Abstract
This study aimed to analyze the taxonomic and functional diversity of the ant fauna in eucalyptus plantations (Corymbia citriodora; Eucalyptus urophylla; and E. saligna) in the Mário Xavier National Forest, state of Rio de Janeiro, Brazil. The E. saligna plantation was adjacent to an urban area, while the others were at least 1 km away. A total of 36 ant species from 14 genera were collected. The largest number of species was found in the C. citriodora plantation (20), followed by E. urophylla (18) and E. saligna (9) plantations. Species composition varied significantly among the areas. Greater numbers of guilds were found in the C. citriodora and E. urophylla plantations (6) compared to the E. saligna plantation (4). These results indicate that environmental attributes and proximity to urban areas can affect the richness and composition of ant species in eucalyptus plantations, consequently influencing the functional diversity.
Keywords:
afforestation and reforestation; conservation; forest entomology
1. INTRODUCTION AND OBJECTIVES
Insects (Class Insecta) are the most species-rich animal group (Lewinsohn & Prado, 2005) and have occupied most terrestrial habitats and adapted to many aquatic habitats (Gullan & Cranston, 2017). Thus, the behavioral diversity of this class of organisms is highly significant (Gallo et al., 2002; Gullan & Cranston, 2017). Regarding feeding behavior, they can be predators, hematophagous, coprophagous, detritivores, and feed on parts of plants or sap; many species are detrimental to cultivated plants, causing significant yield losses (Gallo et al., 2002; Gullan & Cranston, 2017). However, many species are useful for agriculture and forestry, such as pollinators, predators, parasitoids, and species that participate in nutrient cycling in the soil (Apolinario et al., 2019; Rosa et al., 2019; Estrada et al., 2023).
Ants (Hymenoptera: Formicidae) stand out for their abundance, adaptability to different habitats, diverse feeding behaviors, and high species richness, with over 14,000 described species and an estimated total exceeding 30,000 species worldwide (Hölldobler & Wilson, 1990; Baccaro et al. 2015).
A high taxonomic diversity enables ants to form guilds composed of species with diverse functions in ecosystems and cultivated areas (Apolinario et al., 2019; Estrada et al., 2023). Many species are predominantly predators, whether epigeic, hypogeic, and arboreal (Baccaro et al. 2015; Pereira, 2021), and can be useful for agriculture by helping to keep populations of insect pests below economically damaging levels (Eubanks, 2001; Vogt et al., 2001). Additionally, detritivorous ant species contribute to soil nutrient cycling processes (Hölldobler & Wilson, 1990; Baccaro et al. 2015). However, some ant species cause damage to cultivated plants, including leafcutter ants of the genera Amoimyrmex, Atta, and Acromyrmex (Mota Filho, 2022; Stefanelli, 2022). They are among insects that cause the highest production losses in forest plantations in Brazil (Santos et al., 2008; Zanetti et al., 2000). Some ant species protect phytophagous hemipterans from natural enemies in cultivated areas, resulting in an ecological interaction that can increase the damage caused by pest insects (Marchiori et al., 2023). The diversity of ant species found in cultivated areas in Brazil, including forest plantations, can positively or negatively affect plant production, which highlights the importance of comprehensive information on this topic (Martins et al., 2011; Apolinario et al., 2019; Estrada et al., 2023; Marchiori et al., 2023).
Tree species commonly known as eucalyptus (genus Eucalyptus and the species Corymbia citriodora (Hook.) K.D.Hill & L.A.S.Johnson) are widely grown in Brazil, occupying 7,295,309 ha, corresponding to approximately 77% of the forestry production area in 2021 (IBGE, 2023). Eucalyptus plantations are grown for various purposes, including fence posts, construction, firewood, charcoal, furniture manufacturing, plywood, medium density fiberboard (MDF), paper, and essential extracts (Ribeiro, 2020).
Eucalyptus plantations in Brazil are typically grown as monocultures on large areas, which leads to an increase in pest insect populations (Barbosa, 2021). The low structural complexity of these environments tends to generate a restricted species richness associated with the cultivated area (Martins et al., 2011). Furthermore, environmental impacts from areas adjacent to habitats can contribute to reducing the myrmecofauna diversity (Nascimento, 2005). However, forest plantations containing native trees and shrub species among eucalyptus trees can create a more heterogeneous environment, consequently increasing ecological niche availability and species richness (Apolinario et al., 2019). This environmental heterogeneity also impacts the composition of ant species and their guilds, affecting behavioral diversity (Apolinario et al., 2019; Pereira, 2021; Pereira & Almeida, 2023; Estrada et al., 2023). Therefore, the ant fauna depends on the environmental structure of the cultivated area and the characteristics of its surroundings.
Cultivated areas with greater structural heterogeneity have a greater species richness of plant-beneficial organisms, such as predators, parasitoids, pollinators, and species that participate in soil nutrient cycling, resulting in redundancy of ecological functions beneficial to crops (Pereira, 2021). From an agroecological perspective, increased biodiversity in eucalyptus plantations may result in a self-regulating system and a greater production sustainability, with healthier trees and reduced dependence on external inputs (Altieri, 2004, Pereira, 2021). Additionally, reducing the use of synthetic chemical insecticides is essential for maintaining environmental quality (Lopes & Albuquerque, 2018).
Tropical forests are highly heterogeneous environments with a great species richness (Primack & Rodrigues, 2001; Almeida & Vargas, 2017), including ant species (Baccaro et al. 2015; Pereira et al., 2016), which is the case of native forests in the Atlantic Forest Biome (Martins et al., 2011; Montine et al., 2014; Lobo et al., 2023). The Mário Xavier National Forest (FLONA Mário Xavier) in Seropédica, Rio de Janeiro, Brazil, protects rare remnants of lowland Atlantic Forest adjacent to eucalyptus plantations (Souza, 2017), focusing on integrating forestry production and research with biodiversity conservation (Brazil, 2000). Eucalyptus plantations integrated with a significant variety of native plant species can help in conserving a portion of native biodiversity (Apolinario et al., 2019) and providing conditions for maintaining healthy trees. Thus, this work aimed to analyze the taxonomic and functional diversity of the ant fauna in Eucalyptus forests in the Mário Xavier National Forest.
2. MATERIALS AND METHODS
2.1. Study area
The study was conducted in the Mário Xavier National Forest (Mário Xavier FLONA) (22°43’51.15”S; 43°42’30.52”W), in Seropédica, Rio de Janeiro, Brazil. Seropédica is part of the Rio de Janeiro Metropolitan Region, with an area of 265,189 km² and a population of 80,596 inhabitants (IBGE, 2023). According to the Köppen classification, the region’s climate is Aw, tropical rainy, with an average annual precipitation of 1,212.7 mm, ranging from 28.4 (July) to 194.0 mm (January), and an average annual air temperature of 23.5 °C, ranging from 20.5 (July) to 26.8 °C (February) (Paula et al., 2012). According to Paula et al. (2012), the soils of the area were classified as Typic Hapludult and Typic Haploxerult (Argissolo Vermelho-Amarelo and Planossolo Háplico; Santos et al., 2018).
The Mário Xavier FLONA, created by Decree Law No. 93,369 of 1986, has an area of 493.68 ha, with a native vegetation classified as Lowland Dense Ombrophylous Forest, a characteristic vegetation physiognomy of the Atlantic Forest Biome (ICMBIO, 2022). The first eucalyptus plantations in this FLONA were established approximately 80 years ago (ICMBIO, 2022). This conservation unit area has undergone several environmental conflicts, including invasions for sand and wood extraction, use of land for grazing, pollution from external sources, and impacts due to its proximity to urban areas; additionally, it is intersected by two busy federal highways: Presidente Dutra (BR 116) and Arco Metropolitano do Rio de Janeiro (BR-493) (Souza, 2017). Despite these factors and long history of land use, the Mário Xavier FLONA territory still harbors endemic and endangered species (Souza, 2017).
Ant fauna collection was carried out in three eucalyptus plantation areas: Corymbia citriodora (Hook.) K.D.Hill & L.A.S.Johnson (1.28 ha); Eucalyptus urophylla S.T.Blake (28.1 ha); and E. saligna Sm. (estimated area of 5.23 ha). The C. citriodora plantation was established approximately 27 years ago; the average spacing between trees at the time of data collection was 3 × 5 m (660 trees ha-1). The E. urophylla plantation was established approximately 15 years ago; the current average spacing between trees was 3 × 2.5 m (approximately 1333 trees ha-1). The average spacing was obtained in rows and between planting rows close to the ant collection site. The E. saligna plantation was established approximately 80 years ago, but had not been managed for decades, resulting in widely spaced trees. All study areas are located close to fragments of native forests within the FLONA. Additionally, the E. saligna plantation is adjacent to an urban area, whereas the sites where the myrmecofauna was sampled in the E. urophylla and C. citriodora plantations were 1 and 1.2 km from urban areas. The presence of various species of herbaceous plants, shrubs, and other tree species were observed in all eucalyptus plots. However, the E. saligna plantation had a significantly higher grass density than the other areas.
2.2. Data collection
Ants were collected in January 2023 using 21 pitfall traps in each eucalyptus plantation area, following the methodology adapted from Almeida et al. (2007). Each trap consisted of a 300 mL plastic cup filled with 100 mL of 70% alcohol solution, maintained active for 48 hours. The traps installed at each study area were arranged in seven groups (replications) of three traps each; the traps of each group were positioned at the vertices of an equilateral triangle, spaced 2 m apart. The distance between trap groups was 10 meters. The collected ants were sent to the Laboratory of Environmental Sciences (Três Rios Institute at the Federal Rural University of Rio de Janeiro - UFRRJ), where they were sorted, dry-mounted on paper triangles, and identified at the genus level using dichotomous key by Baccaro et al. (2015). Subsequently, the ants were grouped into morphospecies and, when whenever possible, identified to the species level through comparison with reference specimens and specific identification keys for the identified genera, following a standard methodology used for surveying ant species (Apolinário et al. 2019; Estrada et al., 2023; Lobo et al. 2023; Pereira & Almeida, 2023). The ants were further classified into guilds based on the methodology described by Apolinário et al. (2019) and Lobo et al. (2023). The ants were deposited in the Entomological Collection of the Três Rios Institute, UFRRJ.
The following environmental variables were collected next to each trap: temperature at ground level, using a digital thermo-hygrometer; and leaf litter depth, with a graduated ruler.
2.3. Data analysis
The species richness means for each eucalyptus plantation area were compared using analysis of covariance (ANCOVA), considering leaf litter depth as a covariate. Total species richness was analyzed using the species accumulation curve (Mao Tau) based on the frequency of species in traps. Species diversity was compared using the Shannon diversity index t-test and the evenness index was also calculated to quantify the distribution of abundance among species, considering in these analyses the frequency of the ant species in the samples (Amaral et al., 2019; Estrada et al., 2023). Air temperature in the eucalyptus plantations was evaluated using analysis of variance (ANOVA).
Species composition was analyzed using the Jaccard coefficient in non-metric multidimensional scaling ordination (NMDS). Similarity Analysis (ANOSIM) was then used to verify the existence of significant differences in species composition between the study areas. All analyses were performed using the Past software (Hammer et al., 2001).
3. RESULTS AND DISCUSSION
A total of 36 ant species from 14 genera and four subfamilies were collected (Table 1). The subfamily with the largest number of species was Myrmicinae (23 species), followed by Formicinae (6), Ectatomminae (4), and Ponerinae (3). The genera with the greatest species richness were Pheidole (9), followed by Solenopsis (6), Camponotus (4), and Ectatomma (4). Surveys conducted throughout Brazil, including the state of Rio de Janeiro, have shown a high species richness among the subfamilies Myrmicinae and Formicinae and the genera Pheidole, Solenopsis, and Camponotus, which is a pattern widely observed both in cultivated areas and natural ecosystems (Almeida et al., 2007; Martins et al., 2011; Estrada et al., 2019; Lobo et al., 2023).
Frequency (number of samples) of the ant fauna sampled in three eucalyptus plantations (Corymbia citriodora, Eucalyptus urophylla, and E. saligna) in the Mário Xavier National Forest, Seropédica, state of Rio de Janeiro, Brazil. AO: arboreal omnivorous; LC: leafcutters; FG: fungus-growers; SLD: soil or litter dominants; SLO: soil or litter dominant true omnivorous; LOS: litter omnivores and scavengers; LGP: litter generalist predators; LEP: litter specialist predators.
The largest number of species was found for the C. citriodora plantation (20), followed by E. urophylla (18) and E. saligna (9) plantations. Eucalyptus plantations, typically established as monocultures, are environments with relatively low structural heterogeneity, consequently tending to have lower diversity of ecological niches and species richness than natural forests (Martins et al. 2011; Apolinário et al. 2019). Martins et al. (2011) used attractive baits to collect 24 ant species in an ecological reforestation area in the state of Rio de Janeiro and found 20 species in a native forest fragment and 17 species in plantations with E. urophylla and Eucalyptus pellita F. Muell. Apolinário et al. (2019) also surveyed ant species in the state of Rio de Janeiro, using 24 pitfall traps per area, and collected 29 species in a secondary forest fragment and 25 species in a C. citriodora plantation. In both studies, a greater number of ant species was found in eucalyptus forests compared to pastures, indicating that eucalyptus forests may provide conditions for a more diverse ant fauna than other land uses in simpler environments.
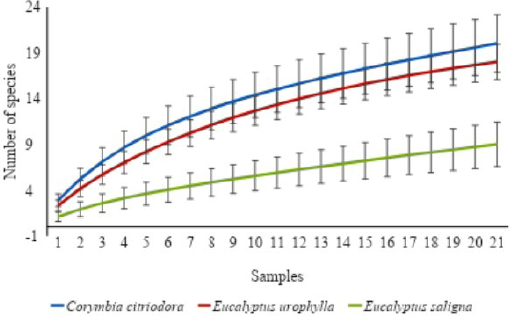
Total species richness was significantly lower in the E. saligna plantation compared to the other two areas (Figure 1). However, no significant difference was found between C. citriodora and E. urophylla plantations. The species accumulation curves did not reach an asymptote, which is a common result in ant species surveys across Brazil, even in cultivated areas, due to the high diversity of ant communities in terms of taxonomy and behavior (Apolinário et al., 2019; Estrada et al., 2019; Marchiori, 2020; Pereira & Almeida, 2023). The mean number of ant species varied significantly among the studied areas (ANCOVA; F = 17.59; P < 0.01), which was significantly lower for E. saligna than for C. citriodora plantation (Figure 2).
Species accumulation curves (Mao-Tau method) for ant fauna sampled in eucalyptus plantations (Corymbia citriodora, Eucalyptus urophylla, and E. saligna) in the Mário Xavier National Forest, Seropédica, state of Rio de Janeiro, Brazil.
Mean number (± standard deviation) of ant species collected in eucalyptus plantations (Corymbia citriodora, Eucalyptus urophylla, and E. saligna) in the Mário Xavier National Forest, Seropédica, state of Rio de Janeiro, Brazil (ANCOVA; F = 17.59; P < 0.01). Different letters indicate a significant difference according to the Tukey test at a 5% probability.
Thus, the characteristics of the evaluated eucalyptus plantations affected ant species richness. Studies have indicated that the vegetation structure can significantly influence the ant fauna in cultivated areas (Amaral et al., 2019; Estrada et al., 2019; Pereira & Almeida, 2023). The leaf litter depth and diversity are closely connected to type of vegetation in the area. However, the mean species richness was not affected by leaf litter depth (ANCOVA; F = 0.54. P = 0.59). Leaf litter is important for epigeic ants, as it provides nesting sites and food sources; thus, environments with deeper leaf litter may favor a greater ant species richness (Estrada et al., 2019; Lobo et al., 2023). However, species richness might be more connected to the diversity of leaf litter components than the total amount of litter material (Vargas et al., 2017). The presence of a significant diversity of other tree and shrub species among eucalyptus trees can promote a higher diversity of ant species due to a more structurally heterogeneous vegetation, which provides a wider range of ecological niches for ants compared to simpler environments (Apolinario et al., 2019). The dominance of grasses in the E. saligna plantation may have negatively affected ant species richness by generating a more homogeneous leaf litter, consequently with less diversity of resources than the other evaluated areas. Furthermore, the myrmecofauna can be negatively affected by several anthropogenic impacts (Delabie et al., 2006; Rocha et al., 2015), mainly those from nearby urban areas. Thus, the number of ant species may be greater in areas further away from urban centers (Nascimento, 2005). The E. saligna plantation is adjacent to the Seropédica urban area, which may have contributed to the negative effects on ant species richness.
Air temperature affects insects, as they are poikilotherms and have a development speed often accelerated by increases in temperature, consequently affecting population growth (Almeida & Gonçalves, 2007; Almeida & Gonçalves, 2009). Additionally, temperature affects the ant activity, which can influence the probability of capturing individuals in pitfall traps. However, the average air temperature did not differ significantly between C. citriodora (33.1 ± 1.3 °C), E. urophylla (34.0 ± 0.4 °C), and E. saligna (33.0 ± 0.4 °C) plantations (ANOVA; F = 2.95; p = 0.08).
Ant species diversity did not differ significantly between C. citriodora (2.45) and E. urophylla (2.68) (t = 0.39; p = 0.70) plantations. However, a significant difference was found between C. citriodora and E. saligna (1.91) (t = 3.41; p < 0.01) and between E. urophylla and E. saligna (t = 3.03; p < 0 .01). The evenness index was higher for the ant fauna in the E. urophylla plantation (1.02), followed by C. citriodora (0.97) and E. saligna (0.94), showing the same trend found for species richness and diversity. The E. saligna plantation had the lowest uniformity due to the dominance of Atta sexdens (Linnaeus, 1758) and, mainly, Pheidole sp.8 in this area. Ant species such as Ectatomma sp.2 and Wasmannia. auropunctata (Roger, 1863) were relatively abundant in the C. citriodora plantation, whereas several other species were found in only one sampling unit. In the E. urophylla plantation, Pheidole sp.1 had a significantly higher frequency of occurrence than other species, but most species had similar frequencies.
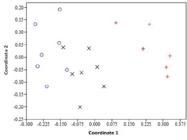
Ant species composition also differed significantly among the eucalyptus plantations (ANOSIM; R = 0.73; P < 0.01; Figure 3). Significant differences were found between C. citriodora and E. urophylla (R = 0.37; P < 0.01,) between C. citriodora and E. saligna (R = 0.84; P < 0. 01), and between E. urophylla and E. saligna plantations (R = 0.91; P < 0.01). Interestingly, the proximity of C. citriodora and E. urophylla plantations did not necessarily result in a homogeneous ant fauna, denoting variations in the availability of resources and ecological niches between these areas. Furthermore, the distance to urban areas might be another important factor, as already discussed.
Non-metric multidimensional scaling ordination (NMDS) for similarity (Jaccard coefficient) of ant fauna sampled in eucalyptus plantations with Corymbia citriodora (X), Eucalyptus urophylla (O), and E. saligna (+) in the Mário Xavier National Forest, Seropédica, state of Rio de Janeiro, Brazil (ANOSIM; R = 0.73; P < 0.01).
The collected ant species were grouped into eight guilds, with the largest number of species found for the “litter omnivores and scavengers” guild, followed by “soil or litter dominant true omnivorous “ (Table 2). This result is consistent with those found for native forest fragments (Lobo et al., 2023) and eucalyptus plantations in state of Rio de Janeiro (Apolinario et al., 2019). The collection technique used is focused on capturing mainly epigeic species, however, an “arboreal omnivorous” guild was recorded. The use a wider range of collection techniques, including traps for arboreal and hypogeic ants, may result in a greater diversity of guilds (Lobo et al., 2023).
Ant species richness (R) and frequency (F) for ant guilds in eucalyptus plantations (Corymbia citriodora, Eucalyptus urophylla, and E. saligna) in the Mário Xavier National Forest, Seropédica, state of Rio de Janeiro, Brazil.
The results of functional richness reflected the taxonomic richness, with a greater number of guilds in the C. citriodora and E. urophylla plantations (each with 6 guilds) than in the E. saligna plantation (4). Additionally, the study areas showed significant differences in the abundance of species within guilds. Variations in guild richness and composition may be reflected in variations in ecological functions of the myrmecofauna in cultivated areas, including ecological interactions potentially beneficial for wood production, such as predation on insects harmful to cultivated plants (Apolinario et al., 2019; Estrada et al., 2019). Therefore, further studies are needed to provide comprehensive understanding of factors affecting different ant guilds and the conditions favoring the abundance of beneficial guilds to eucalyptus plantations, such as predator species. Only leafcutter guilds can include eucalyptus pest species, but this guild was only represented by A. sexdens in the present study. However, most of the collected ant species can play beneficial roles in eucalyptus production.
4. CONCLUSIONS
Environmental variations in eucalyptus plantations can affect the richness, diversity, and composition of ant communities, consequently impacting their functional diversity. Additionally, proximity to urban areas can reduce species richness and diversity, also affecting ant fauna composition.
Although the leafcutter species Atta sexdens is an important eucalyptus pest in Brazil, most ant species found in the studied eucalyptus plantations can play beneficial roles in eucalyptus production.
ACKNOWLEDGEMENTS
“This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001”. The authors thank the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), especially to the managers of the Mário Xavier National Forest.
REFERENCES
- Altieri MA. Agroecologia: a dinâmica produtiva da agricultura sustentável. 4.ed., Porto Alegre: Editora da UFRGS; 2004.
- Almeida FS, Gonçalves L. Efeitos da temperatura na reprodução de Dysdercus maurus Distant, 1901 (Hemiptera: Pyrrhocoridae). Revista Brasileira de Zoociências 2009; 11, 113-117.
- Almeida FS, Gonçalves L. Efeitos da temperatura e do alimento no desenvolvimento de Dysdercus maurus Distant (Hemiptera, Pyrrhocoridae). Revista Brasileira de Entomologia 2007; 51, 506-511.
- Almeida FS, Vargas AB. Bases para a gestão da biodiversidade e o papel do Gestor Ambiental. Diversidade e Gestão 2017; 1, 10-32.
- Almeida FS, Queiroz JM, Mayhé-Nunes AJ. Distribuição e abundância de ninhos de Solenopsis invicta Buren (Hymenoptera: Formicidae) em um agroecossistema diversificado sob manejo orgânico. Floresta e Ambiente 2007; 14, 33 - 43.
- Amaral GC, Vargas AB, Almeida FS. Efeitos de atributos ambientais na biodiversidade de formigas sob diferentes usos do solo. Ciência Florestal 2019; 29, 660.
- Apolinario LCMH, Queiroz JM, Vargas AB, Almeida AA, Almeida FS. Diversity and Guilds of Ants in Different Land-Use Systems in Rio de Janeiro State, Brazil. FLORAM 2019; v. 26, p. 1-11,
- Baccaro F, Feitosa RM, Fernandez F, Fernandes IO, Izzo TJ, Souza JLP, Solar R. Guia para os gêneros das Formigas do Brasil. Editora INPA, Manaus; 2015.
- Barbosa LR, Queiroz DL, Nickele MA, Queiroz EC, Reis Filho W, Iede ET, Penteado SRC. Pragas de eucaliptos, 751-780. In: Oliveira EB, Pinto Junior JE. (Ed.). O eucalipto e a Embrapa: quatro décadas de pesquisa e desenvolvimento. Brasília, DF: Embrapa; 2021,
-
Brazil; 2000. Sistema Nacional de Unidades de Conservação da Natureza - SNUC, Lei No 9.985, de 18 de julho de 2000. Available at: Available at: https://www.planalto.gov.br/ccivil_03/leis/l9985.htm [Accessed 18rd Auguste 2023].
» https://www.planalto.gov.br/ccivil_03/leis/l9985.htm - Delabie JH, Paim VRDM, Nascimento ICD, Campiolo S, Mariano CDS. As formigas como indicadores biológicos do impacto humano em manguezais da costa sudeste da Bahia. Neotropical Entomology 2006; 35, 602-615.
- Estrada MA, Almeida AA, Vargas AB, Almeida FS. Diversidade, riqueza e abundância da mirmecofauna em áreas sob cultivo orgânico e convencional. Acta Biológica Catarinense 2019; 6, 87-103.
- Estrada MA, Pereira JR, Almeida AA, Vargas AB, Almeida FS. Ant functional groups and their effects on other insects in organic and conventional cropping areas. Entomobrasilis 2023; 16, e1018.
- Eubanks MD. Estimates of the direct and indirect effects of red imported fire ants on biological control in field crops. Biological Control 2001; 21, 35-43.
- Gallo D, Nakano O, Silveira Neto S, Carvalho RPL, Baptista GC, Berti Filho E, Parra JRP, Zucchi RA, Alves SB, Vendramim JD, Marchini LC, Lopes JRS, Omoto C. Entomologia agrícola. Piracicaba, FEALQ; 2002.
- Gullan PJ, Cranston PS. Insetos: fundamentos da entomologia. 5 ed. Rio de Janeiro: Roca; 2017.
- Hammer O, Harper DAT, Ryan PD. PAST - Paleontological Statistics software package for education and data analysis. Palaeontologia Electronica 2001; 41, 1-9.
- Hölldobler B, Wilson EO. The ants. Cambridge, Belknap/Harvard University; 1990.
-
IBGE - Instituto Brasileiro de Geografia e Estatística. PEVS - Produção da Extração Vegetal e da Silvicultura; 2023a. Available at: Available at: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9105-producao-da-extracao-vegetal-e-da-silvicultura.html?=&t=resultados [Accessed 18rd Auguste 2023].
» https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9105-producao-da-extracao-vegetal-e-da-silvicultura.html?=&t=resultados -
IBGE - Instituto Brasileiro de Geografia e Estatística. Seropédica. 2023b. Available at: Available at: https://cidades.ibge.gov.br/brasil/rj/seropedica/panorama [Accessed 22rd Auguste 2023].
» https://cidades.ibge.gov.br/brasil/rj/seropedica/panorama -
ICMBIO - Instituto Chico Mendes de Conservação da Biodiversidade. Plano de Manejo da Floresta Nacional Mário Xavier. Seropédica: Ministério do Meio Ambiente; 2022. Available at: Available at: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/mata-atlantica/lista-de-ucs/flona-mario-xavier/arquivos/pm_fn_mario_xavier_versao_versao_final-cleaned-1.pdf [Accessed 22rd Auguste 2023].
» https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/mata-atlantica/lista-de-ucs/flona-mario-xavier/arquivos/pm_fn_mario_xavier_versao_versao_final-cleaned-1.pdf - Lewinsohn TM, Prado PI. Quantas espécies há no Brasil? Megadiversidade 2005; 1,36-42.
- Lobo NCR, Ribeiro LM, Pereira JR, Almeida AA, Almeida FS. Efeitos de fatores ambientais sobre as assembleias de formigas arborícolas e epigéicas na Floresta Estacional Semidecidual. Ciência Florestal 2023; 33, e67579-24,
- Lopes CVA, Albuquerque GSC. Agrotóxicos e seus impactos na saúde humana e ambiental: uma revisão sistemática. Saúde Debate 2018; 42, 518-534.
- Marchiori JJP. Mirmecofauna e suas interações com hemípteros fitófagos em áreas cultivadas. Dissertation (Master’s degree in Plant Health and Applied Biotechnology) - Federal Rural University of Rio de Janeiro; 2020.
- Marchiori JJP, Almeida FS, Mayhe-Nunes AJ, Nobre RVL, Paulo HH. Interactions between ants and mealybugs in sugarcane: species and effects on insect pests. Revista Caatinga 2023; 36, 731-739.
- Martins L, Almeida FS, Mayhe-Nunes AJ, Vargas AB. Efeito da complexidade estrutural do ambiente sobre as comunidades de formigas (Hymenoptera: Formicidae) no município de Resende, RJ, Brasil. Revista Brasileira de Biociências 2011; 9, 174-179.
- Mota Filho TMM. Controle da formiga cortadeira, Atta sexdens (Hymenoptera: Formicidae), utilizando inseticidas: estudo sobre os mecanismos comportamentais. Dissertation (Master’s in Agronomy - Plant Protection), Faculty of Agricultural Sciences, São Paulo State University, Botucatu Campus; 2022.
- Montine PSM, Viana NF, Almeida FS, Dátillo WFC, Santanna AS, Martins L, Vargas AB. Seasonality of Epigaeic Ant Communities in a Brazilian Atlantic Rainforest. Sociobiology 2014; 61, 178-183.
- Nascimento RP. Conservação de invertebrados em áreas urbanas: um estudo de caso com formigas no Cerrado brasileiro. Dissertation (Master’s in Biological Sciences) - Federal University of Uberlândia, Uberlândia. 2005; 71 f.
- Paula RR, Pereira MG, Santiago RR, Amorim HB. Propriedades Edáficas e Desenvolvimento de Eucalipto em Topossequência na Flona Mário Xavier-RJ. Floresta e Ambiente 2012; 19, 344-35.
- Pereira LPC, Almeida FS, Vargas AB, Araujo MS, Mayhe-Nunes AJ, Queiroz JM. Seasonal Analysis of Taxonomic and Functional Diversity of Poneromorph Ant Assemblages in the Amazon Forest. Sociobiology 2016; 63, 941.
- Pereira JR. Diversidade, composição e guildas de formigas epigéicas e arborícolas em áreas cultivadas no município de Bom Despacho, Estado de Minas Gerais. Dissertation (Master’s degree in Plant Health and Applied Biotechnology) - Federal Rural University of Rio de Janeiro; 2021.
- Pereira JR, Almeida FS. Influência da heterogeneidade ambiental sobre a mirmecofauna em diferentes usos do solo no município de Bom Despacho, estado de Minas Gerais. Ciência Florestal 2023; 33, e64534-25.
- Primack RB, Rodrigues E. Biologia da conservação. Londrina: Editora Rodrigues; 2001.
- Ribeiro LLB. Análise do resultado financeiro da produção de eucalipto das cidades do território do alto Rio Pardo - MG. Editora Científica: São Paulo; 2020.
- Rocha WDO, Dorval A, Peres Filho O, Vaez CDA, Ribeiro ES. Formigas (Hymenoptera: Formicidae) Bioindicadoras de Degradação Ambiental em Poxoréu, Mato Grosso, Brasil. Floresta e Ambiente 2015; 22, 88-98.
- Rosa JM, Arioli CJ, Nunes SP, Mello FR. Desaparecimento de abelhas polinizadoras nos sistemas naturais e agrícolas: Existe uma explicação? Revista de Ciências Agroveterinárias 2019; 18, 154-162.
- Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Araujo Filho JC, Oliveira JB, Cunha TJF. Brazilian Soil Classification System. 5th ed. Brasília, DF: Embrapa ; 2018.
- Santos GP, Zanuncio JC, Zanuncio TV, Pires EM. Pragas do eucalipto. Informe Agropecuário, Belo Horizonte 2008; 29(242), 43-64.
- Souza RLN. Restauração da Mata Atlântica: potencialidades, fragilidades, e os conflitos ambientais na Floresta Nacional Mário Xavier, Seropédica/RJ. Dissertação (Mestrado em Geografia, Área de Concentração em Espaço, Questões Ambientais e Formação em Geografia), Curso de Pós-Graduação em Geografia, Universidade Federal Rural do Rio de Janeiro, Seropédica; 2017.
- Stefanelli LEP. Controle biológico microbiano: Beauveria bassiana e Trichoderma harzianum em Atta sexdens rubropilosa, e Metarhizium rileyi em Spodoptera litura. Tese (Doutorado em Agronomia - Proteção de Plantas), Faculdade de Ciências Agronômicas, Universidade estadual Paulista, Câmpus de Botucatu; 2022.
- Vargas AB, Amaral GC, Almeida FS. Quais fatores influenciam a riqueza de espécies de formigas na serapilheira: frequência ou riqueza de plantas? Acta Scientiae Et Technicae 2017; 5, 7-10,
- Vogt JT, Grantham RA, Smith WA, Arnold DC. Prey of the red imported fire ant (Hymenoptera: Formicidae) in Oklahoma peanuts. Biological Control 2001; 30, 123-128.
- Zanetti R, Jaffé K, Vilela EF, Zanuncio JC, Leite HG. Efeito da densidade e do tamanho de sauveiros sobre a produção de madeira em eucaliptais. Anais da Sociedade Entomológica do Brasil 2000; 29, 105-112.
Edited by
-
Associate editor:
Fernando Gomes http://orcid.org/0000-0003-0363-4888
Data availability
The dataset supporting the results of this study is not publicly available.
Publication Dates
-
Publication in this collection
11 July 2025 -
Date of issue
2025
History
-
Received
31 May 2025 -
Accepted
08 June 2025

 Myrmecofauna in eucalyptus plantations in the Mário Xavier National Forest, Rio de Janeiro State, Brazil
Myrmecofauna in eucalyptus plantations in the Mário Xavier National Forest, Rio de Janeiro State, Brazil