Abstract
INTRODUCTION: The etiology of preeclampsia is not fully established. A few studies have shown a relationship between natural coagulation inhibitors and preeclampsia. OBJECTIVES: The purpose of this study was to investigate the status of natural coagulation inhibitors and active protein C resistance (APC-R) in preeclampsia. PATIENTS AND METHODS: We studied 70 women with preeclampsia recruited consecutively and 70 healthy pregnant and 70 nonpregnant women as controls. Plasma protein C (PC), free protein S (fPS), antithrombin III (ATIII) and APC-R were evaluated. RESULTS: ATIII values were found to be significantly lower in preeclamptic patients than in the control groups (p< 0.001). Nevertheless, there was no significant difference between the healthy pregnant and nonpregnant women groups (p=0.141). The fPS values of the preeclamptic and healthy pregnant groups were lower than that of the nonpregnant group (p< 0.001), and the fPS value of the preeclamptic pregnant women was lower than that of healthy pregnant women (p<0.001). The PC value of the preeclamptic pregnant women was lower than that of the control groups (p< 0.001). The PC value of the healthy pregnant women was lower than that of the nonpregnant women (p< 0.001). The mean APC activity values were lower in the preeclamptic patients than that of the control groups (p< 0.001, p< 0.001). The APC-R positivity rates of the preeclamptic groups were higher than that of the control groups (p<0.001). CONCLUSIONS: This study demonstrated that ATIII, fPS, PC values and APC resistance were lower and APC-R positivity was higher in preeclamptic women than in normal pregnant and nonpregnant women.
Natural Coagulation Inhibitors; APC-R; Risk; Pathogenesis; Preeclampsia
CLINICAL SCIENCE
Natural coagulation inhibitors and active protein c resistance in preeclampsia
Cengiz DemirI; Imdat DilekII
IDepartment of Hematology, Medical Faculty, Yuzuncu Yil University, Van, Turkey
IIDepartment of Hematology, Ataturk Training and Research Hospital, Ankara, Turkey. Email: drcengizdemir@hotmail.com. Tel: 90 432 2150474
ABSTRACT
INTRODUCTION: The etiology of preeclampsia is not fully established. A few studies have shown a relationship between natural coagulation inhibitors and preeclampsia.
OBJECTIVES: The purpose of this study was to investigate the status of natural coagulation inhibitors and active protein C resistance (APC-R) in preeclampsia.
PATIENTS AND METHODS: We studied 70 women with preeclampsia recruited consecutively and 70 healthy pregnant and 70 nonpregnant women as controls. Plasma protein C (PC), free protein S (fPS), antithrombin III (ATIII) and APC-R were evaluated.
RESULTS: ATIII values were found to be significantly lower in preeclamptic patients than in the control groups (p< 0.001). Nevertheless, there was no significant difference between the healthy pregnant and nonpregnant women groups (p=0.141). The fPS values of the preeclamptic and healthy pregnant groups were lower than that of the nonpregnant group (p< 0.001), and the fPS value of the preeclamptic pregnant women was lower than that of healthy pregnant women (p<0.001). The PC value of the preeclamptic pregnant women was lower than that of the control groups (p< 0.001). The PC value of the healthy pregnant women was lower than that of the nonpregnant women (p< 0.001). The mean APC activity values were lower in the preeclamptic patients than that of the control groups (p< 0.001, p< 0.001). The APC-R positivity rates of the preeclamptic groups were higher than that of the control groups (p<0.001).
CONCLUSIONS: This study demonstrated that ATIII, fPS, PC values and APC resistance were lower and APC-R positivity was higher in preeclamptic women than in normal pregnant and nonpregnant women.
Keywords: Natural Coagulation Inhibitors, APC-R, Risk, Pathogenesis, Preeclampsia.
INTRODUCTION
Preeclampsia is a clinical manifestation characterized by hypertension, proteinuria and edema that occurs after the 20th week of pregnancy. It is identified as a cause of maternal and fetal morbidity and mortality. Although the etiology of preeclampsia remains unknown, it is suggested that preeclampsia is associated with intervillous and spiral artery thrombosis, vascular endothelium damage and abnormalities of coagulation, leading to inadequate maternal, fetal and placental circulation.1 Immunologic adaptation disorders, abnormal increase of vasoconstrictor tone, nutritional factors, and genetic factors are some other theories.2-4 Preeclampsia and its association with thrombophilia remain controversial. Several investigators have reported an association between thrombophilia and adverse pregnancy outcomes caused by uteroplacental thrombosis, i.e., severe intrauterine growth restriction and placental abruption. Other groups, however, have failed to confirm this association.5-7
Protein S (PS) deficiency and active protein C resistance (APC-R) have been found in severe preeclamptic women.8 Antithrombin III (ATIII) deficiency is associated with recurrent miscarriages.9 Nevertheless, the extent of coagulation problems induced by hypertensive disorders of pregnancy is not clear yet. The primary objective of our study was to determine the status of natural coagulation inhibitors and APC-R in preeclampsia.
PATIENTS AND METHODS
Preeclamptic and healthy pregnant women who presented to the Obstetrics and Gynecology department of the Medical Faculty Hospital of Yuzuncu Yil University and Van Maternity Hospital were included in this study. Seventy preeclamptic pregnant women (25 mild and 45 severe) in the third trimester and a control group consisting of 70 healthy pregnant and 70 healthy nonpregnant women were included in the study. The healthy non-pregnant group was recruited from the healthy relatives of patients coming for check-ups at the Clinic of Internal Medicine, Faculty of Medicine, Yuzuncu Yil University. The healthy pregnant group was recruited from the pregnant women coming to the Obstetrics Clinic at the Yuzuncu Yil University, Faculty of Medicine.
The study design was approved by the Ethics Committee of Yuzuncu Yil University, and written informed consent was obtained from all participants.
Exclusion criteria for the preeclamptic group included a history of any of the following: hypertension, smoking, deep venous thrombosis in the patient and/or her family history, and use of any kind of vitamin K antagonist or anticoagulant drugs.
Preeclamptic cases were classified as mild and severe according to the criteria of the American College of Obstetricians and Gynecologists.10 Mild preeclampsia was diagnosed in the presence of two criteria after the 20th week of pregnancy: a blood pressure of 140/90 mm/hg or higher (after two consecutive measurements with an interval of six hours) and proteinuria (300 mg of protein or higher in a 24-hour urine specimen). In a pregnant woman who was formerly normotensive, the presence of edema was not considered to be a diagnostic criterion. Severe preeclampsia was diagnosed when there were two or more of the following severe preeclampsia diagnostic criteria: (a) systolic blood pressure exceeding 160 mm/Hg, diastolic blood pressure exceeding 110 mm/Hg (blood pressure findings must be observed twice at intervals of at least six hours); (b) proteinuria measured with greater than 5 gr/24 hours or three or four positive proteinuria findings detected with dipstick; (c) oliguria (urine output of < 400 ml/24 hours); (d) frontal headache or visual disturbances; (e) abnormal liver function tests (alanine aminotransferase and aspartate aminotransferase); (f) thrombocytopenia; and (g) pulmonary edema or cyanosis.
Care was taken to match the age and week of pregnancy of the healthy pregnant group and the preeclamptic group. Pregnant women who had chronic renal disease, vascular disease, had received anticoagulant treatment during pregnancy or had a previous thromboembolic complication were excluded from the study. Inclusion criteria for nonpregnant healthy group were similar with preeclamptic group for age; not using oral contraceptive and anticoagulant drug, being non-smoker or absence of thromboembolic disease in the subject and her family history.
Blood was drawn from the antecubital veins of each of the three groups of the study and placed into test tubes containing 1 ml of 3.8% sodium-citrate. After centrifugation of these specimens for ten minutes at room temperature and 2500xg, the plasma specimens were placed into 1.5 ml tubes and kept at -80 °C in the deep-freezer until analysis.
APC-R, PC, fPS and ATIII levels were studied in the hematology laboratory of Yuzuncu Yil University Medical Faculty Research and Practice Hospital using the fully automatic coagulometer equipment (Diagnostica stago STA Compact; France). The PC and ATIII values were determined by the colorimetric method using a kit (STA- stachrom; France), and the results are given as percentages for both PC and ATIII. To measure the fPS levels, the immuno-turbidimetric method was applied using a kit (STA-Liatest, France), and the results are given as percentages. APC-R was determined by the coagulation method (STA-Staclot APC-R method) using a specific kit (Diagnostica Stago, France), and the results were given in seconds. The tests were performed according to the manufacturer's guidelines. The laboratory reference spectrum was designated as 70-130% for PC, 60-140% for fPS and 80-120% for ATIII. The normal range in healthy subjects analyzed at our laboratory was 120-300 seconds for active protein C (APC) activity, and results under 120 seconds were accepted as APC-R.
Statistical Analysis
The sizes of the groups were equal to produce more reliable and effective results. One-way ANOVA variance analysis was used to compare the characteristics of the groups, and the Duncan multiple comparison test was used to identify the different groups. The designated level of statistical significance was p<0.05.
RESULTS
The mean ages of the nonpregnant group, the healthy pregnant group and the preeclamptic pregnant group were 25.94±2.78, 25.37±4.40 and 26.12±3.32 years, respectively. There were no statistical differences among the groups (p=0.426). The mean gestational weeks of the preeclamptic pregnant and healthy pregnant groups were 32.91±1.63 and 33.20±1.76 weeks, respectively. There was no statistical difference between the groups (p=0.322).
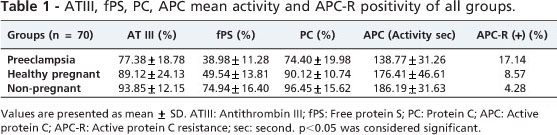
The mean values of ATIII for the preeclamptic, healthy pregnant and nonpregnant groups are given in Table 1. There was no significant difference between the healthy pregnant and nonpregnant women (p=0.141). When the preeclamptic patients were compared to the healthy pregnant and nonpregnant groups, however, the ATIII value was found to be lower (p<0.001 and p<0.001, respectively).
The mean values of fPS for the preeclamptic, healthy pregnant and nonpregnant groups are given in Table 1. The fPS values for the healthy pregnant and preeclampsia groups were lower than that of the nonpregnant group (p<0.001). The fPS value of the preeclamptic pregnant women was lower than that of the healthy pregnant women (p<0.001).
The mean PC values of preeclamptic, healthy pregnant and nonpregnant groups are given in Table 1. The PC value of preeclamptic pregnant women was lower than those of the other two groups (p<0.001). The PC level of the healthy pregnant women was lower than that of the nonpregnant women (p<0.001).
The mean APC values of the preeclamptic, healthy pregnant and nonpregnant groups are given in Table 1. There was no significant difference between the healthy pregnant and nonpregnant groups (p=0.120). The APC activity of the preeclamptic patients was significantly lower than those of the healthy pregnant and nonpregnant patients (p<0.001). Positivity rates of APC-R of the preeclamptic and healthy pregnant groups are given in Table 1. The APC-R positivity of preeclamptic patients was significantly higher than those of the healthy pregnant and the nonpregnant groups (p<0.001). There was no significant difference between the healthy pregnant and the nonpregnant groups (p=0.150).
The mean ATIII values of mild and severe preeclamptic pregnant women are given in Table 2. The decrease in ATIII in severe preeclamptic patients was significant compared to that of mild preeclamptic patients (p<0.001). Other parameters (age, gestational age, ATIII, fPS, PC and APC values) were not significantly different between these two groups (Table 2).
DISCUSSION
During pregnancy, there is an increased tendency toward coagulation and a decrease in fibrinolysis in the homeostatic system.11 PS levels decrease during healthy pregnancy, but coagulation inhibitors, such as ATIII and PC, remain at normal levels.12-14 We found that the PC activity of the healthy pregnant group was significantly lower than that of the nonpregnant control group. The data provided by Uchikova et al. for healthy pregnant and non-pregnant women were consistent with our results.15 PC results in preeclamptic pregnant women are contradictory. Aznar et al. found that the PC levels of severe preeclamptic women were significantly decreased compared to those of healthy pregnant women.16 Gilabert et al. reported similar findings.17 They emphasized that the decrease in PC levels might be related to increased consumption. We found that the PC activity of preeclamptic pregnant women was lower than that of healthy pregnant and non-pregnant women. In contrast, there were no significant differences between the PC activities of the preeclamptic and healthy pregnant groups in two studies.18,19
It is suggested that total and free PS levels decrease gradually in healthy pregnant women during pregnancy, and this condition may be caused both by increased protein C resistance and increased levels of coagulation factors.20,21 Consistent with the literature, we found that in healthy pregnant women, the PS level was lower than in nonpregnant controls. In two studies conducted on the PS state in preeclampsia, the total and fPS levels of preeclamptic cases did not decrease more than those of healthy pregnant women.17,22 In contrast, we found that the fPS activity of preeclamptic pregnant women was lower than that of healthy pregnant women. Similarly, in the study by Sayin et al., there was a significant decrease in PS activity in the preeclamptic group.18
In a study of the homeostatic system of healthy pregnancy, the ATIII levels were reported to decrease slightly.23 In our study, the ATIII levels of healthy pregnant women were lower than those of nonpregnant women, but it was not statistically significant. In studies performed on preeclamptic pregnant women, ATIII levels were significantly lower than those of healthy pregnant women.24-26 Consistent with the literature, our study found that the ATIII activity of preeclamptic pregnant women was lower than that of healthy pregnant women. Pekonen et al. reported that ATIII activity decreased only in severe preeclamptic patients.27 Terao et al. also reported that ATIII activity was decreased in severe preeclamptic patients.28 In our study, we found decreased ATIII activity in mild preeclamptic women compared to healthy pregnant women. Moreover, the ATIII activity in severe preeclamptic cases was lower than that of mild preeclamptic cases. Weiner et al. emphasized that the decrease in plasma ATIII activity was correlated with the stage of the disease, and it could also be used as a test to distinguish preeclampsia and other hypertensive diseases of pregnancy.29 According to our study, the lower ATIII activity level may be related to a more severe disease stage.
Dekker et al. found that the frequency of APC-R positivity in severe preeclamptic cases was 16%.8 Lindoff et al. found that the APC-R frequency six weeks after delivery in women with severe preeclampsia and healthy pregnant women was 22% and 10%, respectively, and thus, APC-R increases the risk of preeclampsia.30 In another study of severe preeclamptic and healthy pregnant patients, APC-R positivity in preeclamptic pregnant patients in the third trimester and at three months and nine months after delivery were 84.4%, 71.9% and 15.6%, respectively, and these findings were higher than those of healthy pregnant women.31 Thus, we tested APC-R in preeclamptic pregnant women. Similarly, in a study of severe preeclamptic and healthy control groups, Pampus et al. found APC-R positivity as 11.3% and 1.5%, respectively, and the factor V Leiden mutation was present in only half of APC-R-positive patients.32 They did not find any difference in the frequency of FV Leiden mutation in both groups. These studies indicate that APC-R may be acquired. Moreover, fPS is a cofactor of APC; therefore, temporary acquired APC-R of preeclampsia may be related to fPS deficiency. In a study designed similarly to our study, the APC-R frequency was found to be 31% in preeclamptic pregnant women, 16.6% in healthy pregnant women, and 7.6% in the control group.33 In our study, the APC-R positivity of preeclamptic patients was significantly higher than that of the healthy pregnant and nonpregnant groups. We did not find a significant difference between the healthy pregnant group and the healthy control group. This finding is consistent with a previous study.34
In conclusion, preeclamptic women have more pronounced deficiencies of PC, PS, ATIII and more APC resistance compared to normal pregnant women. More studies are needed to clarify whether these changes are secondary to preeclampsia or are involved in pathogenesis of the condition.
Received for publication on May 05, 2010
First review completed on June 11, 2010
Accepted for publication on August 18, 2010
- 1. Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53-6.
- 2. Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003-10.
- 3. Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175:1365-70.
- 4. Brenner B, Blumenfeld Z. Thrombophilia and fetal loss. Blood Rev. 1997;11:72-9.
- 5. Many A, Elad R, Yaron Y, Eldor A, Lessing JB, Kupferminc MJ. Third-trimester unexplained intrauterine fetal death is associated with inherited thrombophilia. Obstet Gynecol. 2002;99:684-7.
- 6. Foka ZJ, Lambropoulos AF, Saravelos H, Karas GB, Karavida A, Agorastos T, et al. Factor V leiden and prothrombin G20210A mutations, but not methylenetetrahydrofolate reductase C677T, are associated with recurrent miscarriages. Hum Reprod. 2000; 15:458-62.
- 7. Hefler L, Jirecek S, Heim K, Grimm C, Antensteiner G, Zeillinger R, et al. Genetic polymorphisms associated with thrombophilia and vascular disease in women with unexplained late intrauterine fetal death: a multicenter study. J Soc Gynecol Investig. 2004;11:42-4.
- 8. Dekker GA, de Vries JI, Doelitzch PM, Huijgens PC, von Blomberg BM, Jakops C, et al. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995;173:1042-8.
- 9. Preston FE, Rosendaal FR, Walker ID, Briët E, Berntorp E, Conard J, et al. Increased fetal loss in women with heritable thrombophilia. Lancet. 1996;348:913-6.
- 10. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. In: ACOG Technical Bulletin No: 219. ACOG, Washington DC, 1996.
- 11. Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409-14.
- 12. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153-68.
- 13. Bremme K, Ostlund E, Almqv1st I, Heinonen K, Blomback M. Enhanced trombin generation and fibrinolytic activity in normal pregnancy and the puerperium. Obstet Gynecol. 1992;80:132-7.
- 14. Vodnik T, Ignjatovic S, Majkic-Singh N. Changes in the plasma levels of protein C system parameters in pregnancy. Scand J Clin Lab Invest. 2003;63:481-8.
- 15. Uchikova EH, Ledjev II. Changes in haemostasis during normal pregnancy. Eur J Obstet Gynecol Reprod Biol 2005;119:185-188.
- 16. Aznar J, Gilabert J, Estelles A, Espana F. Fibrinolytic activity and protein C in preeclampsia. Thromb Haemost. 1986;55:314-7.
- 17. Gilabert J, Fernandez JA, Espana F, Aznar J, Estelles A. Physiological coagulation inhibitors (protein S, protein C and antithrombin III) in severe preeclamptic states and in users of oral contraceptives. Thromb Res. 1988;49:319-29.
- 18. Sayin M, Varol FG, Sayin NC. Evaluation of natural coagulation inhibitor levels in various hypertensive states of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005;123:183-7.
- 19. Osmanaaolu MA, Topçuolu K, Ozeren M, Bozkaya H. Coagulation inhibitors in preeclamptic pregnant women. Arch Gynecol Obstet. 2005;271:227-30.
- 20. Walker MC, Garner PR, Keely EJ, Rock GA, Reis MD. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162-9.
- 21. Comp PC, Thurnau GR, Welsh J, Esmon CT. Functional and immunologic protein S levels are decreased during pregnancy. Blood. 1986;68:881-5.
- 22. de Boer K, ten Cate JW, Sturk A, Borm JJ, Treffers PE. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989;160:95-100.
- 23. Wickström K, Edelstam G, Löwbeer CH, Hansson LO, Siegbahn A. Reference intervals for plasma levels of fibronectin, von Willebrand factor, free protein S and antithrombin during third-trimester pregnancy. Scand J Clin Lab Invest. 2004;64:31-40.
- 24. Halligan A, Bonnar J, Sheppard B, Darling M, Walshe J. Haemostatic, fibrinolytic and endothelial variables in normal pregnancies and pre-eclampsia. Br J Obstet Gynaecol. 1994;101:488-92.
- 25. Tanjung MT, Siddik HD, Hariman H, Koh SC. Coagulation and fibrinolysis in preeclampsia and neonates. Clin Appl Thromb Hemost. 2005;11:467-73.
- 26. Ho CH, Yang ZL. The predictive value of the hemostasis parameters in the development of preeclampsia. Thromb Haemost. 1992;67:214-8.
- 27. Pekonen F, Rasi V, Ammälä M, Viinikka L, Ylikorkala O. Platelet function and coagulation in normal and preeclamptic pregnancy. Thromb Res. 1986;43:553-60.
- 28. Terao T, Kobayashi T, Imai N, Oda H, Karasawa T. Pathological state of the coagulatory and fibrinolytic system in preeclampsia and the possibility of its treatment with AT III concentrate. Asia Oceania J Obstet Gynecol. 1989;15:25-32.
- 29. Weiner CP, Brandt J. Plasma antithrombin III activity: an aid in the diagnosis of preeclampsia-eclampsia. Am J Obstet Gynecol. 1982;142:275-81.
- 30. Lindoff C, Ingermarsson I, Martinsson G, Segelmark M, Thysell H, et al. Preeclampsia is associated with a reduced response to activated protein C. Am J Obstet Gynecol. 1997;176:457-60.
- 31. Cetin M, Güçer S, Serin IS, Eser B, Tayyar M, Unal A. Activated protein C resistance in Turkish women with severe preeclampsia. Gynecol Obstet Invest. 2001;52:168-72.
- 32. van Pampus MG, Dekker GA, Wolf H, Huijgens PC, Koopman MM, von Blomberg BM, et al. High prevalence of hemostatic abnormalities in women with a history of severe preeclampsia. Am J Obstet Gynecol. 1999;180:1146-50.
- 33. Cagirgan S, Donmez A, Ispahi C. Activated protein C resistance in preeclampsia. Clin Exp Obstet Gynecol. 2004;31:59-62.
- 34. Peek MJ, Neslon-Piercy C, Manning RA, de Swiet M, Letsky EA. Activated protein C resistance in normal pregnancy. Br J Obstet Gynaecol. 1997;104:1084-6.
Publication Dates
-
Publication in this collection
05 Jan 2011 -
Date of issue
2010
History
-
Received
05 May 2010 -
Accepted
18 Aug 2010