Abstracts
The aim of this work was to evaluate the effect of processing and roasting on the antioxidant activity of coffee brews. Brews prepared with light, medium and dark roasted coffees were analyzed. The pH, total solids content, polyphenols content, reducing substances and chlorogenic acids content were determined. The antioxidant activity of aqueous extracts, the guaicol decolorizing and the capacity to inhibit lipid peroxidation were also analyzed. The antioxidant activity of coffee brews were concentration-dependent. A progressive antioxidant activity and polyphenols content was observed decreasing with roasting. The light roasted coffee showed the highest antioxidant activity and dark roasted coffee showed the lowest antioxidant activity. The results indicate that the ingestion of coffee brews prepared with light and medium roasted coffees might protect cells from oxidative stress damages.
beverage; antioxidant activity; roasting; coffee; processing
O objetivo deste trabalho foi avaliar o efeito do processamento e grau de torração sobre a atividade antioxidante da bebida de café. Foram analisadas bebidas preparadas com café nos graus de torração claro, médio e escuro. Foram determinados o pH, o conteúdo de sólidos totais, o conteúdo de polifenóis, o conteúdo de substâncias redutoras e o conteúdo de ácidos clorogênicos. Além disto, foram analisadas a atividade antioxidante dos extratos aquosos, a descoloração do guaiacol e a capacidade de inibição da formação de peróxidos lipídicos. A atividade antioxidante mostrou ser dependente da concentração da bebida de café. Foi observada redução progressiva da atividade antioxidante e de compostos fenólicos com o grau de torração. O café submetido à torra clara apresentou atividade antioxidante máxima e o café com maior grau de torra apresentou a menor atividade antioxidante. Os resultados indicam que a ingestão de bebidas preparadas com cafés de torras clara e média pode proteger a célula contra os efeitos do estresse oxidativo.
bebida; atividade antioxidante; torração; café; processamento
Effect of processing and roasting on the antioxidant activity of coffee brews
Efeito do processamento e da torração sobre a atividade antioxidante da bebida de café
Stella Maris da Silveira DuarteI; Celeste Maria Patto de AbreuII; Hilary Castle de MenezesIII; Marcelo Henrique dos SantosI; Cibele Marli Cação Paiva GouvêaI, * * A quem a correspondência deve ser enviada.
IEscola de Farmácia e Odontologia de Alfenas. End.: Centro Universitário Federal, Alfenas-MG. CEP: 37130-000, Brazil. Tel: +55-35-32991305; Fax: +55-35-32991067. E-mail: cibelegouvea@hotmail.com
IIUniversidade Federal de Lavras, Lavras-MG, CEP: 37200-000, Brazil
IIIUniversidade Estadual de Campinas, Campinas-SP, CEP: 13083-970 , Brazil
SUMMARY
The aim of this work was to evaluate the effect of processing and roasting on the antioxidant activity of coffee brews. Brews prepared with light, medium and dark roasted coffees were analyzed. The pH, total solids content, polyphenols content, reducing substances and chlorogenic acids content were determined. The antioxidant activity of aqueous extracts, the guaicol decolorizing and the capacity to inhibit lipid peroxidation were also analyzed. The antioxidant activity of coffee brews were concentration-dependent. A progressive antioxidant activity and polyphenols content was observed decreasing with roasting. The light roasted coffee showed the highest antioxidant activity and dark roasted coffee showed the lowest antioxidant activity. The results indicate that the ingestion of coffee brews prepared with light and medium roasted coffees might protect cells from oxidative stress damages.
Keywords: beverage; antioxidant activity; roasting; coffee; processing.
RESUMO
O objetivo deste trabalho foi avaliar o efeito do processamento e grau de torração sobre a atividade antioxidante da bebida de café. Foram analisadas bebidas preparadas com café nos graus de torração claro, médio e escuro. Foram determinados o pH, o conteúdo de sólidos totais, o conteúdo de polifenóis, o conteúdo de substâncias redutoras e o conteúdo de ácidos clorogênicos. Além disto, foram analisadas a atividade antioxidante dos extratos aquosos, a descoloração do guaiacol e a capacidade de inibição da formação de peróxidos lipídicos. A atividade antioxidante mostrou ser dependente da concentração da bebida de café. Foi observada redução progressiva da atividade antioxidante e de compostos fenólicos com o grau de torração. O café submetido à torra clara apresentou atividade antioxidante máxima e o café com maior grau de torra apresentou a menor atividade antioxidante. Os resultados indicam que a ingestão de bebidas preparadas com cafés de torras clara e média pode proteger a célula contra os efeitos do estresse oxidativo.
Palavras-chave: bebida; atividade antioxidante, torração; café; processamento.
1 - INTRODUCTION
Coffee is one of the most popular beverages consumed daily throughout the world. However the types of coffee beverages and amount consumed depends on the social habits, and cultures of the country.
Besides that, the coffee beverage is a very complex mixture of several chemicals which are either naturally occurring or induced by the roasting process. Green beans contain a variet and large amount of phenolic acids. The chlorogenic acids include some isomer groups as caffeoylquinic acids, dicaffeoylquinic acids and feruloylquinic acids. Those acids are important to the formation of pigments, taste and flavor of coffee beverages [7,14]. Coffee is the major source of chlorogenic acid in human diet [17] and there are reports showing its antioxidant activity in vitro [23]. The content of the chlorogenic acid in the coffee beverage is dependent on the species, the variety, and the processing conditions of the coffee beans [7,13] .
Differences in green bean composition, roasting conditions, and extraction procedures adopted for the coffee beverages preparation result in great diversity of the chemical composition of the final product [3], which could account for differences in the biological activities of coffee brews.
During roasting, the green beans are heated at 200-240ºC for 10-15min depending on the degree of roasting required, which is generally evaluated by color [1]. It leads to profound changes in the chemical composition of coffee, such as protein, amino acids, reducing sugars, sucrose, trigonelline, chlorogenic acid and water decreasing and melanoidins formation, many of which are due to the Maillard reaction. It occurs when sugars condense with free amino acids, peptides or proteins, and leads to the formation of a wide variety of compounds reported to possess antioxidant activity or even prooxidant properties [16].
The processing method used on a coffee is usually the single largest contributor to the flavor profile of a coffee. The dry-process, also known as the natural method, produces coffee that is heavy in body, sweet, smooth, and complex. The dry-process is often used in countries where rainfall is scarce and long periods of sunshine are available to dry the coffee properly. Most coffees from Indonesia, Ethiopia, Brazil, and Yemen are dry-processed. The semi-dry or pulped natural method consists of pulping a coffee, but omitting the fermentation stage to remove the silverskin. This results in a beverage that has characteristics of both a dry- and wet-processed coffee. It is often sweeter than wet-processed coffees, has some of the body of a dry-processed coffee, but also retains some of the acidity of a wet-processed coffee. This type of processing can only occur in countries where the humidity is low and the coffee covered in the sweet mucilage can be dried rapidly without fermenting. Brazil has made this method famous and produces some of the best pulped natural coffees in the world [11].
The aim of the present study was to evaluate the effect of processing and roasting on the antioxidant activity of coffee beverages, prepared as commonly consumed in Brazil.
2.1 - Coffee samples
Green coffee (Coffea Arabica L.) cv. Mundo Novo, harvested in 2001/2002, sieve 17/18, without imperfections, were kindly supplied by Ipanema Agro Indústria Ltda. (Alfenas, MG). The beans obtained by the dry process, which gives natural coffees, and by the semi-dry process were roasted in a two-step laboratory roaster of 1kg capacity at 200 ºC, during time sufficient to give light, medium and dark roasted samples. The beans were finely commercially ground and packed in non-permeable polypropylene/aluminum/polyethylene bags hermetically sealed under vacuum, and stored at 20°C until use.
2.2 - Color analysis
Color analysis of the ground coffee was carried out using a tristimulus colorimeter (Chromameter-2 Reflectance, Minolta, Osaka, Japan) equipped with a CR-300 measuring head. The instrument was standardized against a white tile before each measurement. Color was expressed in L*, a*, and b* Comission Internationale de Eclairage (CIE) scale parameters. Five measurements were carried out on each sample, and the coefficients of variation, expressed as the percentage ratio between the standard deviation and the mean value, which was less than 5 %.
2.3 - Coffee brews preparation
Coffee brews were prepared by solid-liquid extraction with deionized water. The coffee powder was percolated with 100mL deionized water at 90ºC, on a Whatman n.3 filter. All analyses were performed with freshly prepared coffee brews.
2.4 - pH measurement
The pH of 10g% coffee beverages was determined using pH meter equipped with a combination of glass eletrode and a temperature probe. Five measurements of each sample were obtained.
2.5 - Polyphenols quantification
Polyphenols were extracted from 10g% coffee beverages using 50% methanol as extractor, and were identified by the method of Folin-Denis, using tannic acid as standard. The polyphenols content was expressed as g equivalents of tannic acid per 100g of the sample [10]. Five measurements of each sample were obtained.
2.6 - Total solids determination
The total solids were determined in 0.02g% coffee beverages using a refratometer Abbe model 2 WAJ [12]. Five measurements of each sample were obtained.
2.7 - Determination of caffeoylquinic acid (CQA) concentration
The content of 3 CQA, 4 CQA, and 5CQA of 10g% coffee brews was determined by HPLC [9]. Briefly, the separation of each CQA isomers was achieved using a Pharmacia-LKB gradient system equipped with an RT-18 column and UV detector at 315nm. Samples were analysed using a gradient of methanol in 0.01M tripotassium citrate solution (pH 2.5), increased from 20 to 70% with 5 min linear segments. The peaks of 3 CQA, 4 CQA, and 5CQA were identified based on the time of retention of 3 CQA, 4 CQA, and 5CQA standards (Sigma Chem Co). To confirm the identification of CQA each standard was added to the coffee samples. The content of CQA was expressed as %dry weight. Three measurements of each sample were obtained.
2.8 - Reducing substances determination
The reducing substances were determined as described by DAGLIA et al. [7], with modifications. Briefly0.01mL aliquot of each coffee beverage (containing 0.02g%) were diluted in 1mL ethanol and placed in test tubes containing 2.5mL of 0.2M phosphate buffer and 2.5mL of 1% potassium hexacyanoferrate (III). Samples were mixed and incubated in a water-bath for 20 min at 45ºC and 2.5mL of 10% (w/v) trichloroacetic acid were added to the mixture. Aliquots of 5.0mL of the mixture were transferred to a test tube containing 5.0mL of MilliQ water and 1.0mL of 0.1% iron chloride (III). Sample absorbance was read in a spectrophotometer Femto 810 at 700nm. The reducing substances were expressed as the percentage of BHT, used as standard. Five measurements of each sample were obtained.
2.9 - Guaicol decolorization method
We developed a rapid and sensitive method for screening antioxidant activity of coffee brews using the guaiacol decolorization assay. Guaiacol is an electron donor to peroxidase for the reduction of hydrogen peroxide. When oxidized the guaiacol develops a brown color. In this method, the guaiacol oxidation was measured by continuous assay at 25 ºC in 0.1M succinic acid (pH5.0) with 1mM guaiacol, 1mM H2O2 , and 170U horseradish peroxidase. Absorbance values were recorded at 450 nm, after exactly 1 min and then every minute for 5 min, for the control and samples, in a Femto 810 spectrophotometer. BHT was used as standard. Each coffee brew was tested in the following concentration: 0.016; 0.033; 0.066 and 0.100g%. Appropriated controls were also run. Five measurements of each sample were obtained. The coffee brew antioxidant activity was considered as the percentage of guaiacol oxidation inhibition, and was calculated in the following way:
where:
AA% is the percentage of the antioxidant activity;
Ac5min is the absorbance of the control after 5min of reaction;
Ac1min is the absorbance of the control after 1min of reaction;
As5min is the absorbance of the coffee brew sample after 5min of reaction;
As1min is the absorbance of the coffee brew sample after 1min of reaction.
2.10 - Inhibition of lipid peroxide formation
The reaction mixture was prepared by adding 25% (w/v) rat brain homogenate containing 2.5mg of protein, 0.01mL of various concentrations of coffee brews or BHT controls in phosphate buffer, pH 7.2 in a final volume of 1mL. It should be noted that extract and BHT controls have their own control reactions (containing all related reagents except the test compounds). The mixture was then incubated at 37°C for 30min. The lipid peroxide formation was measured by the method of DAGLIA et al.[7]. For this, the mixture was transferred to a test tube containing 1mL of 25% chloridric acid, 1mL of 1% TBA in 0.1M sodium hydroxide and 0.9mL 2% BHT and heated at 100°C for 10min. After the mixture cooled, 1mL of distilled water and 5mL of n-butanol were added, and the mixture was shaken vigorously. After centrifugation at 4000rpm for 10min, the organic layer was taken, and its absorbance at 535nm was measured, using a Femto 810 spectrophotometer. Five measurements of each sample were obtained. Inhibition of lipid peroxide formation was calculated in the following way:
where:
I% is the percentage of the inhibition of lipid peroxide formation;
Ac is the absorbance of the control reaction (containing all reagents except the coffee brew);
As is the absorbance of the sample reaction, containing the coffee brew.
2.11 - Statistical analysis
The Tukey test was applied, considering 5% as significance level, in the Sanest program [24].
3 - RESULTS AND DISCUSSION
According to the L*, a*, and b* CIE parameters our roasted coffee beans were classified as light, medium and dark. Table 1 shows the changes in lightness values (L*) for coffee samples at different roasting degrees. As expected the lightness values decreased with increased roasting. It is in agreement with results reported by DA PORTO et al. [6] and NICOLI, ANESE & PARPINEL [16]. When a* and b*, the chromatic parameters are examined, each stage of the roasting process can be located in a welldefined color zone, as a* and b* indicate the color directions: +a* corresponds to the red color; -a* corresponds to green color; +b* corresponds to yellow color and -b* corresponds to blue color. It is interesting to note that the color of the coffee with different degrees of roasting showed a statiscally significant difference. However, the color of the same degree of roasting was equal (p>0.05) in both semi-dry and natural coffee samples. This indicates that we have obtained the same degree of roasting in the two types of coffee processing, which is important in order to compare the chemical content and biological properties of both coffees.
The pH values, polyphenols and total solids contents in the different coffee brews prepared with ground coffee at different degrees of roasting are presented in Table 2. The pH of the brews increased with increased roasting, they were significantly different when different degrees of roasting were compared, but there was no difference between the semi-dry and natural coffees. Our results are in agreement with DAGLIA et al. [7], who observed also that the pH of beverages increased with roasting. The pH increasing could alter the degree of ionization of the chemical compounds and it can improve the taste of coffee beverage.
The polyphenols concentration decreased with increased roasting. The main components of polyphenols are the tannis, which are water-soluble phenolic compounds with a broad biological and pharmacological activity, including antioxidant activity [4, 5, 19, 20]. As recent evidence shows that plant polyphenols exhibit antioxidant and radical scavenging properties, our results suggest that the light semi-dry coffee could be the best coffee beverage to protect cells against oxidative damage.
The total solids showed no statistically significant difference when the different coffee brews were compared. Our results indicate that we have compared coffee samples with the same concentration.
The CQA content is presented in Table 3. The results obtained for the green coffees showed that the total CQA content is higher in semi-dry than in the natural samples (p<0.001). The major component in both types of coffee processing was the 5-caffeoylquinic acid (5-CQA), which represented 76.68% of the total CQA in semi-dry and 76.75% in the natural. The second major component was the 4-CQA representing 12.93% and 13.17%, of the total CQA, respectively, in semi-dry and natural coffees. The smallest fractions of the total CQA was the 3-CQA, representing 10.39% in semi-dry and 10.08% in the natural coffee.
After roasting, considerable change in the CQA composition occurred in both coffee samples. The green semi-dry coffee had higher 5-CQA content than the natural green coffee and its level decreased steadily with roasting, especially in the semi-dry samples (p<0.05). The 4-CQA and 3-CQA increased in both light semi-dry and natural coffees and decreased in medium and dark roasted samples. This increasing of the 3-isomer after roasting was also observed by TRUGO & MACRAE [21]. The formation of 3-CQA and 4-CQA could arise either from isomerization of the 5-isomer, which decreased sharply with roasting or from partial decomposition of others caffeoylquinic acids, such as dicaffeoylquinic acids.
The CQA is a natural phenolic compound formed by the quinic acid esterification with caffeic acids, and collectively called chlorogenic acids, which perform an important role in the formation of pigments, taste, and flavor of coffee [4]. Taking into consideration that as a phenolic compound it also possesses antioxidant activity [4, 5, 19, 20], our results indicated that even though the dark natural coffee could be considered as the best in terms of taste, its antioxidant activity is diminished, because it loses several phenolic compounds, including CQAs and tannins.
The results on the reducing substances of coffee with different processing and degrees of roasting are presented in Table 4. All coffee samples analyzed presented high values of reducing substances indicating an antioxidant activity. The dark natural coffee presented the lesser value (p<0.05) as compared to other samples.
The reducing substances decreased with the degree of roasting (p<0.01). DAGLIA et al. [7] found also a significant decreasing in the reducing substances with roasting, even though the reducing substances values were lesser than the reducing substances found in the present work. The reducing substances decreasing could be probably due to the different content of polyphenol compounds in the samples studied, particularly decreased after roasting [23]. The increase in the reducing substances of the medium semi-dry samples could arise from the formation of low molecular weight thermolysis products.
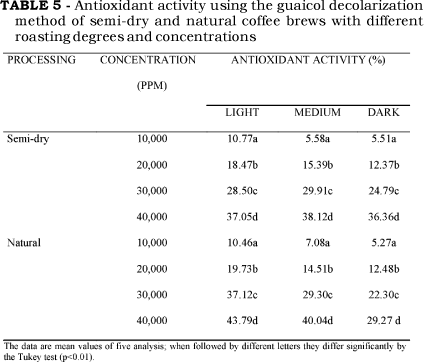
Results of Table 5 show the effect of roasting in the antioxidant activity of different concentrations of coffee brews. All of the coffee brews presented a high antioxidant activity in all tested concentrations, but the antioxidant activity decreased with roasting. At any degree of roasting, the antioxidant activity of semi-dry and natural coffees were quite similar, except for the dark natural, that showed a significantly lesser antioxidant activity than the other samples (p<0.05). The BHT showed a maximum inhibition of guaicol oxidation of 56.94%, and coffee brew maximum inhibition was 43.79%, achieved with light natural coffee.
The antioxidant activity found in the present work is dose-dependent and it agrees with previous reports [7, 15, 22]. A lesser literature agreement is found when the roasting degree is compared [2, 3, 7, 15]. From our data it could be seen that the light coffee presented the highest antioxidant activity (p<0.01). NICOLI et al. [15] found the highest antioxidant activity in the medium-dark roasted coffee brews. However their L, a*, and b* CIE parameters closely matched to our light-roasted coffee parameters. Our antioxidant activity was inversely correlated to the degree of roasting, the same result found by ANESE & NICOLI [2] in ready-to-drink coffee brews. On the other hand DEL CASTILLO, AMES & GORDON [8] and RICHELLI, TAVAZZI & OFFORD [18] found differences between the highest antioxidant activity in relation to the degree of roasting.
The behavior of antioxidant activity depending on the degree of roasting can probably be attributed to the loss of polyphenolic compounds, and to the successive formation of other antioxidant compounds, such as Maillard reaction products, which are lost or undergone pyrolysis when more severe thermal conditions are applied. Our results also showed that coffee beverages maintained high antioxidant activity even at lower concentrations than those consumed daily by a modest coffee drinker. It seems that the relationship between the antioxidant activity and the degree of roasting vary according to the type of coffee, roasting conditions, extraction procedure and antioxidant assay.
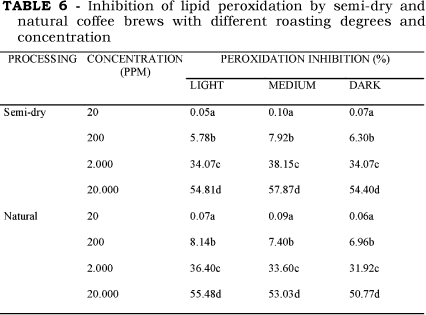
As demonstrated in Table 6 the coffee beverages inhibit lipid damage caused by hydroxyl radicals at the concentrations of 0.01, 0.1, 1.0 and 10.0g%, respectively, 20, 200, 2,000 and 20,000ppm. The concentration of 0.01g% presented no capacity to inhibit lipid peroxidation. No significant difference was observed in the capacity to inhibit lipid peroxidation in either type of coffee processing. The IC50 for the semi-dry coffee was: 0.447g% for the light, 0.435g% for the medium, and 0.479g% for the dark. The values for the natural coffee were: 0.432g% for the light, 0.453g% for the medium and 0.459g% for the dark. As the coffee brew administered to rats corresponds to the ingestion of 200mL of a 10g% coffee brew by a 70-kg-person, the IC50 , in theory, can be achieved by the ingestion of 200mL of a coffee brew in the concentrations of the experimentally IC50 obtained.
Although slight, an inverse correlation was observed between the capacities of coffee brews to inhibit lipid peroxidation and the degree of roasting, except for the medium semi-dry coffee samples. It could be attributed, at least in part, to the loss of polyphenols with roasting. RICHELLE, TAVAZZI & OFFORD [18] also observed that coffee brews can protect against lipid oxidation. In an assay involving measuring low-density lipoproteins oxidation, they demonstrated a decrease in the antioxidant capacity of coffee brews with an increase in the extent of roasting. They suggest that this capacity is not due to a single polyphenolic compound, but is the result of the action of several polyphenolic constituents. NICOLI et al. [15] found that coffee brews can suppress lipid oxidation in the Rancimat test, with the maximum activity in the medium-dark roasted coffee, which corresponds to our light coffee samples. DAGLIA et al. [7] analyzing the coffee brew protective capacity against rat liver microsomal lipid peroxidation found that all of the roasted samples gave complete protection. In contrast the same authors using another test-model, based on coupled oxidation of b-carotene and linoleic acid, found an increasing in the capacity of coffee brews to inhibit lipid peroxidation with roasting. BORRELLI et al. [3] obtained similar results, when the linoleic acid peroxidation in micelles decreased with increased roasting.
It should be pointed out that different assays to evaluate the antioxidant activity of coffee brews can bring different results, probably because simultaneous antioxidant compounds are present in coffee brews, and they may have different mechanisms of action.
4 - CONCLUSIONS
In conclusion, the data presented here suggest that the antioxidant activity of coffee brews decrease with roasting, and it can be attributed, at least in part, to the loss of phenolic compounds during the roasting process. The coffee that presented the lower antioxidant activity was the dark natural. It should be taken into account for a healthy human coffee intake, especially nowadays when the antioxidant activity is claimed to ameliorate and prevent several human diseases.
5 - REFERENCES
6 - ACKNOWLEDGEMENT
We thank CNPq and CAPES for the fellowships and Ipanema Agro Indústria Ltda (Alfenas, MG) for providing the coffee samples.
Recebido para publicação em 22/06/2004. Aceito para publicação em 04/05/2005 (001367).
- [1] ANDRIOT, I.; LE QUÉRÉ, J.-L.; GUICHARD, E. Interactions between coffee melanoidins and flavour compounds: impact of freeze-drying (method and time) and roasting degree of coffee on melanoidins retention capacity. Food Chemistry, v. 85, n. 2, p. 289-294, 2004.
- [2] ANESE, M.; NICOLI, M. C. Antioxidant properties of ready-to-drink coffee brews. Journal of Agricultural and Food Chemistry, v.51, p.942-946, 2003.
- [3] BORRELLI, R. C.; VISCONTI, A.; MENNELLA, C.; ANESE, M.; FOGLIANO, V. Chemical characterization and antioxidant properties of coffee melanoidins. Journal of Agricultural and Food Chemistry, v. 50, p. 6527-6533, 2002.
- [4] CLIFFORD, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Medica, v. 70, n. 12, p. 1103-1114, 2004.
- [5] COLLINS, A.R. Assays for oxidative stress and antioxidant status: applications to research into the biological effectiveness of polyphenols. American Journal of Clinical Nutrition, v. 81, n. 1 Suppl, p. 261S-267S, 2005.
- [6] DA PORTO, C.; NICOLI, M. C.; SEVERINI, C.; SENSIDONI, A.; LERICI, C. R. Study on physical and physicochemical changes of coffee beans during roasting. Note 2. Italian Journal of Food Science, v.3, p.197-207, 1991.
- [7] DAGLIA, M.; PAPETTI, A.; GREGOTTI, C.; BERTÈ, F.; GAZZANI, G. In vitro antioxidant and ex vivo protective activities of green and roasted coffee. Journal of Agricultural and Food Chemistry, v. 48, p.1449-1454, 2000.
- [8] DEL CASTILLO, M. D.; AMES, J. M.; GORDON, M. H. Effect of roasting on the antioxidant activity of coffee brews. Journal of Agricultural and Food Chemistry,v.50, p.3698-3703, 2002.
- [9] FARAH, A.; DE PAULIS, T.; TRUGO, L.C.; MARTIN, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. Journal of Agricultural and Food Chemistry, v. 53, n. 5, p. 1505-13, 2005.
- [10] GEORGE, S.; BRAT, P.; ALTER, P.; AMIOT, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. Journal of Agricultural and Food Chemistry, v. 53, n. 5, p.1370-1373, 2005.
- [11] ILLY, A; VIANI, R. Expresso coffee: the chemistry of quality. London: Academic Press, 1995. 253 p.
- [12] MAEZTU, L.; ANDUEZA, S.; IBANEZ, C.; PAZ DE PENA, M.; BELLO, J.; CID, C. Multivariate methods for characterization and classification of espresso coffees from different botanical varieties and types of roast by foam, taste, and mouthfeel. Journal of Agricultural and Food Chemistry, v. 49, n. 10, p. 4743-4747, 2001.
- [13] MOREIRA, D.P.; MONTEIRO, M.C.; RIBEIRO-ALVES, M.; DONANGELO, C.M.; TRUGO, L.C. Contribution of chlorogenic acids to the iron-reducing activity of coffee beverages. Journal of Agricultural and Food Chemistry, v. 53, n. 5, p. 1399-1402, 2005.
- [14] MOREIRA, R. F. A.; TRUGO, L. C.; DE MARIA, C. A. B.; MATOS, A. G. B.; SANTOS, S. M.; LEITE, J. M. C. Discrimination of Brazilian arabica green coffee samples by chlorogenic acid composition. Archivos. Latinoamericanos de. Nutrición, v.51, p.95-99, 2001.
- [15] NICOLI, M. C.; ANESE, M.; MANZOCCO, L.; LERICI, C. R. Antioxidant properties of coffee brews in relation to the roasting degree. Lebensmitell Wissenschaft und Technologie, v.30, p.292-297, 1997.
- [16] NICOLI, M. C.; ANESE, M.; PARPINEL, M. T. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Science Technological, v.10, p.94-100,1999.
- [17] OLTHOF, M.R.; HOLLMAN, P.C.H.; KATAN, M.B. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. Journal of Nutrion, v. 131, p. 6671, 2001.
- [18] RICHELLE, M.; TAVAZZI, I.; OFFORD, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. Journal of Agricultural and Food Chemistry, v.49, p.3438-3442, 2001.
- [19] SOKMEN, M.; ANGELOVA, M.; KRUMOVA, E.; PASHOVA, S.; IVANCHEVA, S.; SOKMEN, A.; SERKEDJIEVA, J. In vitro antioxidant activity of polyphenol extracts with antiviral properties from Geranium sanguineum L. Life Sciences, v. 76, n. 25, p. 2981-93, 2005.
- [20] STOCLET, J.C.; CHATAIGNEAU, T.; NDIAYE, M.; OAK, M.H.; EL BEDOUI, J.; CHATAIGNEAU, M.; SCHINIKERTH, V.B. Vascular protection by dietary polyphenols. European Journal of Pharmacology, v. 500, n. 1-3, p. 299-313, 2004.
- [21] TRUGO, L. C.; MACRAE, R. A study of the effect of roasting on the chlorogenic acid composition of coffee using HPLC. Food Chemistry, v.15, p.219-227, 1984.
- [22] YANAGIMOTO, K.; LEE, K.G.; OCHI, H.; SHIBAMOTO, T. Antioxidative activity of heterocyclic compounds found in coffee volatiles produced by Maillard reaction. Journal of Agricultural and Food Chemistry, v.50, p.5480-5484, 2002.
- [23] YEN, W.J.; WANG, B.S.; CHANG, L.W.; DUH, P.D. Antioxidant properties of roasted coffee residues. Journal of Agricultural and Food Chemistry, v. 53, n. 7, p. 2658-2663, 2005.
- [24] ZONTA, E.P.; MACHADO, A.A. SANEST 2: Sistema de Análise Estatística para Computadores. Piracicaba: SEI, 1992 (software).
Publication Dates
-
Publication in this collection
17 Aug 2005 -
Date of issue
June 2005
History
-
Accepted
04 May 2005 -
Received
22 June 2004