual resource-based networks, to functionally defined sections of entire ecosystems.
2. New opportunities, new applications
Since biodiversity was established, both scientifically (Wilson 1988WILSON, E. O. (ed.) 1988. Biodiversity. National Academy of Sciences/Smithsonian Institution.) and politically (CBD 1992CBD (UNITED NATIONS ENVIRONMENT PROGRAMME) 1992. Convention on Biological Diversity. United Nations Environment Programme, Nairobi.) as a crucial entity of worldwide concern, the preponderant effort has been vested into assessing and monitoring species diversity, especially of better-known taxa, such as vertebrates and flowering plants. However, many scientists as well as decision makers and social stakeholders have come to realize that other structural and functional components of biodiversity are essential for meaningful assessments and, even more so, for predicting imminent changes and attempting to reduce or recoup losses. Thus, together with the huge store of undescribed species(the so-called Linnean shortfall) other shortfalls have to be addressed in order to better grasp and improve policies for global biodiversity. Among these, the Eltonian shortfall refers specifically to our shortage of knowledge of interactions among species, and their functional implications (Hortal et al. 2015HORTAL, J., DE BELLO, F., DINIZ-FILHO, J.A.F., LEWINSOHN, T.M., LOBO, J.M. & LADLE, R.J. 2015. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46:523–549.).
The incorporation of interaction diversity into routine assessments, monitoring programs and predictive modeling exercises, though still incipient, is advancing swiftly. In fact, well-established protocols for surveying and monitoring diversity of species can easily integrate procedures for the assessment of interaction diversity. This is illustrated in Figure 7, with data obtained in cerrado vegetation in the state of São Paulo. Here, cumulative curves show the total number of species of Asteraceae and of flowerhead herbivores recorded as more localities were successively sampled. These standard collectors’ curves are shown together with a curve for the cumulative number of plant-herbivore species interactions recorded with the samples. We see that the number of plant-herbivore interactions increased steadily, in contrast with the decelerating accumulation of plant and herbivore species. Thus, as further localities were surveyed, more interactions among already-known species were discovered; in other words, the beta diversity of interactions is higher than the beta diversity of species. On the other hand, connectance, a commonly used measure for network structure, stabilized as the number of sampled localities increased.
Collector’s curves for species and interactions in cerrado localities in the state of São Paulo (main localities shown in Figure 2). Five of eight localities were resampled in different seasons, totalling 23 samples (details in Almeida et al. 2005ALMEIDA, A.M., FONSECA, C.R., PRADO, P.I., ALMEIDA-NETO, M., DINIZ, S., KUBOTA, U., BRAUN, M.R., RAIMUNDO, R.L.G., ANJOS, L.A.D., MENDONÇA, T.G., FUTADA, S.M., & LEWINSOHN, T.M. 2005. Diversidade e ocorrência de Asteraceae em cerrados de São Paulo. Biota Neotrop. 5:27–43.). Continuous curves show the total number of plants, insects and interactions found as species accumulate. The dashed line shows the connectance, the proportion of potential links actually recorded in each cumulative sample set. Original figure.
These findings highlight the importance of recording pairwise species interactions.They suggest that habitat loss induces high extinction rates of unique plant-herbivore interactions, in keeping with Janzen (1974)JANZEN, D.H. 1974. The deflowering of Central America. Nat. Hist. 83:49–53. and Thompson (1999)THOMPSON, J.N. 1999. The evolution of species interactions. Science 284:2116–2118.. The procedure, illustrated here at the landscape or regional scale, can equally well be applied to the discovery rates of species and their interactions within a local community.
A major challenge for studies on ecological networks with tropical insect faunas is the high proportion of undescribed species (Stork 2018STORK, N.E. 2018. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63:31–45.), the abovementioned Linnean shortfall, which for a long time has precluded ecological studies of poorly known taxonomic groups. The insects associated with Asteraceae flower heads, for instance, include several recognizable genera of Cecidomyiidae, but since most Neotropical cecidomyiid species have not been described, their taxonomic resolution is constrained to the genus level. Parasitoids are also poorly known, yet highly important, insects in flower heads. In remnants of cerrado vegetation in São Paulo, 192 morphospecies of parasitoid wasps reared from flower heads of 74 Asteraceae species, 95% of which were identified to the genus level, comprising 103 genera from 18 families (Nascimento et al. 2014NASCIMENTO, A.R., ALMEIDA-NETO, M., ALMEIDA, A.M., FONSECA, C.R., LEWINSOHN, T.M. & PENTEADO-DIAS, A.M. 2014. Parasitoid wasps in flower heads of Asteraceae in the Brazilian Cerrado: taxonomical composition and determinants of diversity. Neotrop. Entomol. 43:298–306.). Even for better known groups of insects such as fruit-flies (Tephritidae), 40% of the species and three to six genera were new (Prado et al. 2004PRADO, P.I., NORRBOM, A.L., & LEWINSOHN, T.M. 2004. New species of Tomoplagia Coquillett (Diptera: Tephritidae) from capitula of Asteraceae in Brazil. Neotrop. Entomol. 33:189–211., Norrbom & Prado 2006NORRBOM, A.L. & PRADO, P.I. 2006. New genera and host plant records of Asteraceae-feeding Tephritidae (Diptera) from Brazil. Zootaxa 1139:1–17.).
The development of molecular tools applied to insect identification (Jinbo et al. 2011JINBO, U., KATO, T. & ITO, M. 2011. Current progress in DNA barcoding and future implications for entomology. Entomol. Sci.14:107–124.) is likely to expand the capacity of inventorying and sorting even incompletely described taxa. Even more important for our purposes are recent essays that concomitantly identify herbivores and their food plants (Gómez-Zurita et al. 2016GÓMEZ-ZURITA, J., CARDOSO, A., CORONADO, I., LA CADENA, DE, G., JURADO-RIVERA, J.A., MAES, J.-M., MONTELONGO, T., NGUYEN, D. & PAPADOPOULOU, A. 2016. High-throughput biodiversity analysis: Rapid assessment of species richness and ecological interactions of Chrysomelidae (Coleoptera) in the tropics. ZooKeys 597:3–26.) or parasitoids and their hosts (Šigut et al. 2017ŠIGUT M, KOSTOVČÍK M, ŠIGUTOVÁ H, HULCR J, DROZD P. & HRČEK, J. 2017. Performance of DNA metabarcoding, standard barcoding, and morphological approach in the identification of host–parasitoid interactions. PLOS ONE 12: e0187803., Dong et al. 2020DONG, Y., XI, X., CHEN, H., YANG, Y. & SUN, S. 2020. A protocol to identify the host of parasitoids by DNA barcoding of vestigial tissues. Ann. Zool. Fenn. 57:11–19.) from DNA extracts that include gut contents. Once these methods are refined, given proper reference libraries, the possibilities of studying plant-herbivore-parasitoid networks in megadiverse regions are almost limitless. We can envisage that highly-resolved tritrophic or indeed, multitrophic or multilayer networks (Astegiano et al. 2017ASTEGIANO, J., ALTERMATT, F. & MASSOL, F. 2017. Disentangling the co-structure of multilayer interaction networks: degree distribution and module composition in two-layer bipartite networks. Sci. Rep. 7:15465.) will become more accessible and widely investigated in the coming decades (Bohan et al. 2017BOHAN, D.A., VACHER, C., TAMADDONI-NEZHAD, A., RAYBOULD, A., DUMBRELL, A.J. & WOODWARD, G. 2017. Next-generation global biomonitoring: large-scale, automated reconstruction of ecological networks. Trends Ecol. Evol. 32:477–487.).
Interaction networks can reveal subtle responses in biodiversity driven by environmental changes. As an example from our study system, Asteraceae and their flowerhead herbivores were sampled in Cerrado remnants that ranged from well-preserved to highly impacted (Figure 8). Disturbance was measured as the proportion of cover occupied by invasive grasses which, in turn, was highly correlated with frequency of fires and cattle grazing. The number of interactions increased and then decreased with progressive disturbance, in accordance with the Intermediate Disturbance Hypothesis (Connell 1978CONNELL, J.H. 1978. Diversity in tropical rain forests and coral reefs. Science 199:1302–1310.). In this case, moderate disturbance allows the dispersal and establishment of additional plants and herbivores, whereas more intense disturbance provokes the progressive loss, especially of endemic plants and more specialized herbivore species.
Number of unique interactions between Asteraceous host species and endophagous flowerhead herbivores in 20 cerrado remnants encompassing a gradient of invasion by exotic grasses. The disturbance metric is the mean invasive grass cover (in 45 plots of 30m × 5m per remnant), with the following ordinal scale: (1) 0%; (2) 1 – 25%, (3) 26% – 50%, (4) 51% – 75%, (5) 75% – 100%. Redrawn from Almeida-Neto et al. (2011)ALMEIDA-NETO, M., PRADO, P.I. & LEWINSOHN, T.M. 2011. Phytophagous insect fauna tracks host plant responses to exotic grass invasion. Oecologia 165:1051–1062..
In tropical systems, given the high turnover and prevailing rarity of most taxa, taxonomic composition and richness are often uncertain indicators of ecological integrity. Biotic interactions deserve more attention as markers and indicators of biodiversity responses to various global change drivers (Memmott et al. 2007MEMMOTT, J., CRAZE, P.G., WASER, N.M., & PRICE, M.V. 2007. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 10:710–717., Valiente-Banuet 2014VALIENTE-BANUET, A., AIZEN, M.A., ALCÁNTARA, J.M., ARROYO, J., COCUCCI, A., GALETTI, M., GARCÍA, M.B., GARCÍA, D., GÓMEZ, J.M., JORDANO, P., MEDEL, R., NAVARRO, L., OBESO, J.R., OVIEDO, R., RAMÍREZ, N., REY, P.J., TRAVESET, A., VERDÚ, M. & ZAMORA, R. 2014. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29:299–307.). Network parameters may be more stable than species composition and respond more predictably to environmental change. Furthermore, disturbances may induce shifts in interactions so that networks are rewired even without species loss (Tylianakis et al. 2007TYLIANAKIS, J.M., TSCHARNTKE, T., & LEWIS, O.T. 2007. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445:202–205.). Interactions are also fundamental to predict coextinctions that multiply the consequences of species loss (Vieira & Almeida-Neto 2015VIEIRA, M.C. & ALMEIDA-NETO, M. 2015. A simple stochastic model for complex coextinctions in mutualistic networks: robustness decreases with connectance. Ecol. Lett. 18:144–52.).
To conclude, it is important to point out that observing and monitoring ecological interactions does not necessarily require highly specialized knowledge or equipment. An outstanding example is the growing network of citizen science initiatives to monitor pollinators and pollinator activity that provide an essential ecosystem service (Ghilardi-Lopes & Zattara 2022GHILARDI-LOPES, N.P. & ZATTARA, E.E. (eds.) 2022. Ciência Cidadã e Polinizadores da América do Sul. Cubo Multimídia, São Carlos, SP. Available at https://doi.org/10.4322/978-65-86819-20-5.100001.pt.
https://doi.org/10.4322/978-65-86819-20-...
).
Acknowledgments
We thank the students, colleagues and friends who participated in field and lab work, too many to be listed, but especially Umberto Kubota, Carlos Fonseca, Marcelo Lopes, Bruno Buys, Vinicius Motta, Antônio Carlos Macedo, Flávia Batista and Daniela América de Oliveira. We also thank the entomologists, especially Allen Norrbom, Angelo Prado, Victor Becker, Sergio Vanin, Alma Solis, Raymond Gagné and Cees Gielis; the botanists, Hermógenes Freitas Leitão Filho, João Semir, Nelson Matzenbacher, Mara Magenta, Roberto Esteves, John Pruski and Harold Robinson; beyond identifying our specimens, they made us appreciate and cherish the plants and insects that occupied our lives for many years. Carine Emer and Rafael Pinheiro produced new figures. Hermógenes Leitão Filho, Howard Cornell, John Lawton and Pedro Jordano offered encouragement and inspiration at various stages. FAPESP and the BIOTA-FAPESP program gave essential support through many grants and scholarships, especially FAPESP 363-4/85, 94/02837-2 and 98/05085-4. Additional funding and scholarships were provided by CAPES and by CNPq, including the National Institute for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation (CNPq Grant 465610/2014-5), in which TML and MAN are research associates; the CNPq Research Fellowships to MAN (308641/2020-5), PIP (310885/2017-5) and the Senior Research Fellowship to TML (314133/2020-8). TML acknowledges an EURIAS Grant for the 2018-2019 Fellowship at the Wissenschaftskolleg (Institute of Advanced Studies) in Berlin, which offered time to reflect on conceptual developments summarized in this paper.
-
EthicsThis study did not involve human beings and/or clinical trials that require approval by an Institutional Committee.
References
- ALMEIDA, A.M. 2001. Biogeografia de interações entre Eupatorieae (Asteraceae) e insetos endófagos de capítulos na Serra da Mantiqueira. PhD Thesis, Universidade Estadual de Campinas, Campinas. Available at https://repositorio.unicamp.br/Busca/Download?codigoArquivo=484206
» https://repositorio.unicamp.br/Busca/Download?codigoArquivo=484206 - ALMEIDA, A.M., FONSECA, C.R., PRADO, P.I., ALMEIDA-NETO, M., DINIZ, S., KUBOTA, U., BRAUN, M.R., RAIMUNDO, R.L.G., ANJOS, L.A.D., MENDONÇA, T.G., FUTADA, S.M., & LEWINSOHN, T.M. 2005. Diversidade e ocorrência de Asteraceae em cerrados de São Paulo. Biota Neotrop. 5:27–43.
- ALMEIDA-NETO, M., PRADO, P.I. & LEWINSOHN, T.M. 2011. Phytophagous insect fauna tracks host plant responses to exotic grass invasion. Oecologia 165:1051–1062.
- ALMEIDA-NETO, M., GUIMARÃES, P.R., JR, & LEWINSOHN, T.M. 2007. On nestedness analysis: rethinking matrix temperature and anti-nestedness. Oikos 116:716–722.
- ALMEIDA-NETO, M., GUIMARÃES, P.R., JR, LOYOLA, R.D. & ULRICH, W. 2008. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239.
- ALMEIDA-NETO, M. & ULRICH, W. 2011. A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Modell. Softw. 26:173–178.
- APG IV. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 181:1–20.
- ARAUJO, A.I.L., CORSO, G., ALMEIDA, A.M. & LEWINSOHN, T.M. 2010. An analytic approach to the measurement of nestedness in bipartite networks. Physica A 389:1405–1411.
- ASSIS, A.P.A., THOMPSON, J.N., SANTANA, P.C., JORDANO, P., BASCOMPTE, J. & GUIMARÃES JR., P.R. 2020. Genetic correlations and ecological networks shape coevolving mutualisms. Ecol. Lett. 23:1789–1799.
- ASTEGIANO, J., ALTERMATT, F. & MASSOL, F. 2017. Disentangling the co-structure of multilayer interaction networks: degree distribution and module composition in two-layer bipartite networks. Sci. Rep. 7:15465.
- ATMAR, W. & PATTERSON, B.D. 1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96:373–382.
- BARROS, A.R.A, AZEVEDO, E.B., SILVA, E.S., CASTILHO, R.C. & DE MORAES, G.J. 2021. Diversity of edaphic Gamasina mites (Acari: Mesostigmata) in different ecosystems of the Caatinga biome in northeast Brazil. Syst. Appl. Acarol. 26:1301–1313.
- BASCOMPTE, J., JORDANO, P., MELIÁN, C.J. & OLESEN, J.M. 2003. The nested assembly of plant–animal mutualistic networks. Proc. Natl. Acad. Sci. USA 100: 9383–9387.
- BENSON, W.W. 1978. Resource partitioning in passion vine butterflies. Evolution 32:493–518.
- BERENBAUM, M. 1991. Meat and veg (review of P.W. Price et al. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions). Nature 351:617–618.
- BERENBAUM, M. 2017. Insect biodiversity–millions and millions. In Insect Biodiversity: Science and Society (R.G. Foottit & P.H. Adler, eds). Vol.1, 2nd edition. Wiley, New York, p. 783–792.
- BERGAMO, P.J., WOLOWSKI, M., MARUYAMA, P.K., VIZENTIN-BUGONI, J., CARVALHEIRO, L.G. & SAZIMA, M. 2017. The potential indirect effects among plants via shared hummingbird pollinators are structured by phenotypic similarity. Ecology 98:1849–1858.
- BERNAYS, E.A. & CHAPMAN, R.F. 1994. Host-plant Selection by Phytophagous Insects. Chapman & Hall, New York.
- BEZERRA, C.H., PINHEIRO, L.T., DE MELO, G.C., ZANCHI-SILVA, D., QUEIROZ, M.S., ANJOS, L.A., HARRIS, D.J. & BORGES-NOJOSA, D.M. 2016 Assessing the influence of geographic distance in parasite communities of an exotic lizard. Acta Parasitol. 61:136–143.
- BOHAN, D.A., VACHER, C., TAMADDONI-NEZHAD, A., RAYBOULD, A., DUMBRELL, A.J. & WOODWARD, G. 2017. Next-generation global biomonitoring: large-scale, automated reconstruction of ecological networks. Trends Ecol. Evol. 32:477–487.
- BURIN, G., GUIMARÃES JR, P.R., QUENTAL, T.B. 2021. Macroevolutionary stability predicts interaction patterns of species in seed dispersal networks. Science 372:733–737.
- CADENASSO, M.L., PICKETT, S.T.A. & GROVE, J.M. 2006. Dimensions of ecosystem complexity: Heterogeneity, connectivity, and history. Ecol. Complex. 3:1–12.
- CBD (UNITED NATIONS ENVIRONMENT PROGRAMME) 1992. Convention on Biological Diversity. United Nations Environment Programme, Nairobi.
- CHRISTENHUSZ, M.J. & BYNG, J.W. 2016. The number of known plant species in the world and its annual increase. Phytotaxa 261: 01–217.
- COHEN, J.E. 1977. Food webs and the dimensionality of trophic niche space. Proc. Natl. Acad. Sci. USA 74:4533–4536.
- COLWELL, R.K., CHAO, A., GOTELLI, N., LIN, S.-Y., MAO, C., CHAZDON, R.L. & LONGINO, J. 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 5:3–21.
- CONNELL, J.H. 1978. Diversity in tropical rain forests and coral reefs. Science 199:1302–1310.
- CORNELL, H.V. 1985. Local and regional richness of cynipine gall wasps on California oaks. Ecology 66:1247–1260.
- CORSO, G., CRUZ, C., PINTO, M.P., ALMEIDA, A.M. & LEWINSOHN, T.M. 2015. Binary versus weighted interaction networks. Ecol. Complex. 23:68–72.
- CORSO, G., FERREIRA, G.M.F. & LEWINSOHN, T.M. 2020. Mutual information as a general measure of structure in interaction networks. Entropy 22:1–17.
- DONATTI, C.I., GUIMARÃES, JR., GALETTI, M., PIZO, M.A., MARQUITTI, F.M.D. & DIRZO, R. 2011. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol. Lett. 14:773–781.
- DONG, Y., XI, X., CHEN, H., YANG, Y. & SUN, S. 2020. A protocol to identify the host of parasitoids by DNA barcoding of vestigial tissues. Ann. Zool. Fenn. 57:11–19.
- DUNNE, J.A., WILLIAMS, R.J. & MARTINEZ, N.D. 2002. Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. USA 99:12917–12922.
- EMER, C., GALETTI, M., PIZO, M.A., GUIMARÃES, P.R., JR, MORAES, S., PIRATELLI, A. & JORDANO, P. 2018. Seed-dispersal interactions in fragmented landscapes – a metanetwork approach. Ecol. Lett. 21:484–493.
- EMER, C., GALETTI, M., PIZO, M.A., JORDANO, P. AND VERDÚ, M. 2019. Defaunation precipitates the extinction of evolutionarily distinct interactions in the Anthropocene. Sci. Adv. 5:eaav6699.
- EMER, C., JORDANO, P., PIZO, M.A., RIBEIRO, M.C., DA SILVA, F.R., GALETTI, M. 2020. Seed dispersal networks in tropical forest fragments: Area effects, remnant species, and interaction diversity. Biotropica 52:81– 89.
- FAPESP-CDI. 2022. Fapesp Virtual Library: The referential information source for Research Supported by FAPESP. https://bv.fapesp.br/43922 Accessed on 25 March 2022.
» https://bv.fapesp.br/43922 - FELIX, G.M., PINHEIRO, R.B.P., POULIN, R., KRASNOV, B.R. & MELLO, M.A.R. 2022. The compound topology of host–parasite networks is explained by the integrative hypothesis of specialization. Oikos 2022: e08462.
- FOX, L.R. & MORROW, P.A. 1981. Specialization: species property or local phenomenon? Science 211:887–893.
- FUNK, V.A., SUSANNA, A., STUESSY, T. & BAYER, R. (eds) 2009. Systematics, Evolution and Biogeography of Compositae. IAPT, Vienna
- GASTON, K.J., REAVEY, D. & VALLADARES, G.R. 1992. Intimacy and fidelity: internal and external feeding by the British microlepidoptera. Ecol. Entomol. 17:86–88.
- GHILARDI-LOPES, N.P. & ZATTARA, E.E. (eds.) 2022. Ciência Cidadã e Polinizadores da América do Sul. Cubo Multimídia, São Carlos, SP. Available at https://doi.org/10.4322/978-65-86819-20-5.100001.pt
» https://doi.org/10.4322/978-65-86819-20-5.100001.pt - GÓMEZ-ZURITA, J., CARDOSO, A., CORONADO, I., LA CADENA, DE, G., JURADO-RIVERA, J.A., MAES, J.-M., MONTELONGO, T., NGUYEN, D. & PAPADOPOULOU, A. 2016. High-throughput biodiversity analysis: Rapid assessment of species richness and ecological interactions of Chrysomelidae (Coleoptera) in the tropics. ZooKeys 597:3–26.
- GROOMBRIDGE, B. (ed) 1992. Global Biodiversity – Status of the Earth’s Living Resources. World Conservation Monitoring Centre, London.
- GUIMARÃES, P.R., JR, PIRES, M.M., JORDANO, P., BASCOMPTE, J. & THOMPSON, J.N. 2017. Indirect effects drive coevolution in mutualistic networks. Nature 550:511–514.
- HARPER, J.L. & HAWKSWORTH, D.L. 1995. Preface. In Biodiversity: measurement and estimation, (D.L. Hawksworth, ed), Chapman and Hall, London, p. 5–12.
- HEYWOOD, V.H. (ed) 1995. Global Biodiversity Assessment. United Nations Environment Programme. Cambridge University Press, Cambridge.
- HORTAL, J., DE BELLO, F., DINIZ-FILHO, J.A.F., LEWINSOHN, T.M., LOBO, J.M. & LADLE, R.J. 2015. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46:523–549.
- JANZEN, D.H. 1968. Host plants as islands in evolutionary and contemporary time. Am. Nat. 102:592–595.
- JANZEN, D.H. 1973. Host plants as islands. II. Competition in evolutionary and contemporary time. Am. Nat. 107:786–790.
- JANZEN, D.H. 1974. The deflowering of Central America. Nat. Hist. 83:49–53.
- JINBO, U., KATO, T. & ITO, M. 2011. Current progress in DNA barcoding and future implications for entomology. Entomol. Sci.14:107–124.
- JORGE, L.R., NOVOTNY, V., SEGAR, S.T., WEIBLEN, G.D., MILLER, S.E., BASSET, Y. & LEWINSOHN, T.M. 2017. Phylogenetic trophic specialization: a robust comparison of herbivorous guilds. Oecologia 185: 551–559.
- JORGE, L.R., PRADO, P.I., ALMEIDA-NETO, M. & LEWINSOHN, T.M. 2014. An integrated framework to improve the concept of resource specialisation. Ecol. Lett. 17:1341–1350.
- LAKATOS, I. 1970. Falsification and the methodology of scientific research programmes. In Criticism and the Growth of Knowledge (I. Lakatos and A. Musgrave, eds). Cambridge University Press, Cambridge, p. 91–196.
- LAWTON, J.H. 1984. Non-competitive populations, non-convergent communities, and vacant niches: the herbivores of bracken. In Ecological Communities: Conceptual Issues and the Evidence (D.R. Strong, D. Simberloff, L.G. Abele & A.B. Thistle, eds), Princeton University Press, Princeton, NJ, p. 67–101.
- LAWTON, J.H. 1992. There are not 10 million kinds of population dynamics. Oikos 63:337–338.
- LAWTON, J.H. & PRICE, P.W. 1979. Species richness of parasites on hosts: agromyzid flies on the British Umbelliferae. J. Anim. Ecol. 48:619–637.
- LEES, A.C. & PIMM, S.L. 2015. Species, extinct before we know them? Curr. Biol. 25:R177–R180.
- LEIBOLD, M.A. & MIKKELSON, G.M. 2002. Coherence, species turnover, and boundary clumping: elements of meta-community structure. Oikos 97:237–250.
- LEWINSOHN, T.M. 1991. Insects in flower heads of Asteraceae in southeast Brazil: a tropical case study on species richness. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions (P.W. Price, T.M. Lewinsohn, G.W. Fernandes & W.W. Benson, eds.) Wiley/Interscience, New York, p. 525–560.
- LEWINSOHN, T.M., NOVOTNY, V. & BASSET, Y. 2005. Insects on plants: Diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Evol. Syst. 36:597–620.
- LEWINSOHN, T.M., PRADO, P.I., JORDANO, P., BASCOMPTE, J., & OLESEN, J.M. 2006. Structure in plant-animal interaction assemblages. Oikos 113:174–184.
- LEWINSOHN, T.M. & ROSLIN, T. 2008. Four ways towards tropical herbivore megadiversity. Ecol. Lett.11:398–416.
- LIMA, R.A.F., CONDE, P.A., BANKS-LEITE, C., CAMPOS, R.C., HERNANDEZ, M.I.M., RODRIGUES, R.R. & PRADO, P.I. 2020. Disentangling the effects of sampling scale and size on the shape of species abundance distributions. PloS One 15:e0238854.
- LOVEJOY, T.E. 1980. Foreword. In Conservation Biology (M.E. Soule and B.A. Wilcox, eds). Sinauer, Sunderland, Mass. p. ix–x.
- MARUYAMA, P.K., SONNE, J., VIZENTIN-BUGONI, J., MARTÍN GONZÁLEZ, A.M., ZANATA, T.B., ABRAHAMCZYK, S., ALARCÓN, R., ARAUJO, A.C., ARAÚJO, F.P., BAQUERO, A.C., CHÁVEZ GONZÁLEZ, E., COELHO, A.G., COTTON, P.A., DEHLING, D.M., FISCHER, E., KOHLER, G., LARA, C., LAS CASAS, F.M.G., MACHADO, A.O., MACHADO, C.G., MAGLIANESI, M.A., MALUCELLI, T.S., MARÍN GÓMEZ, O.H., OLIVEIRA, P.E., ORNELAS, J.F., ORTIZ-PULIDO, R., RAMÍREZ BURBANO, M.B., ROCCA, M.A., RODRIGUES, L.C., ROSERO-LASPRILLA, L., RUI, A.M., SANDEL, B., SVENNING, J.-C., TINOCO, B.A., VARASSIN, I.G., WATTS, S., RAHBEK, C., SAZIMA, M., SCHLEUNING, M. & DALSGAARD, B. 2018. Functional diversity mediates macroecological variation in plant–hummingbird interaction networks. Glob. Ecol. Biogeogr. 27:1186–1199.
- MELO, A.S., CIANCIARUSO, M.V. & ALMEIDA-NETO, M. 2014. treeNODF: nestedness to phylogenetic, functional and other tree-based diversity metrics. Methods Ecol. Evol. 5:563–572.
- MACARTHUR, R.H. & WILSON, E.O. 1967. The Theory of Island Biogeography. Princeton University Press, Princeton, NJ.
- MANDEL, J.R., DIKOW, R.B., SINISCALCHI, C.M., THAPA, R., WATSON, L.E. & FUNK, V.A. 2019. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 116:14083–14088.
- MEMMOTT, J., CRAZE, P.G., WASER, N.M., & PRICE, M.V. 2007. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 10:710–717.
- MURDOCH, W., EVANS, F. & PETERSON, C. 1972. Diversity and pattern in plants and insects. Ecology 53:819–829.
- NASCIMENTO, A.R., ALMEIDA-NETO, M., ALMEIDA, A.M., FONSECA, C.R., LEWINSOHN, T.M. & PENTEADO-DIAS, A.M. 2014. Parasitoid wasps in flower heads of Asteraceae in the Brazilian Cerrado: taxonomical composition and determinants of diversity. Neotrop. Entomol. 43:298–306.
- NEWMAN, M. 2003. The structure and function of complex networks. Siam Rev. 45:167–256.
- NORSE, E.A., ROSENBAUM, K.L., WILCOVE, D.S. & WILCOX, B.A. 1986. Conserving Biological Diversity in our National Forests. The Wilderness Society, Washington.
- NORRBOM, A.L. & PRADO, P.I. 2006. New genera and host plant records of Asteraceae-feeding Tephritidae (Diptera) from Brazil. Zootaxa 1139:1–17.
- NOVOTNY, V. 2009. Beta diversity of plant-insect food webs in tropical forests: a conceptual framework. Insect Conserv. Diver. 2:5–9.
- NOVOTNY, V., BASSET, Y., MILLER, S., DROZD, P. & CIZEK, L. 2002. Host specialization of leaf-chewing insects in a New Guinea rainforest. J. Anim. Ecol. 71: 400–412.
- NRC (National Research Council USA) 1980. Research Priorities in Tropical Biology. National Academies Press, Washington, DC.
- PINHEIRO, R.B.P., FELIX, G.M.F., DORMANN, C.F. & MELLO, M.A.R. 2019. A new model explaining the origin of different topologies in interaction networks. Ecology 100:1–30.
- PIRES, M.M., GUIMARÃES, P.R., JR., GALETTI, M. & JORDANO, P. 2018. Pleistocene megafaunal extinctions and the functional loss of long‐distance seed‐dispersal services. Ecography 41:153–163.
- PIRES, M.M., PRADO, P.I., & GUIMARÃES, P.R., JR. 2011. Do food web models reproduce the structure of mutualistic networks? PLoS ONE 6:e27280.
- PICKETT, S.T.A., KOLASA, J. & JONES, C.G. 2007. Ecological Understanding: the Nature ofTheory and the Theory of Nature. 2nd Edition. Elsevier / Academic Press, Amsterdam and Boston.
- PRADO, P.I., NORRBOM, A.L., & LEWINSOHN, T.M. 2004. New species of Tomoplagia Coquillett (Diptera: Tephritidae) from capitula of Asteraceae in Brazil. Neotrop. Entomol. 33:189–211.
- PRADO, P.I. & LEWINSOHN, T.M. 2004. Compartments in insect–plant associations and their consequences for community structure. J. Anim. Ecol. 73:1168–1178.
- PRICE, P.W., DINIZ, I.R., MORAIS, H.C. & MARQUES, E.S.A. 1995. The abundance of insect herbivore species in the tropics: the high local richness of rare species. Biotropica 27:468–478.
- PRICE, P.W., LEWINSOHN, T.M., FERNANDES, G.W. & BENSON, W.W. (eds.) 1991. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Wiley/Interscience, New York.
- RAVEN, P.H. & WILSON, E.O. 1992. A fifty-year plan for biodiversity surveys. Science 258:1099–1100.
- RICO-GRAY, V. & OLIVEIRA, P.S. 2007. The Ecology and Evolution of Ant-plant Interactions. University of Chicago Press, Chicago.
- ROQUE, N., TELES, A.M. & NAKAJIMA, J.N. (eds.) 2017. A Família Asteraceae no Brasil: Classificação e Diversidade. EDUFBA, Salvador. Available at: https://doi.org/10.7476/9788523219994
» https://doi.org/10.7476/9788523219994 - ROQUE, N. et al. 2020. Asteraceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Available at: <https://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB55>. Accessed on: 07 March 2022
» https://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB55 - SENDOYA, S.F., BLUETHGEN, N., TAMASHIRO, J.Y., FERNANDEZ, F. & OLIVEIRA, P.S. 2016. Foliage-dwelling ants in a neotropical savanna: effects of plant and insect exudates on ant communities. Arthropod-Plant Interact. 10:183–195.
- ŠIGUT M, KOSTOVČÍK M, ŠIGUTOVÁ H, HULCR J, DROZD P. & HRČEK, J. 2017. Performance of DNA metabarcoding, standard barcoding, and morphological approach in the identification of host–parasitoid interactions. PLOS ONE 12: e0187803.
- SILVA, W.R., DE MARCO, P., HASUI, E., & GOMES, V.S.M. 2002. Patterns of fruit-frugivores interactions in two Atlantic Forest bird communities of South-eastern Brazil: implications for conservation. In: Seed dispersal and frugivory: ecology, evolution and conservation (eds. Levey, D.J, Silva, W.R, and Galetti, M.). CAB International, Wallingford, p.423-435.
- SOUTHWOOD, T. 1961. The number of species of insect associated with various trees. J. Anim. Ecol. 30:1–8.
- STORK, N.E. 2018. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63:31–45.
- STROGATZ, S. 2001. Exploring complex networks. Nature 410:268–276.
- STRONG, D.R., JR. 1974. Rapid asymptotic species accumulation in phytophagous insect communities: the pests of cacao. Science 185:1064–1066.
- STRONG, D.R., JR. 1977. Insect species richness: hispine beetles of Heliconia latispatha. Ecology 58:573–582.
- STRONG, D.R., JR., LAWTON, J.H. & SOUTHWOOD, T.R. 1984. Insects on Plants: Community Patterns and Mechanisms. Blackwell, Oxford.
- THOMPSON, J.N. 1996. Evolutionary ecology and the conservation of biodiversity. Trends Ecol. Evol. 11:300–303.
- THOMPSON, J.N. 1991. An exuberance of life (review of Plant-Animal Interactions; Peter W. Price, Thomas M. Lewinsohn, G. Wilson Fernandes & Woodruff W. Benson, eds). Science 252:1435–1435.
- THOMPSON, J.N. 1997. Conserving interaction biodiversity. In: The Ecological Basis of Conservation: Heterogeneity, Ecosystems, and Biodiversity (S.T.A. Pickett, R.S. Ostfeld, M. Shachak & G.E. Likens, eds). Chapman & Hall, New York, p. 285–293.
- THOMPSON, J.N. 1999. The evolution of species interactions. Science 284:2116–2118.
- THOMPSON, R., BROSE, U., DUNNE, J., HALL, R., HLADYZ, S., KITCHING, R., MARTINEZ, N., RANTALA, H., ROMANUK, T., STOUFFER, D. & TYLIANAKIS, J. 2012. Food webs: reconciling the structure and function of biodiversity. Trends Ecol. Evol. 27:689–697.
- TYLIANAKIS, J.M., TSCHARNTKE, T., & LEWIS, O.T. 2007. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445:202–205.
- VALIENTE-BANUET, A., AIZEN, M.A., ALCÁNTARA, J.M., ARROYO, J., COCUCCI, A., GALETTI, M., GARCÍA, M.B., GARCÍA, D., GÓMEZ, J.M., JORDANO, P., MEDEL, R., NAVARRO, L., OBESO, J.R., OVIEDO, R., RAMÍREZ, N., REY, P.J., TRAVESET, A., VERDÚ, M. & ZAMORA, R. 2014. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29:299–307.
- VIEIRA, M.C. & ALMEIDA-NETO, M. 2015. A simple stochastic model for complex coextinctions in mutualistic networks: robustness decreases with connectance. Ecol. Lett. 18:144–52.
- VIEIRA, D.H.M.D., NARCISO, R.B., DE AZEVEDO, R.K., & DA SILVA, R.J. 2021. Description of two novel Henneguya (Cnidaria: Myxosporea) infecting curimatid fish, using morphological, histological, and molecular analyses Acta Parasitol. 67:233–243.
- VIZENTIN-BUGONI, J., MARUYAMA, P.K., DEBASTIANI , V.J., DUARTE, L.S., DALSGAARD, B. & SAZIMA, M. 2016. Influences of sampling effort on detected patterns and structuring processes of a Neotropical plant-hummingbird network. J. Anim. Ecol. 85:262–272.
- VIZENTIN-BUGONI, J., MARUYAMA, P.K., SOUZA, C.S.D., OLLERTON, J., RECH, A.R. & SAZIMA, M. 2018. Plant-pollinator networks in the tropics: a review. In Ecological Networks in the Tropics (W. Dáttilo & V. Rico-Gray, eds), Springer, Cham, Switzerland, p. 73–91.
- VIDAL, M.M., HASUI, E., PIZO, M.A., TAMASHIRO, J.Y., SILVA, W.R. & GUIMARÃES P.R., JR. 2014. Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95:3440–3447.
- WHITTAKER, R.H. 1977. Evolution of species diversity in land communities. Evol. Biol. 10:1–67.
- WILSON, E. O. (ed.) 1988. Biodiversity. National Academy of Sciences/Smithsonian Institution.
- WILSON, E.O. & WILLIS, E.O. 1975. Applied biogeography. In Ecology and Evolution of Communities, (M.L. Cody & J.M. Diamond, eds). Harvard Univ. Press, Cambridge, MA, p. 522–34.
- ZWÖLFER, H. 1987. Species richness, species packing, and evolution in insect-plant systems. Ecol. Stud. 61:301–319. Springer, Berlin.
Edited by
Publication Dates
-
Publication in this collection
16 Sept 2022 -
Date of issue
2022
History
-
Received
25 July 2022 -
Accepted
07 Aug 2022