Abstract
OBJECTIVES:
The purpose is to study the effects of hyperbaric oxygen therapy and autologous platelet concentrates in healing the fibula bone of rabbits after induced fractures.
METHODS:
A total of 128 male New Zealand albino rabbits, between 6-8 months old, were subjected to a total osteotomy of the proximal portion of the right fibula. After surgery, the animals were divided into four groups (n = 32 each): control group, in which animals were subjected to osteotomy; autologous platelet concentrate group, in which animals were subjected to osteotomy and autologous platelet concentrate applied at the fracture site; hyperbaric oxygen group, in which animals were subjected to osteotomy and 9 consecutive daily hyperbaric oxygen therapy sessions; and autologous platelet concentrate and hyperbaric oxygen group, in which animals were subjected to osteotomy, autologous platelet concentrate applied at the fracture site, and 9 consecutive daily hyperbaric oxygen therapy sessions. Each group was divided into 4 subgroups according to a pre-determined euthanasia time points: 2, 4, 6, and 8 weeks postoperative. After euthanasia at a specific time point, the fibula containing the osseous callus was prepared histologically and stained with hematoxylin and eosin or picrosirius red.
RESULTS:
Autologous platelet concentrates and hyperbaric oxygen therapy, applied together or separately, increased the rate of bone healing compared with the control group.
CONCLUSION:
Hyperbaric oxygen therapy and autologous platelet concentrate combined increased the rate of bone healing in this experimental model.
Hyperbaric Oxygenation; Platelet-rich Plasma; Fibula, Fracture Consolidation
Introduction
Loss of osseous continuity due to fracture is a disabling condition, even momentarily, that often deprives the individual of his or her social and professional lifestyle. Therefore, as a medical specialty, orthopedics has invested in studies that promote reduced bone healing time and, consequently, reduced time required to return to normal or almost normal function.
Although most fractures do not present healing problems under normal conditions, in some situations, the poorly vascularized bone tissue does not respond adequately to treatment, resulting in delayed consolidation or non-consolidation (11. Chiang CC, Su CY, Huang CK, Chen WM, Chen TH, Tzeng YH. Early experience and results of bone graft enriched with autologous platelet gel for recalcitrant nonunions of lower extremity. J Trauma. 2007;63(3):655-61.). Thus, developing new techniques to help the bone healing process and stimulate fracture consolidation is very important.
Several studies have focused on reducing bone healing time using low-intensity pulsed ultrasound, low-energy lasers, electrical stimulation, and platelet-rich concentrates (22. Taylor MA, Norman TL, Clovis NB, Blaha JD. The response of habbit patellar tendon after autologous blood injection. Med Sci Sports Exerc. 2002;34(1):70-3.).
Platelet-rich concentrate (PRC) is an autologous concentration of platelets that releases growth factors. Platelets and the release of these proteins are essential for clot formation in the physiological process of tissue repair. A normal clot consists of 95% red blood cells, 4% platelets, and 1% white blood cells. However, an analysis of platelet-enriched clots reveals 95% platelets, 4% red blood cells, and 1% white blood cells (22. Taylor MA, Norman TL, Clovis NB, Blaha JD. The response of habbit patellar tendon after autologous blood injection. Med Sci Sports Exerc. 2002;34(1):70-3.).
Platelet-derived growth factor (PDGF) has defined periods of activity. PDGF is a major factor during the onset of tissue repair and acts directly on cell differentiation and angiogenesis. According to classic studies by Heldin & Westmark (33. Heldin CH, Westemark B. PDGF-like growth factors in autocrine stimulation of growth. J Cell Physiol Suppl. 1987;Suppl 5:31-4, http://dx.doi.org/10.1002/jcp.1041330407.
http://dx.doi.org/10.1002/jcp.1041330407...
), PDGF is the first growth factor that initiates repair by stimulating mitogenesis and increasing the local populations of mesenchymal stem cells and osteoprogenitor cells. At concentrations of 20 to 100 ng/ml, bone formation and alkaline phosphatase activity increase in the demineralized matrices of rats (44. Andrew JG, Hoyland JA, Freemont AJ, March DL. Platelet delivery growth factor expression in normally healing human fractures. Bone.1995;16:455-60.).
Transforming growth factor beta (TGF-β) is a cytokine synthesized in bone tissue and platelets that stimulates the proliferation of osteoblast precursor cells, promotes collagen synthesis, increases the number of cells that express the osteoblast genotype, increases osteoclast apoptosis, and activates endothelial cells for angiogenesis and chondroprogenitor cells in cartilage formation. However in vitro, TGF-β alone does not induce bone formation in an animal model (55. Beck LS, Deguzman L, Lee WP, Xu Y, McFratridge LA, Gillett NA, et al. Rapid publication. TGF-beta 1 induces bone closure of skull defects. J Bone Miner Res. 1991;6(11):1257-65.).
Methods for obtaining platelet growth factors should consider the platelet concentrate rate, processing technique, concentration of secreted proteins, handling and application, and especially the clinical application (66. Eppley BL, Pietrzak WS, Blanton M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plast Reconstr Surg. 2006;118(6):147e-159e.). Platelet-rich plasma (PRP) is undoubtedly the most widely used serum; however, its indiscriminate use has been criticized, and it is still not approved by the Food and Drug Administration (FDA) (77. Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18(18):1201-25, http://dx.doi.org/10.1016/S0142-9612(97)00050-1.
http://dx.doi.org/10.1016/S0142-9612(97)...
).
Autologous platelet concentrate (APC) is a platelet-rich concentrate (PRC) that is similar to PRP but has certain methodological advantages in terms of how it is obtained. In addition to being approved by the FDA (using the double-syringe method), APC can be withdrawn and used during a surgical procedure for fracture fixation and is easily processed in a few minutes, which is important because PDGF and TGF-β have half-lives of only a few minutes outside the vascular system (77. Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18(18):1201-25, http://dx.doi.org/10.1016/S0142-9612(97)00050-1.
http://dx.doi.org/10.1016/S0142-9612(97)...
).
The basic difference between PRP and APC is that PRP is an PRC, with a platelet concentration over 2 times greater than that of plasma. APC is a PRC that combines PRP with platelet-poor plasma (PPP); according to the manufacturer, APC contains 2 times the platelet concentration of blood plasma. According to studies by Graziane et al. (88. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212-9, http://dx.doi.org/10.1111/j.1600-0501.2005.01203.x.
http://dx.doi.org/10.1111/j.1600-0501.20...
), this platelet concentration is sufficient to stimulate osteoblast proliferation in vitro. In addition, obtaining APC using the double-syringe method eliminates leukocytes, proteases, and hydrolases, which may be able to degrade APC (99. Anitua E, Sánchez M, Orive G, Andía I. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008;29:37-41, http://dx.doi.org/10.1016/j.tips.2007.10.010.
http://dx.doi.org/10.1016/j.tips.2007.10...
).
Hyperbaric oxygen therapy (HBO) is another treatment that may have positive effects on the bone healing process.
According to the Undersea and Hyperbaric Medical Society, HBO is defined as intermittently inhaling 100% oxygen inside a chamber at pressures greater than 1 atmosphere absolute (ATA). This therapy has been first-choice treatment for decompression sickness in divers since the 1920s; however, only since the 1950s has it been used to treat gas embolism, carbon monoxide poisoning, gangrene gas, compartment syndrome, soft tissue infection, osteomyelitis, and osteoradionecrosis (1010. Williams STB. The role of hyperbaric oxygen therapy in trauma. Trauma. 2010;12(1):13-20, http://dx.doi.org/10.1177/1460408609354430.
http://dx.doi.org/10.1177/14604086093544...
).
The advantage of HBO is that it is a safe treatment. According to the literature, the complication rate is 1.83%, of which 94% are considered minor complications. The disadvantages of HBO include the high cost of the hyperbaric chamber and the technical training necessary, considering the number of circumstances under which it can be used with some level of evidence. However, the delayed consolidation or non-consolidation of fractures has significant financial impacts on society and represents a serious clinical challenge for which few treatment options are available. Various, often necessary surgical procedures are accompanied with patient morbidity and reduced quality of life (1111. Huang KC, Hsu WH, Peng KT, Huang TJ, Hsu RWJ. Hyperbaric oxygen therapy in orthopedic conditions: an evaluation of safety. J Trauma. 2006;61(4):913-7.).
This study was designed to test the hypothesis that many patients with fractures or delayed consolidations could benefit from APC, HBO, or a combination of both treatments because the literature indicates that the osteogenic action of growth factors depends directly on the tissue oxygen tension (1212. Gospodarowicz D. Growth factors and their action in vivo and in vitro. J Pathol.1983;141(3):201-33, http://dx.doi.org/10.1002/path.1711410304.
http://dx.doi.org/10.1002/path.171141030...
).
MATERIALS AND Methods
Sample
This study was approved by the Ethics Committee of Universidade Federal de São Paulo (0163/08). All animals were handled according to "The Ethical Principles of Animal Experiments of the International Union for the Protection of Animals" 11.794/2008 Act of October 8, 2008.
A total of 128 6-8-month-old male New Zealand albino rabbits (Oryctolagus cuniculus), with an average weight of 3,000 grams, were subjected to an osteotomy of the right fibula after an adaptation period.
After surgery, the animals were randomized into 4 groups of 32 rabbits according to the postsurgical treatment used, as follows:
-
Control Group (CG) - animals subjected only to osteotomy of the right fibula, without further treatment
-
APC Group (APCG) - animals subjected to osteotomy of the right fibula and treated with APC
-
HBO Group (HBOG) - animals subjected to osteotomy of the right fibula and treated with consecutive daily HBO sessions, totaling 9 sessions
-
APC and HBO group (APC+HBOG) - animals subjected to osteotomy of the right fibula and treated with APC, combined with consecutive daily HBO sessions, totaling 9 sessions
Each group was further divided into 4 subgroups of 8 animals each, according to a pre-determined euthanasia time point at 2, 4, 6, and 8 weeks postoperatively.
Operative procedure
All animals were food- and water-fasted for 6 hours pre-operatively and then weighed; all the fur was shaved at the leg region.
Pre-anesthesia was induced with ketamine hydrochloride solution at a dose of 30 mg/kg, xylazine at a dose of 3 mg/kg, and tramadol hydrochloride at a dose of 5 mg/kg intramuscularly in the gluteal region.
The animals were anesthetized with spinal anesthesia using 2% lidocaine without a vasoconstrictor at a dose of 0.5 mg/kg. Venoclysis of the right auricular vein was performed for the infusion of the necessary drugs. Antisepsis was then performed with polyvinylpyrrolidone, and the operative field was isolated with sterile field cloths, delimiting a restricted area for the surgery.
Then, a 2-cm lateral longitudinal incision was made along the right leg and dissected by following the muscular planes and localization of the fibular diaphyseal region. A complete transverse osteotomy of the fibula was performed using a no. 15 scalpel blade at the proximal third of the tibial tuberosity.
The surgery was completed by suturing the planes, aponeurosis, subcutaneous tissue, and skin with 4-0 nylon thread. Tramadol hydrochloride analgesic was administered at a dose of 2 mg/kg intramuscularly and maintained every 12 hours for 3 consecutive days, in combination with 4 intramuscular antibiotic treatments with benzathine penicillin and G potassium at doses of 20,000 IU/kg every 48 hours, in all animals.
Immediately after the osteotomy of the fibula, the animals were randomized according to the pre-determined treatment groups under study and to their respective subgroups. The hind limb did not need to be immobilized because the distal portion of the fibula in rabbits exhibits fusion with the tibia.
During the post-operative period, at the pre-determined time for each subgroup (2, 4, 6, or 8 weeks), the animals were anesthetized again according to the previous protocol, and the fibulae were resected, including the osseous callus, using a no. 15 scalpel blade. Euthanasia was induced during deep anesthesia with xylazine.
Application of autologous platelet concentrate
This procedure was performed during the surgery. Before the surgical procedure, 2.5 ml of blood was withdrawn from the auricular vein using a 10-ml double-syringe (ACP-double syringe, Arthrex, Naples, FL, USA) containing 0.5 ml of acid citrate dextrose anticoagulant, and the syringe was gently agitated to ensure the adequate mixing of the blood with the anticoagulant. The syringe containing the anti-coagulated blood was then stored in a suitable tube and centrifuged (Rotofix 32A, Arthrex, USA) at 1500 RPM for 5 minutes to separate the red blood cells from the plasma.
After centrifugation, the plasma was removed from the tube using the same syringe, while the internal syringe collected only the concentrate, leaving the red blood cells. Using this procedure, which was provided by the manufacturer, we obtained approximately 1.5 ml of concentrate, with a high concentration of autologous platelets enriched with growth factors.
The platelet concentrate was applied directly to the fracture (osteotomy) in the proximal third of the rabbit fibula before closing the muscular fascia.
Hyperbaric oxygen therapy
HBO sessions were conducted using a veterinary hyperbaric chamber model (AN - 500, Sechrist, USA) with a 14-rabbit capacity (average weight of 2,500 grams each). The sessions were conducted daily, 8 animals at a time, and began on the first post-operative day for 90 minutes at 3 ATA (1313. Tobey RE, Kelly JF. Osteoradionecrosis of the jaws. Otolaryngol. Clin North Am. 1979;12(1):183-6.). We opted for 9 sessions in the HBO and APC+HBO groups because the growth factor acts primarily during the first 7 post-operative days (1414. Delgado R, Bonatelli APF, Alves MTS. Estudo sobre a associação de cerâmica a Plasma Rico em Plaquetas na coluna vertebral de ratos. Acta Ortop Bras. 2009;17(5):282-5, http://dx.doi.org/10.1590/S1413-78522009000500006.
http://dx.doi.org/10.1590/S1413-78522009...
).
The beginning of the session included a compression period. After 15 minutes, the chamber manometer indicated 2 atm of pressure; assuming that the manometer in the hyperbaric chamber measures the pressure difference between the chamber and the surrounding environment, the barometric pressure and environmental pressure (1 ATA) were added to convert the values into absolute atmospheres (ATA). Therefore, the pressure applied by the chamber was 3 ATA. This absolute pressure (3 ATA) was maintained for 60 minutes, followed by decompression for 15 minutes; thus, each session ended after 90 minutes.
Histomorphometric analysis
Immediately after the experiment, the proximal third of the fibula from each animal, containing the osseous callus region, was surgically removed and placed in 10% buffered formalin for 48 hours. After this period, the decalcification process was begun in ethylenediaminetetraacetic acid (EDTA); after 6 days, the pieces were dehydrated in increasing concentrations of ethyl alcohol, diaphanized in xylene, impregnated in histological paraffin, and embedded in paraffin.
Paraffin blocks were cut with a Minot microtome (Leica, Model RM 2145) with semi-serial 4 ìm cuts. After drying for 6-8 hours in an oven at 60°C, some sections were stained with hematoxylin and eosin (HE), and others were stained with picrosirius red. The slides were observed under a light microscope (Carl Zeiss®) at 4 to 100x.
To determine the concentration of bone cells (osteocytes, osteoblasts, and osteoclasts) in the fibula, an 1800x1600 μM rectangle was initially determined, drawn using a mouse, in two central regions adjacent to the healing bone; one was on the diaphysis side, and the other was on the epiphyseal side. Then, the cells were individually counted within the rectangles, excluding cells that touched the rectangles. For each animal, 6 slides were evaluated. We recorded the average number of cells counted in the rectangles for each animal.
The thickness of the cartilage region within the remaining osseous callus was obtained at 3 points in each slide (6 slides for each animal) using a mouse. We recorded the average number of measurements in μM for each animal.
Determining the percentages of collagen
The cuts stained with picrosirius red were used to determine the percentage of collagen formed. Initially, the sections were dewaxed in xylene and then hydrated in a decreasing ethanol gradient. Afterward, elastin was blocked with 0.2% phosphomolybdic acid for 10 minutes. The sections were washed in distilled water, immersed in a 0.1% sirius red solution, and dissolved in saturated aqueous picric acid for 90 minutes. Then, the sections were washed in 0.01 N HCl for 2 minutes. Later, the sections were dehydrated in an increasing ethanol gradient, cleared in xylene, and mounted with Entellan®.
Photomicrographs of the picrosirius red-stained sections from the rabbit fibulae of different groups were taken under a polarized light microscope (Axiolab Standard 2.0, Carl Zeiss®) coupled to a high-resolution video camera (AxionCam, Carl Zeiss®) to identify the distribution of collagen fibers in the bone matrix. Then, the micrographs were analyzed on a computer with the Imagelab® program.
Statistical analysis
For quantitative variables, descriptive statistics were calculated. Analysis of variance (ANOVA) and multiple comparisons by the Tukey test were used as inferential analyses to either confirm or refute the evidence found in the descriptive analysis.
For all the results obtained via inferential analyses, a significance level of α = 5% was used. All statistical analyses were performed with the R program software, version 2.11.1.
RESULTS
Information referring to the percentages of collagen, numbers of osteoblasts and osteocytes, and lesion thickness were important measurements assessed among groups and euthanasia time periods (subgroups). Table 1 and Figure 1 (A - D) summarize the behavior of these measurements according to the groups and euthanasia periods (subgroup).
-Distribution of the average percentage of collagen (A), average number of osteoblasts (B) and osteocytes (C), and average lesion thickness (D), according to groups and euthanasia periods (subgroups).
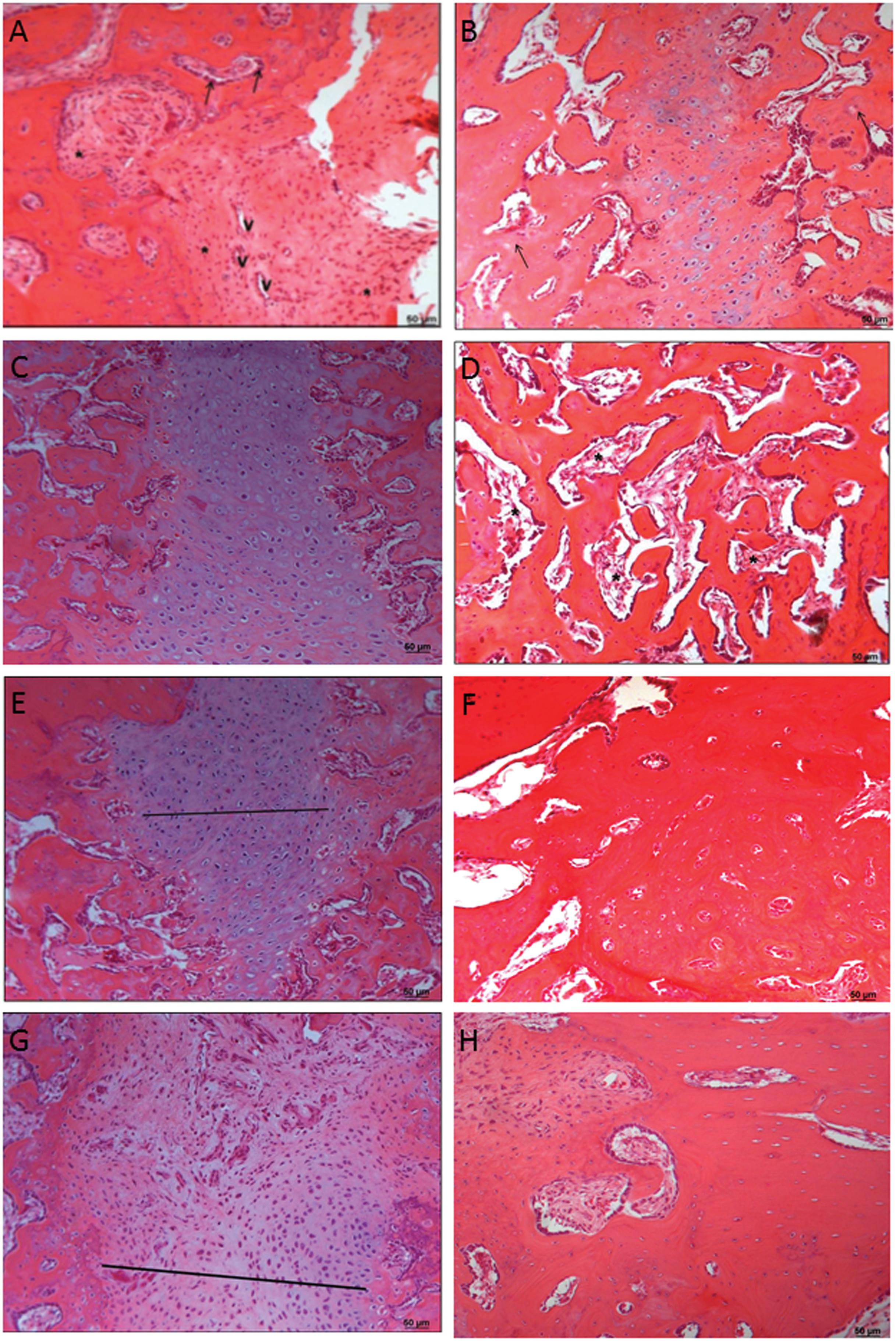
Table 2 shows the results of comparisons of the percentages of collagen, numbers of osteoblasts and osteocytes, and lesion thicknesses among groups and euthanasia periods. Figure 2 (A - H) shows the HE slides from the groups and subgroups (2th and 6th weeks).
-A) Photomicrograph of rabbit fibula of the CG showing the region of the lesion after 2 weeks. Note that there are many connective tissue cells (*), blood vessels (V), and osteoblasts (arrows) (HE). B) Photomicrographs of the rabbit fibulae of the CG 6 weeks after the injury. Note the cartilaginous tissue and small regions of cartilage calcified into newly formed bone within the tissue (arrows) (HE). C) Photomicrograph of rabbit fibulae of the APCG 2 weeks after the injury (HE). D) Photomicrographs of the rabbit fibulae of the ACPG after 6 weeks showing the damaged areas. Note that the bone tissue contains numerous cavities (*) (HE). E) Photomicrographs of the rabbit fibulae of the HBOG showing the region of the lesions after 2 weeks. Note the cartilaginous tissue (straight line) (HE). F) Photomicrographs of the rabbit fibulae of the HBOG showing the region of the lesion after 6 weeks (HE). G) Photomicrographs of the rabbit fibulae of the APC + HBOG after 2 weeks showing the region of the injury. Note that the site of the injury is filled with cartilage tissue (straight line) (HE). H) Photomicrographs of the rabbit fibulae of the APC + HBOG after 6 weeks showing the region of the injuries. Note the absence of injury and the bone tissue that formed (HE). HBOG was superior to the APCG across all periods for this variable.
DISCUSSION
The results obtained from this experimental model indicate a higher rate of bone healing in response to APC combined with HBO. No previous study in the literature was found to have used this combination in an experimental study.
Qualitative analysis highlighted the accelerated bone healing in the APC+HBOG compared with the CG during all weeks studied; additional data on the percentages of collagen and lesion thickness confirm this assessment. In 1983, a review article found that low oxygen tension reduced the action of autologous growth factors (1212. Gospodarowicz D. Growth factors and their action in vivo and in vitro. J Pathol.1983;141(3):201-33, http://dx.doi.org/10.1002/path.1711410304.
http://dx.doi.org/10.1002/path.171141030...
). Similarly, the outcomes shown here suggest that the higher oxygen tension caused by HBO application could potentiate the effects of the growth factors offered by APC therapy on bone healing.
Although there was no significant difference between the numbers of osteoblasts during any of the periods studied or between the number of osteocytes counted during the 2nd and 8th weeks, relative to the groups treated with APC and HBO combined, quantitative data on the average lesion thickness showed that the APCG, HBOG, and APC+HBOG were superior to the CG. The APC+HBOG was superior to the other groups across all periods, whereas the HBOG was superior to the APCG across all periods for this variable.
The fact that PRC use (in this study, APC) led to a superior rate of bone healing compared with the CG corroborates the outcomes of other studies. Marx et al. (1515. Marx R.E, Garg A.K. Bone structure, metabolism, and physiology: its impact on dental implantology. Implant Dent. 1998;7(4):267-75, http://dx.doi.org/10.1097/00008505-199807040-00004.
http://dx.doi.org/10.1097/00008505-19980...
) performed the first and most convincing study using PRP combined with autogenous bone grafts. The authors evaluated the effect of PRP on the reconstruction of mandibular bone defects resulting from tumor removal. The authors stated that bone grafts with PRP showed more than double the maturity of the controls.
Another study by Anitua produced excellent outcomes using autogenous bone grafts combined with PRP (1616. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-35.). The authors observed a total alveolar bone regeneration in 8 of 10 patients who were treated with PRP and bone graft. Biopsies of the respective areas showed compact mature bone with normal morphology and well-organized trabecular regions, while the control patients showed connective tissue, mostly filled with alveoli, and no mature bone.
However, some studies do not support the effect of PRC on the rate of bone healing. Recently, Broggini et al. (1717. Broggini N, Hofstetter W, Hunziker E, Bosshardt DD, Bornstein MM, Seto I, et al. The Influence of PRP on Early Bone Formation in Membrane Protected Defects. A Histological and Histomorphometric Study in the Rabbit Calvaria. Clin Implant Dent Relat Res. 2011;13(1):1-12, http://dx.doi.org/10.1111/j.1708-8208.2009.00266.x.
http://dx.doi.org/10.1111/j.1708-8208.20...
) evaluated bone repairs in the monocortical defects in the calvaria of 15 New Zealand rabbits. The authors concluded that PRP, alone or combined with an autogenous graft, did not lead to a higher rate of bone regeneration compared with basal clot during the initial healing.
Currently, the literature is more concerned with quantifying the concentration of growth factors present in some of the most widely used PRCs, especially PRP. A study using this approach stated that the concentration of growth factors in PRP can reach up to 7 and 30 times the basal levels of TGF-β and PDGF, respectively, in a basal clot (1818. Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet rich plasma: a literature review. Implant Dent. 2009;18(4):303-10, http://dx.doi.org/10.1097/ID.0b013e31819e8ec6.
http://dx.doi.org/10.1097/ID.0b013e31819...
). According to the manufacturer's data on the double-syringe method used in this study, APC can have up to 4 and 25 times the levels of TGF-β and PDGF, respectively. Although the concentrations of growth factors found in APC were lower than in PRP, this study demonstrated that this difference does not seem to be a disadvantage. However, it is important to note that functional and non-harmful amounts of these factors in tissue repair have not been well determined.
Regarding our outcomes on the effect of HBO on the rate of bone healing, HBO increased the bone consolidation rate compared with the CG, which could be observed through the varying percentages of collagen during each week, the number of osteocytes during the 6th and 8th weeks, and the lesion thickness across all weeks.
Our search for relevant publications led us to the observation that the first studies on the effects of HBO on bone healing date back to the 1960s. After almost 50 years, there are still few studies on this subject.
In a Cochrane review study (1919. Bennett MH, Standford R, Turner R. Hiperbaric Oxigen Therapy for promoting fracture healing and treating fracture nonunion. Chrocane Dapabase of Systematic Reviews (1) CD004712,2008.), the authors concluded that there is insufficient evidence to support or refute HBO use for fracture treatment or as a therapy for non-consolidated fractures within an expected time. This conclusion was based on 9 potentially relevant articles among the 68 references identified. However, none of the articles met the review's inclusive criteria: any randomized or quasi-randomized studies that compared clinical results obtained with HBO use to those obtained without HBO use (no treatment).
Although the literature did not include clinical results that met the methodology described above, our outcomes and the studies cited indicate that HBO is beneficial to the fracture healing process.
In 1998, Ueng et al. (2020. Ueng SW, Lee SS, Lin SS, Wang CR, Liu SJ, Yang HF, et al. Bone healing of tibial lengthening is enhanced by hyperbaric oxygen therapy: a study of bone mineral density and torsional strength on rabbits. J Trauma. 1998;44(4):676-81.) studied bone mineral density and tensile strength in a rabbit tibial-lengthening model. The bone segments from HBO-treated animals had higher mineral densities and superior biomechanical properties compared with the control animals. Using the same experimental model, this group obtained similar findings, showing that HBO is also advantageous during the early stages of tibial fracture healing (2121. Wang IC, Wen-Neng Ueng S, Yuan LJ, Tu YK, Lin SS, et al. Early administration of hyperbaric oxygen therapy in distraction osteogenesis- a quantitative study in New Zealand rabbits. J Trauma. 2005;58(6):1230-5.).
Chen et al. (2222. Chen WJ, Lai PL, Chang CH, Lee ML, Chen CH, Tai CL. The effect of hyperbaric oxygen therapy on spinal fusion: using the model of posterolateral intertransverse fusion in rabbits. J Trauma. 2002;52(2):333-8.) studied the effect of HBO in rabbits subjected to fusion of the vertebrae with a bone graft. According to these authors, the incidence of bone non-consolidation for this procedure in humans ranges from 6-35% of cases and 40-50% in rabbits. After 8 weeks, the HBO group showed a 100% consolidation of the fractures, which were identified radiologically and by torsional biomechanical tests.
The vast majority of the studies combining HBO and bone repair refer to osteoradionecrosis treatment. Radiation therapy results in progressive obliterative endarteritis, which leads to ischemia and a pathological fracture of the bone with reduced healing ability (2323. Karamitros AE, Kalentzos VN, Soucacos PN. Electric stimulation and hyperbaric oxygen therapy in the treatment of nonunions. Injury. 2006;37 Suppl 1:S63-73.). Muhonen et al. (2424. Muhonen A, Haaparanta M, Grönroos T, Bergman J, Knuuti J, Hinkka S, et al. Osteoblastic activity and neoangiogenesis in distracted bone of irradiated rabbit mandible with or without hyperbaric oxygen treatment. Int J Oral Maxillofac Surg. 2004;33(2):173-8, http://dx.doi.org/10.1054/ijom.2003.0489.
http://dx.doi.org/10.1054/ijom.2003.0489...
) evaluated the angiogenic and osteogenic responses after radiation and HBO in a rabbit mandibular distraction model. The authors concluded that the HBO group improved in the bone formation pattern.
Most experimental studies have found a positive effect from HBO use in the fracture healing process. This effect may be directly associated with the increased formation of new vessels, which increases blood inflow and, thereby, bone formation. Wu et al. (2525. Wu D, Malda J, Crawford RW, Xiao Y. Effects of hyperbaric oxygen on proliferation and differentiation of osteoblasts derived from human alveolar bone. Connect Tissue Res. 2007;48(4):206-13, http://dx.doi.org/10.1080/03008200701458749.
http://dx.doi.org/10.1080/03008200701458...
) presented the outcomes of an in vitro study that investigated the effects of HBO on the proliferation and differentiation of human osteoblasts and concluded that HBO also stimulates initial cell proliferation and acts directly on osteogenic differentiation in osteoblasts.
Jan et al. (2626. Jan AM, Sándor GK, Iera D, Mhawi A, Peel S, Evans AW, et al. Hyperbaric oxygen results in an increase in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(2):144-9.) presented the results of a study that assessed the effect of HBO on a rabbit calvaria fracture model. After radiographic and histomorphometric evaluations of the lesion after 6 and 12 weeks, the authors concluded that bone regeneration was significantly improved in the group of HBO-treated animals. It is noteworthy that the type of lesion caused in this experiment was considered critical, and bone healing was unlikely to occur.
Yeh et al. (2727. Yeh WL, Lin SS, Yuan LJ, Lee KF, Lee MY, Ueng SW. Effects of hyperbaric oxygen treatment on tendon graft and tendon-bone integration in bone tunnel: biochemical and histological analysis in rabbits. J Orthop Res. 2007;25(5):636-45, http://dx.doi.org/10.1002/jor.20360.
http://dx.doi.org/10.1002/jor.20360...
) studied the effects of HBO on the neovascularization of the tendon-bone junction, on the behavior of collagen fibers within the graft, and on the incorporation process of the graft into the bone tunnel of rabbits subjected to knee arthroscopy for the reconstruction of the anterior cruciate ligament with a tendon graft. The graft is incorporated into the bone tunnel through callus formation and the posterior ossification of the tunnel (2828. Weiler A, Hoffman RF, Bail HG, Rehm O, Südkamp NP. Tendon Healing in a Bone Tunnel. Part II: Histologic Analysis After Biodegradable Interference Fit Fixation in a Model ofAnterior Cruciate Ligament Reconstruction in Sheep. Arthroscopy. 2002;18(2):124-35.). In the HBO-treated animals, collagen fibers showed greater organization surrounding the graft and had an early mineralization of fibrocartilage compared with the control animals. The biomechanical test also indicated that HBO-treated animals exhibited greater resistance to the tensile strength of the graft at 18 weeks post-operation than the control animals.
The benefits of HBO are related to its proven therapeutic safety and the possible use of the equipment to treat a wider range of diseases. Its disadvantages include the high cost of the equipment and the low level of scientific evidence for HBO use in treating these diseases (1919. Bennett MH, Standford R, Turner R. Hiperbaric Oxigen Therapy for promoting fracture healing and treating fracture nonunion. Chrocane Dapabase of Systematic Reviews (1) CD004712,2008.).
However, in choosing a treatment method, one must consider many variables, such as the cost of the equipment, the specialized personnel, the methodological complexity, and the characteristics of the fracture to be treated. Although the mechanisms behind the actions of HBO and APC on bone repair may be different, the variables in this study showed only a few differences between the two methods of treatment. Although qualitative assessment highlights APC's superiority to HBO in relation to the consolidation rate, further studies should be conducted comparing the two therapies and their effects on bone healing.
In this study, we observed that osteoblastic activity was high in the CG during the 4th and 6th weeks but was higher in all other groups during the 2nd week. We also observed that osteoblastic activity was higher in the APC group than in the HBO group during the 2nd and 4th weeks, while no difference was found between these groups in relation to the number of osteoblasts during the 6th and 8th weeks. These findings corroborate previous knowledge that osteoblastic actions are strongly associated with higher concentrations of growth factors. We wish to note that this action was stronger in the first weeks with APC treatment and that it was superior to treatment with HBO at this stage.
Despite the differences observed in relation to the osteoblastic activity among the subgroups during the first experimental weeks, counting the number of osteocytes during the 8th week does not indicate the quantity of newly formed bone present in each subgroup, as advocated by other authors (2929. Haga M, Nozawa-Inoue K, Li M, Oda K, Yoshie S, Amizuka N, Maeda T. A morphological analysis on the osteocytic lacunar canalicular system in bone surrounding dental implants. Anat Rec (Hoboken). 2011;294(6):1074-82, http://dx.doi.org/10.1002/ar.21391.
http://dx.doi.org/10.1002/ar.21391...
). We believe that the number of osteocytes associated with qualitative analysis and other variables, such as lesion thickness and percentage of collagen, better suggest the quantity of newly formed bone. In this study, APC+HBO was superior in producing a greater quantity of new bone compared with the APC, HBO, and control treatments during the 8th week.
The importance of this study lies in the possibility of combining these two treatments, the effects of which potentiate increased bone formation. Additional studies with other variables must be conducted to deepen our knowledge of the effects of each of these treatments on bone healing; further studies should examine the economic impact of these combinations.
The analysis of these outcomes allows us to state that APC and HBO combined increased the rate of bone healing in this experimental model. In addition, the rate of bone repair in the groups treated with APC or HBO were similar, and both rates were higher than in the CG.
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #104698).
REFERENCES
-
1Chiang CC, Su CY, Huang CK, Chen WM, Chen TH, Tzeng YH. Early experience and results of bone graft enriched with autologous platelet gel for recalcitrant nonunions of lower extremity. J Trauma. 2007;63(3):655-61.
-
2Taylor MA, Norman TL, Clovis NB, Blaha JD. The response of habbit patellar tendon after autologous blood injection. Med Sci Sports Exerc. 2002;34(1):70-3.
-
3Heldin CH, Westemark B. PDGF-like growth factors in autocrine stimulation of growth. J Cell Physiol Suppl. 1987;Suppl 5:31-4, http://dx.doi.org/10.1002/jcp.1041330407.
» http://dx.doi.org/10.1002/jcp.1041330407 -
4Andrew JG, Hoyland JA, Freemont AJ, March DL. Platelet delivery growth factor expression in normally healing human fractures. Bone.1995;16:455-60.
-
5Beck LS, Deguzman L, Lee WP, Xu Y, McFratridge LA, Gillett NA, et al. Rapid publication. TGF-beta 1 induces bone closure of skull defects. J Bone Miner Res. 1991;6(11):1257-65.
-
6Eppley BL, Pietrzak WS, Blanton M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plast Reconstr Surg. 2006;118(6):147e-159e.
-
7Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18(18):1201-25, http://dx.doi.org/10.1016/S0142-9612(97)00050-1.
» http://dx.doi.org/10.1016/S0142-9612(97)00050-1 -
8Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212-9, http://dx.doi.org/10.1111/j.1600-0501.2005.01203.x.
» http://dx.doi.org/10.1111/j.1600-0501.2005.01203.x -
9Anitua E, Sánchez M, Orive G, Andía I. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008;29:37-41, http://dx.doi.org/10.1016/j.tips.2007.10.010.
» http://dx.doi.org/10.1016/j.tips.2007.10.010 -
10Williams STB. The role of hyperbaric oxygen therapy in trauma. Trauma. 2010;12(1):13-20, http://dx.doi.org/10.1177/1460408609354430.
» http://dx.doi.org/10.1177/1460408609354430 -
11Huang KC, Hsu WH, Peng KT, Huang TJ, Hsu RWJ. Hyperbaric oxygen therapy in orthopedic conditions: an evaluation of safety. J Trauma. 2006;61(4):913-7.
-
12Gospodarowicz D. Growth factors and their action in vivo and in vitro. J Pathol.1983;141(3):201-33, http://dx.doi.org/10.1002/path.1711410304.
» http://dx.doi.org/10.1002/path.1711410304 -
13Tobey RE, Kelly JF. Osteoradionecrosis of the jaws. Otolaryngol. Clin North Am. 1979;12(1):183-6.
-
14Delgado R, Bonatelli APF, Alves MTS. Estudo sobre a associação de cerâmica a Plasma Rico em Plaquetas na coluna vertebral de ratos. Acta Ortop Bras. 2009;17(5):282-5, http://dx.doi.org/10.1590/S1413-78522009000500006.
» http://dx.doi.org/10.1590/S1413-78522009000500006 -
15Marx R.E, Garg A.K. Bone structure, metabolism, and physiology: its impact on dental implantology. Implant Dent. 1998;7(4):267-75, http://dx.doi.org/10.1097/00008505-199807040-00004.
» http://dx.doi.org/10.1097/00008505-199807040-00004 -
16Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-35.
-
17Broggini N, Hofstetter W, Hunziker E, Bosshardt DD, Bornstein MM, Seto I, et al. The Influence of PRP on Early Bone Formation in Membrane Protected Defects. A Histological and Histomorphometric Study in the Rabbit Calvaria. Clin Implant Dent Relat Res. 2011;13(1):1-12, http://dx.doi.org/10.1111/j.1708-8208.2009.00266.x.
» http://dx.doi.org/10.1111/j.1708-8208.2009.00266.x -
18Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet rich plasma: a literature review. Implant Dent. 2009;18(4):303-10, http://dx.doi.org/10.1097/ID.0b013e31819e8ec6.
» http://dx.doi.org/10.1097/ID.0b013e31819e8ec6 -
19Bennett MH, Standford R, Turner R. Hiperbaric Oxigen Therapy for promoting fracture healing and treating fracture nonunion. Chrocane Dapabase of Systematic Reviews (1) CD004712,2008.
-
20Ueng SW, Lee SS, Lin SS, Wang CR, Liu SJ, Yang HF, et al. Bone healing of tibial lengthening is enhanced by hyperbaric oxygen therapy: a study of bone mineral density and torsional strength on rabbits. J Trauma. 1998;44(4):676-81.
-
21Wang IC, Wen-Neng Ueng S, Yuan LJ, Tu YK, Lin SS, et al. Early administration of hyperbaric oxygen therapy in distraction osteogenesis- a quantitative study in New Zealand rabbits. J Trauma. 2005;58(6):1230-5.
-
22Chen WJ, Lai PL, Chang CH, Lee ML, Chen CH, Tai CL. The effect of hyperbaric oxygen therapy on spinal fusion: using the model of posterolateral intertransverse fusion in rabbits. J Trauma. 2002;52(2):333-8.
-
23Karamitros AE, Kalentzos VN, Soucacos PN. Electric stimulation and hyperbaric oxygen therapy in the treatment of nonunions. Injury. 2006;37 Suppl 1:S63-73.
-
24Muhonen A, Haaparanta M, Grönroos T, Bergman J, Knuuti J, Hinkka S, et al. Osteoblastic activity and neoangiogenesis in distracted bone of irradiated rabbit mandible with or without hyperbaric oxygen treatment. Int J Oral Maxillofac Surg. 2004;33(2):173-8, http://dx.doi.org/10.1054/ijom.2003.0489.
» http://dx.doi.org/10.1054/ijom.2003.0489 -
25Wu D, Malda J, Crawford RW, Xiao Y. Effects of hyperbaric oxygen on proliferation and differentiation of osteoblasts derived from human alveolar bone. Connect Tissue Res. 2007;48(4):206-13, http://dx.doi.org/10.1080/03008200701458749.
» http://dx.doi.org/10.1080/03008200701458749 -
26Jan AM, Sándor GK, Iera D, Mhawi A, Peel S, Evans AW, et al. Hyperbaric oxygen results in an increase in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(2):144-9.
-
27Yeh WL, Lin SS, Yuan LJ, Lee KF, Lee MY, Ueng SW. Effects of hyperbaric oxygen treatment on tendon graft and tendon-bone integration in bone tunnel: biochemical and histological analysis in rabbits. J Orthop Res. 2007;25(5):636-45, http://dx.doi.org/10.1002/jor.20360.
» http://dx.doi.org/10.1002/jor.20360 -
28Weiler A, Hoffman RF, Bail HG, Rehm O, Südkamp NP. Tendon Healing in a Bone Tunnel. Part II: Histologic Analysis After Biodegradable Interference Fit Fixation in a Model ofAnterior Cruciate Ligament Reconstruction in Sheep. Arthroscopy. 2002;18(2):124-35.
-
29Haga M, Nozawa-Inoue K, Li M, Oda K, Yoshie S, Amizuka N, Maeda T. A morphological analysis on the osteocytic lacunar canalicular system in bone surrounding dental implants. Anat Rec (Hoboken). 2011;294(6):1074-82, http://dx.doi.org/10.1002/ar.21391.
» http://dx.doi.org/10.1002/ar.21391
Publication Dates
-
Publication in this collection
Sept 2013
History
-
Received
27 Feb 2013 -
Reviewed
25 Mar 2013 -
Accepted
22 Apr 2013