Abstract
PdCl2 immobilized in polyacrylamide (PAM), named Pd/PAM, produced at an extremely low cost, was found to be an efficient catalyst for Suzuki-Miyaura cross-coupling reactions. Iodo- and bromoarenes may couple with phenylboronic acid under eco-friendly conditions (i.e., phosphine-free and with ethanol as the solvent) to give excellent product yields. Aryl chlorides, in contrast, were found to be unsuitable reagents in this context, yielding modest results. The recyclability of Pd/PAM is limited because of PdII leaching, which leaves only the base polymer on the surface after six runs. The Pd/PAM catalyst was initially prepared via the formation of a PAM hydrogel using an aqueous PdCl2 solution. After drying, the solid Pd/PAM was characterized by scanning electron microscopy (SEM), electron-dispersive spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), and inductively coupled plasma optical emission spectrometry (ICP OES).
Keywords:
PdCl2; polyacrylamide; Suzuki-Miyaura cross-coupling
Introduction
Suzuki-Miyaura cross-coupling is a versatile reaction in organic chemistry that is typically catalyzed by palladium salts with auxiliary ligands.11 Negishi, E.; Handbook of Organopalladium Chemistry for Organic Synthesis, 1st ed.; John Wiley & Sons: New York, 2002; Diederich, F.; Meijere, A.; Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, 2004; Miyaura, N.; Suzuki, A.; Chem. Rev.
1995, 7, 2457. Currently, among the most common catalysts for this reaction are PdCl2(PPh3)2, PdCl2L2, and the mixture of Pd(OAc)2 with phosphine ligands since they are stable precursors.22 Biajoli, A. F. P.; Schwalm, C. S.; Limberger, J.; Claudino, T. S; Monteiro, A. L.; J. Braz. Chem. Soc.
2014, 25, 2186. Historically, tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4), a commercial product, was and remains a very popular catalyst in the Suzuki-Miyaura coupling.11 Negishi, E.; Handbook of Organopalladium Chemistry for Organic Synthesis, 1st ed.; John Wiley & Sons: New York, 2002; Diederich, F.; Meijere, A.; Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, 2004; Miyaura, N.; Suzuki, A.; Chem. Rev.
1995, 7, 2457.
2 Biajoli, A. F. P.; Schwalm, C. S.; Limberger, J.; Claudino, T. S; Monteiro, A. L.; J. Braz. Chem. Soc.
2014, 25, 2186.-33 Huang, Z. Y.; Yang, J. F.; Chen, Q.; Cao, R. J.; Huang, W.; Hao, G. F.; Yang, G. F.; RSC Adv.
2015, 5, 75182. This homogeneous catalyst has disadvantages such as high cost, low recyclability, and air sensitivity. Other Pd sources such as palladacycles,44 Dupont, J.; Pfeffer, M.; Palladacycles - Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, 2008; Rosa, G. R.; Rosa, C. H.; Rominger, F.; Monteiro, A. L.; Dupont, J.; Inorg. Chim. Acta
2006, 359, 1947; Alonso, D. A.; Nájera, C.; Chem. Soc. Rev. 2010, 39, 2891. palladium-containing organometallics,55 Li, Y.; Liu, G.; Cao, C.; Wang, S.; Li, Y.; Pang, G.; Shi, Y.; Tetrahedron
2013, 69, 6241; Tang, X.; Huang, Y. T.; Liu, H.; Liu, R. Z.; Shen, D. S.; Liu, N.; Liu, F. S.; J. Organomet. Chem.
2013, 729, 95. and Pd nanoparticles were also tested in this reaction.66 Puthiaraj, P.; Ahn, W. S.; Catal. Commun.
2015, 65, 91. However, the majority of catalytic systems operate under drastic conditions, i.e., with an inert atmosphere, toxic ligands, and unsustainable solvents, which make these systems environmentally unfriendly. Great advances were recently reported in the development of new "green" catalytic systems for Suzuki-Miyaura cross-coupling, such as microwave heating,77 Yılmaz, Ü.; Küçükbay, H.; Deniz, S.; Şireci, N.; Molecules
2013, 18, 2501. activation by sonication,88 Azua, A.; Mata, J. A.; Heymes, P.; Peris, E.; Lamaty, F.; Martinez, J.; Colacino, E.; Adv. Synth. Catal.
2013, 355, 1107. and the use of less toxic bases and solvents.99 Hariprasad, E.; Radhakrishnan, T. P.; ACS Catal.
2012, 2, 1179. However, reports of "green" systems in which the catalyst used is cheap, efficient, and reloaded (recycled) are still scarce. Our group has investigated the use of polymers to support Pd nanoparticles, with a particular focus on optimizing new catalytic systems for eco-friendly cross-coupling reactions.1010 Faria, V. W.; Oliveira, D. G. M.; Kurz, M. H. S.; Gonçalves, F. F.; Scheeren, C. W.; Rosa, G. R.; RSC Adv.
2014, 4, 13446. We believe that this approach has great potential in the development of sustainable catalysts for cross-coupling reactions. Polyacrylamide (PAM) is a very accessible and inexpensive polymer that has been little-explored as a catalyst support in coupling reactions. The literature teaches the use of cross-linked polyacrylamide modified to support Pd salts (for Heck-Mizoroki coupling)1111 Mahdavi, H.; Zirakzadeh, A.; Amani, J.; React. Funct. Polym.
2007, 67, 716. as well as Pd nanoparticles (for Heck-Mizoroki and Suzuki-Miyaura couplings);1212 Tamami, B.; Ghasemi, S.; J. Mol. Catal. A: Chem.
2010, 322, 98. however, modifying the polymer incurs an additional cost.
Herein, we report the development of PdCl2 supported on polyacrylamide hydrogel, named Pd/PAM, as an efficient catalyst for Suzuki-Miyaura reaction produced at an extremely low cost.
Experimental
Preparation of Pd/PAM catalyst
Twenty milligrams of PdCl2 (Synth) were dissolved in 10 mL of distilled water acidified with 0.3 mL of HCl p.a. (37%). Into the resulting solution were added 2.8 g of PAM purchased in the form of gel for planting, i.e., partially hydrolyzed sodium polyacrylamide (Forth). Subsequently, the pH was raised to 7 for hydrogel formation. Finally, the hydrogel was desiccated in an oven at 50 ºC until constant weight.
Scanning electron microscopy (SEM) and electron-dispersive spectroscopy (EDS) elemental analysis
The morphology of the solid Pd/PAM was analyzed using a JEOL SEM JSM 6610 with 20 kV and 500, 1000 and 1500 magnification values. The same instrument was used for the EDS analysis with a Noran detector (20 kV with an acquisition time of 100 s and 1500 magnification). The sample was carbon coated for analysis.
Fourier transform infrared spectroscopy (FTIR)
FTIR analyses were performed using a PerkinElmer Spectrum 100 Precisely FTIR. The samples were analyzed in hydrated gel form using the attenuated total reflectance (ATR) mode with 45 scans.
Inductively coupled plasma optical emission spectrometry (ICP OES)
Prior to Pd determination, Pd/PAM was digested using operational conditions adapted from the literature.1313 Soares, B. M.; Vieira, A. A.; Lemões, J. S.; Santos, C. M. M.; Mesko, M. F.; Primel, E. G.; D'Oca, M. G. M.; Duarte, F. A.; Bioresour. Technol. 2012, 110, 730. The palladium level was determined (emission line at 340.458 nm) using an ICP OES (model Optima 4300 DV, PerkinElmer,) with an axial view configuration. A GemConeTM nebulizer coupled to a cyclonic spray chamber was used. The radiofrequency power was set at 1400 W and argon (99.996%, White Martins) was used for plasma generation. Plasma gas flow rates were set at 15, 0.2, and 0.70 L min-1 for the principal, auxiliary and nebulizer gases, respectively. Other operational parameters were selected as recommended by the instrument manufacturer.
Suzuki-Miyaura cross-couplings
Experimental
All reactions were conducted under an argon atmosphere in a Schlenk reactor. K2CO3, K3PO4, CsF, MgSO4, Et2O, 1,4-dioxane, ethanol, and N,N-dimethylformamide (DMF) were purchased from Synth. Phenylboronic acid and aryl halides were purchased from Sigma-Aldrich. All chemicals were used without further purification. Nuclear magnetic resonance (NMR) spectra were recorded on a Varian XL300 spectrometer. Mass spectra (MS) were obtained on a Shimadzu QP-5050 gas chromatograph (GC)-MS (electron ionization (EI), 70 eV). Gas chromatography was performed on a PerkinElmer Clarus 400 GC equipped with a flame ionization detector (FID) and a 30 m Elite-1 capillary column (PerkinElmer, diameter 0.25 mm, thickness 0.25 µm) with a dimethylpolysiloxane stationary phase. GC operating conditions: N2 (vector gas, 2.7 mL min-1); H2 pressure = 2 bar; air pressure = 3 bar; injector temperature = 250 ºC; detector temperature = 250 ºC; temperature program: beginning 100 ºC for 1 min, heating 15 ºC min-1 to 250 ºC and permanence at this temperature for 9 min; internal standard = undecane.
Typical procedure for the Suzuki-Miyaura cross-coupling reaction
A Schlenk reactor was charged with base (2 mmol), phenylboronic acid (187 mg, 1.5 mmol), aryl halide (1 mmol), Pd/PAM catalyst (120 mg, 0.3 mol% of Pd), undecane (internal standard, 10 µL), and solvent (3 mL). The reaction mixture was stirred at 100 ºC until complete. The solution was then allowed to cool to room temperature, taken up in ether (20 mL) and washed with aqueous NaOH (1 mol L-1, 5 mL) and brine (2 × 5 mL). The organic layer was dried over MgSO4, filtered, concentrated in vacuum, and then the crude material was purified by flash chromatography on silica gel. The corresponding biaryl products were characterized by 1H and 13C NMR and by GC-MS.
4-Methoxybiphenyl
White solid; m.p. 81-83.5 ºC (lit.1414 Protti, S.; Fagnoni, M.; Mella, M.; Albini, A.; J. Org. Chem. 2004, 69, 3465. 77-78.5 ºC); 1H NMR (300 MHz, CDCl3) δ 7.58 (t, 3H, J 8.1, 6.9 Hz, Ph-H), 7.40 (t, 2H, J 8.4, 6.3 Hz, Ph-H), 7.34-7.26 (dd, 2H, J 11.7, 1.5 Hz, Ph-H), 6.98 (d, 2H, J 12.0 Hz, Ph-H), 3.86 (s, 3H, Me); 13C NMR (75.4 MHz, CDCl3) δ 159.4, 141.1, 134.0, 129.0, 128.4, 127.0, 126.9, 114.5, 55.6; GC-MS (EI, 70 eV) (%) 184 (100, [M+]), 169 (55), 141 (47), 115 (34), 185 (13), 63 (11), 139 (10), 76 (10).
Biphenyl
White solid; m.p. 68-73 ºC (lit.1515 Riggleman, S.; DeShong, P.; J. Org. Chem. 2003, 68, 8106. 69 ºC); 1H NMR (300 MHz, CDCl3) δ 7.62 (d, 4H, J 7.8 Hz, Ph-H), 7.42 (t, 4H, J 7.2, 7.5 Hz, Ph-H), 7.35 (d, 2H, J 7.5 Hz, Ph-H); 13C NMR (75.4 MHz, CDCl3) δ 141.2, 128.7, 127.2, 127.1.
4-Aminobiphenyl
Orange solid; m.p. 50-52 ºC (lit.1616 Inamoto, K.; Kuroda, J. I.; Sakamoto, T.; Hiroya, K.; Synthesis 2007, 18, 2853. 52-54 ºC); 1H NMR (300 MHz, CDCl3) δ 7.53 (d, 2H, J 6.3 Hz, Ph-H), 7.40 (dd, 4H, J 8.4, 2.1 Hz, Ph-H), 7.26 (dd, 1H, J 8.1, 1.2 Hz, Ph-H), 6.71 (dd, 2H, J 11.1, 1.8 Hz, Ph-H), 3.58-3.44 (s, 2H, NH2); 13C NMR (75.4 MHz, CDCl3) δ 145.8, 141.0, 131.4, 128.6, 127.9, 126.3, 126.2, 115.3.
4-Chlorobiphenyl
Solid; m.p. 79-82 ºC (lit.44 Dupont, J.; Pfeffer, M.; Palladacycles - Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, 2008; Rosa, G. R.; Rosa, C. H.; Rominger, F.; Monteiro, A. L.; Dupont, J.; Inorg. Chim. Acta 2006, 359, 1947; Alonso, D. A.; Nájera, C.; Chem. Soc. Rev. 2010, 39, 2891. 77-78 ºC); 1H NMR (300 MHz, CDCl3) δ 7.65-7.30 (m, 9H, Ph-H); 13C NMR (75.4 MHz, CDCl3) δ 139.9, 139.6, 133.3, 128.9, 128.8, 128.3, 127.5, 126.9.
4-Acetylbiphenyl
Solid; m.p. 115-118 ºC (lit.44 Dupont, J.; Pfeffer, M.; Palladacycles - Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, 2008; Rosa, G. R.; Rosa, C. H.; Rominger, F.; Monteiro, A. L.; Dupont, J.; Inorg. Chim. Acta 2006, 359, 1947; Alonso, D. A.; Nájera, C.; Chem. Soc. Rev. 2010, 39, 2891. 116-118 ºC); 1H NMR (300 MHz, CDCl3) δ 8.08 (d, 2H, J 8.4 Hz, Ph-H), 7.66 (d, 2H, J 6.9 Hz, Ph-H), 7.59 (d, 2H, J 6.6 Hz, Ph-H), 7.43 (m, 3H, Ph-H), 2.67 (s, 3H, Me); 13C NMR (75.4 MHz, CDCl3) δ 198.1, 146.1, 140.1, 136.1, 129.2, 129.1, 128.5, 127.5, 127.4, 26.9; GC-MS (EI, 70 eV) (%) 181 (100), 152 (54), 196 (49, [M+]), 153 (40), 76 (39), 151 (15), 182 (14), 51 (12).
2,4,6-Trimethylbiphenyl
Oil; 1H NMR (300 MHz, CDCl3) δ 7.40-7.26 (m, 3H, Ph-H), 7.13 (d, 2H, J 6.6 Hz, Ph-H), 6.92 (s, 2H, Ph-H), 2.31 (s, 3H, p-Me), 1.99 (s, 6H, o-Me); 13C NMR (75.4 MHz, CDCl3) δ 141.1, 139.0, 136.4, 135.9, 129.2, 128.3, 128.0, 126.4, 20.9, 20.7.
4-Nitrobiphenyl
Brown solid; m.p. 109-111.5 ºC (lit.1515 Riggleman, S.; DeShong, P.; J. Org. Chem. 2003, 68, 8106. 112-113 ºC); 1H NMR (300 MHz, CDCl3) δ 8.29 (d, 2H, J 9.0 Hz, Ph-H), 7.73 (d, 2H, J 9.0 Hz, Ph-H), 7.63 (dd, 2H, J 6.6, 1.8 Hz, Ph-H), 7.54 (dd, 3H, J 7.5, 6.6 Hz, Ph-H); 13C NMR (75.4 MHz, CDCl3) δ 147.9, 147.3, 139.0, 129.5, 129.2, 128.0, 127.7, 124.4; GC-MS (EI, 70 eV) (%) 152 (100), 199 (95, [M+]), 169 (37), 151 (30), 76 (28), 141 (27), 153 (26), 51 (26).
Results and Discussion
Synthesis and characterization of Pd/PAM
The Pd/PAM solid obtained after the drying step exhibited a color change from white to brown because of the incorporated PdCl2 (Figure 1). The Pd/PAM was characterized by SEM-EDS, FTIR, and ICP OES analyses.
A SEM micrograph of a cross-section of the Pd/PAM catalyst is shown in Figure 2. The Pd/PAM solid appears to have a porous structure, and PdCl2, which appeared as needle-like crystals, is homogeneously distributed over the polymeric solid. EDS analysis of the Pd/PAM sample (Figure 3) indicated the presence of palladium metal.
FTIR was employed for further characterization of Pd/PAM and the interactions between PdII and the PAM organic groups. All recorded spectra showed a very broad band at ca. 3300 cm-1, representing the O-H stretching vibration of water and the symmetric/asymmetric N-H vibrational modes (Figure 4). The PAM gel spectrum exhibited a strong absorption peak at 1637.95 cm-1, which can be attributed to the C=O stretching vibration in the -CONH2 group. In the Pd/PAM spectrum, the C=O stretching vibration is positively shifted to 1658.32 cm-1, suggesting the coordination of the amide carbonyl group to the PdII metal center. Similar behavior was described for the complexation of PdII with a carboxymethylcellulose derivative, displaying negative shifts of the carboxylate group upon coordination.1717 Xiao, J.; Lu, Z.; Li, Y.; Ind. Eng. Chem. Res. 2015, 54, 790.
ICP OES analysis was also performed on the prepared Pd/PAM sample. The Pd loading in Pd/PAM was 2.85 ± 0.03 mg g-1. Thus, the polymeric solid Pd/PAM was characterized, and its catalytic properties in Suzuki-Miyaura cross-coupling reactions were investigated.
Catalytic properties of the Pd/PAM catalyst
The catalytic properties of Pd/PAM were evaluated for the Suzuki-Miyaura cross-coupling of 4-iodoanisole with phenylboronic acid (Scheme 1). The reaction conditions, including the solvent (1,4-dioxane, DMF, or ethanol) and base (CsF, K2CO3, or K3PO4), were optimized using 0.3 mol% of Pd at 100 ºC for 4 h (Table 1). The bases and solvents were selected from previous studies that supported our investigation.44 Dupont, J.; Pfeffer, M.; Palladacycles - Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, 2008; Rosa, G. R.; Rosa, C. H.; Rominger, F.; Monteiro, A. L.; Dupont, J.; Inorg. Chim. Acta
2006, 359, 1947; Alonso, D. A.; Nájera, C.; Chem. Soc. Rev. 2010, 39, 2891.
5 Li, Y.; Liu, G.; Cao, C.; Wang, S.; Li, Y.; Pang, G.; Shi, Y.; Tetrahedron
2013, 69, 6241; Tang, X.; Huang, Y. T.; Liu, H.; Liu, R. Z.; Shen, D. S.; Liu, N.; Liu, F. S.; J. Organomet. Chem.
2013, 729, 95.
6 Puthiaraj, P.; Ahn, W. S.; Catal. Commun.
2015, 65, 91.
7 Yılmaz, Ü.; Küçükbay, H.; Deniz, S.; Şireci, N.; Molecules
2013, 18, 2501.
8 Azua, A.; Mata, J. A.; Heymes, P.; Peris, E.; Lamaty, F.; Martinez, J.; Colacino, E.; Adv. Synth. Catal.
2013, 355, 1107.
9 Hariprasad, E.; Radhakrishnan, T. P.; ACS Catal.
2012, 2, 1179.-1010 Faria, V. W.; Oliveira, D. G. M.; Kurz, M. H. S.; Gonçalves, F. F.; Scheeren, C. W.; Rosa, G. R.; RSC Adv.
2014, 4, 13446.
The results outlined in Table 1 indicate that the best result (entry 9) is obtained when K3PO4 is used as the base and ethanol as the solvent. However, the base is very hygroscopic; thus, K2CO3 was selected for ease of handling (entry 8a). The result is surprising and represents a Suzuki-Miyaura cross-coupling reaction that is free of phosphine ligands and quaternary ammonium salts.99 Hariprasad, E.; Radhakrishnan, T. P.; ACS Catal. 2012, 2, 1179. The catalytic system development employed a green solvent (ethanol) and used a low load of Pd (0.3 mol%). When the same reaction was repeated without PAM but with the same PdCl2 load, the yield decreased to 23% (entry 8b). On the other hand, the coupling reaction in the absence of PdCl2 but with the same PAM load gave a significantly lower yield (entry 8c). These data confirmed the catalytic efficiency of Pd/PAM. By varying the reaction temperature in reaction 8 it was observed that modest yields are found at temperatures below the boiling point of solvent (entries 8d and 8e) as shown in our previous work.1010 Faria, V. W.; Oliveira, D. G. M.; Kurz, M. H. S.; Gonçalves, F. F.; Scheeren, C. W.; Rosa, G. R.; RSC Adv. 2014, 4, 13446.
Optimization of reaction conditions for the Suzuki-Miyaura cross-coupling of Pd/PAM with phenylboronic acid and 4-iodoanisolea a Reaction conditions: 4-iodoanisole (1 mmol), phenylboronic acid (1.5 mmol), Pd/PAM catalyst (120 mg, 0.3 mol% of Pd), base (2 mmol), solvent (3 mL), 4 h (time not optimized), 100 oC, yields determined by GC (average of two runs);
After optimizing the catalytic system for the Suzuki-Miyaura reaction, we performed the cross-coupling reaction using various aryl halides (Table 2).
Excellent yields for the coupling of aryl iodides (more reactive partners) were observed with short reaction times; for example, the coupling reaction with iodobenzene occurred in only 2 h (entry 1). The catalytic system tolerates a variety of functional groups on the aromatic ring of the aryl bromides and furnishes the cross-coupled products in very good yields (entries 3-8); however, a long reaction time is necessary to deactivated aryl bromides and orto-substituted ones (entries 6-8). Because the main goal of our efforts regarding the Suzuki-Miyaura catalytic system was to enable the use of aryl chlorides because they are the cheapest aryl halides, we performed reactions using chloroarenes and did not obtain acceptable yields. Even when we used 4-chloronitrobenzene, an electron-poor aryl chloride, the yield was less than 50% (entry 9). Thus, electron-rich aryl chlorides were found to be unsuitable partners in this context, even after a 36 h reaction time (entry 11). In addition, increases in reaction time were effective in facilitating product formation, as shown in the cross-coupling of aryl bromides; however, we prefer reaction times of less than 24 h.
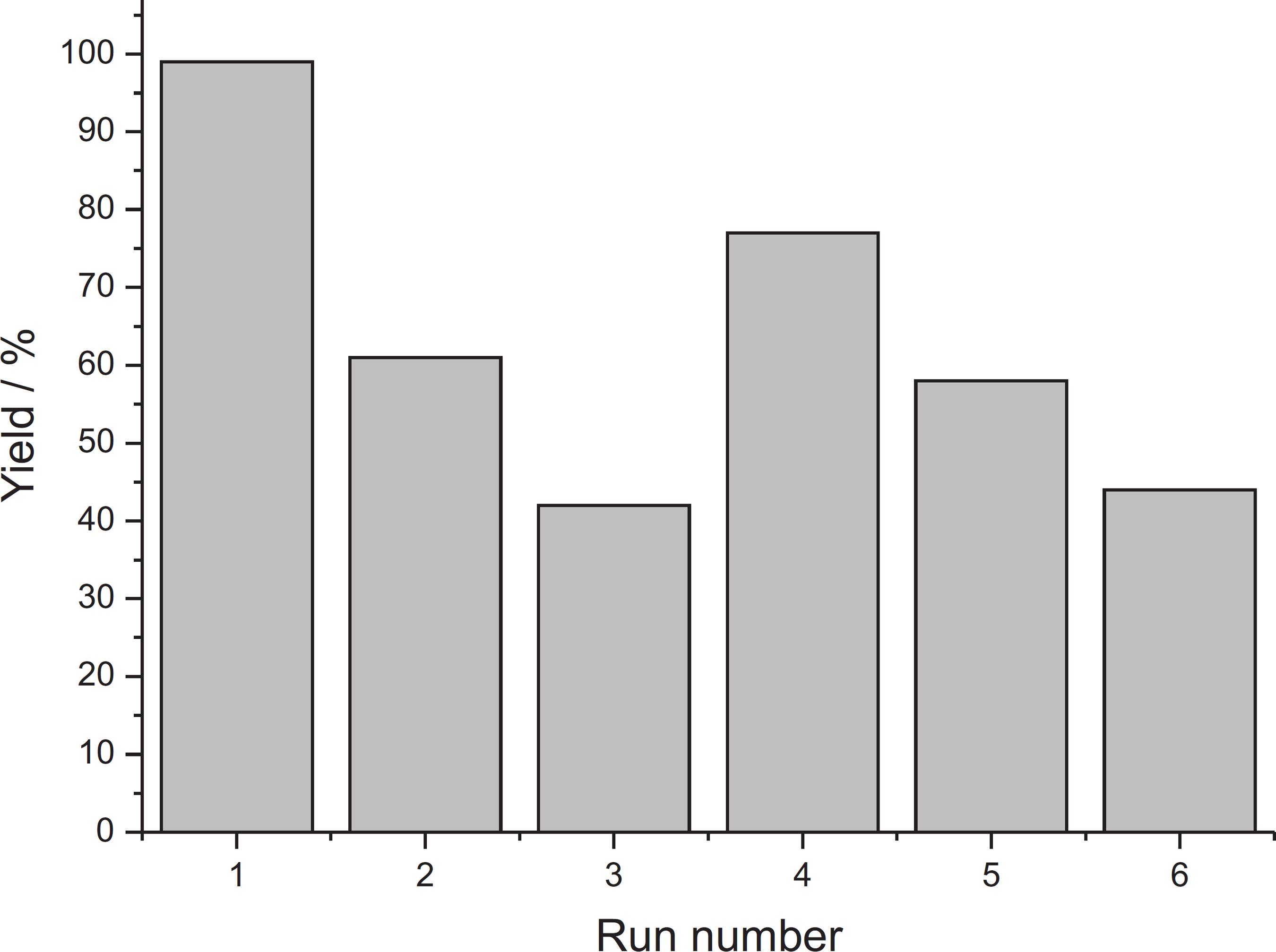
To conduct a preliminary investigation into the recycling capability of Pd/PAM, we selected the reaction of 4-iodoaniline with phenylboronic acid (Table 2, entry 2). An approximately 100% yield was obtained in the first run within 4 h. After the first run, 20 mL of Et2O were added in a Schlenk reactor to extract the organic compounds. The K2CO3 and Pd/PAM remaining in the reactor were loaded into another reaction. The same procedure was repeated in other runs. After six runs, the yields varied nonlinearly (Figure 5). This result may indicate that the polymer suffered deformation during the prolonged reaction time. Additionally, after the last run (number 6), only 44% of the cross-coupling product was found, which was somewhat higher than that reported in our previous study.1010 Faria, V. W.; Oliveira, D. G. M.; Kurz, M. H. S.; Gonçalves, F. F.; Scheeren, C. W.; Rosa, G. R.; RSC Adv. 2014, 4, 13446. Figure 5 shows a loss of catalytic activity; after the fourth recycle, the activity again increased, with the same profile observed in Pd0 nanoparticles in cellulose acetate thin film.1010 Faria, V. W.; Oliveira, D. G. M.; Kurz, M. H. S.; Gonçalves, F. F.; Scheeren, C. W.; Rosa, G. R.; RSC Adv. 2014, 4, 13446. This fact may be related to the leaching of the superficial PdII cations, resulting in the release of the catalytically active PdII species.
Yields obtained after 4 h of reaction time in repeated runs of the Suzuki-Miyaura cross-coupling of phenylboronic acid with 4-iodoaniline in ethanol using K2CO3 as the base and the same load of Pd/PAM catalyst.
Thus, we investigated the possibility of losing (leaching) PdII to the reaction medium by ICP OES analyses of Pd/PAM catalyst after six runs. The results show that Pd loading in Pd/PAM (after six runs) was 2.67 ± 0.03 mg g-1. However, if PdII was there, why did it not work? Was it shielded inside the solid? To further investigate the presence of PdII on the catalyst surface, we analyzed the FTIR spectrum of the recycled Pd/PAM (Figure 6).
FTIR spectra (ATR mode) recorded for the PAM hydrogel, Pd/PAM recycled after six runs, and unused Pd/PAM.
Figure 6 shows that the FTIR spectrum of the recycled Pd/PAM was quite similar to that recorded for the PAM hydrogel, with the C=O stretching vibration appearing at 1637.98 cm-1. This result suggests the loss of surface PdII ions by leaching after its use as a catalyst, leaving only the initial polymer on the surface. Although analogous to the carboxymethylcellulose-PdII complex cited in literature as catalyst, we had no evidence of the formation of metallic nanoparticles during the catalytic cycle.1717 Xiao, J.; Lu, Z.; Li, Y.; Ind. Eng. Chem. Res. 2015, 54, 790. The temperature applied during the Suzuki-Miyaura reactions apparently caused a thermal deformation of the polyacrylamide surface with the consequent leaching of PdII ions. The PdCl2 in the Pd/PAM complex stays catalytically inactive after six runs because of the presence of a polymeric PAM membrane, which accounts for its poor recyclability.
Conclusions
In conclusion, we developed a simple method for the preparation of a PdII catalyst dispersed in PAM, Pd/PAM, as an eco-friendly catalyst for Suzuki-Miyaura reactions in ethanol. Very good yields were obtained for the coupling of phenylboronic acid with a wide variety of functional groups on the aromatic ring of iodo- and bromoarenes. Aryl chlorides, in contrast, were found to be unsuitable reagents in this context, yielding only modest results. The recyclability of Pd/PAM is limited because PdII is leached, leaving only the base polymer on the surface after six runs. Our group continues to investigate the applications of Pd/PAM in other cross-coupling reactions.
Supplementary Information
Supplementary data (copies of NMR spectra) are available free of charge at http://jbcs.sbq.org.br as PDF file.
https://minio.scielo.br/documentstore/1678-4790/FqvxfgN5SjQ8H7WpdQ9wXMh/cc8493f00de40df2ba04da39d6ceced4c194f8bc.pdf-
FAPERGS/CAPES has sponsored the publication of this article.
Acknowledgements
This work was supported by grants from CNPq, FAPERGS and CAPES (Brazil). The authors would like to thank CEME-SUL FURG for the SEM microscopy analyses.
References
-
1Negishi, E.; Handbook of Organopalladium Chemistry for Organic Synthesis, 1st ed.; John Wiley & Sons: New York, 2002; Diederich, F.; Meijere, A.; Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, 2004; Miyaura, N.; Suzuki, A.; Chem. Rev. 1995, 7, 2457.
-
2Biajoli, A. F. P.; Schwalm, C. S.; Limberger, J.; Claudino, T. S; Monteiro, A. L.; J. Braz. Chem. Soc. 2014, 25, 2186.
-
3Huang, Z. Y.; Yang, J. F.; Chen, Q.; Cao, R. J.; Huang, W.; Hao, G. F.; Yang, G. F.; RSC Adv. 2015, 5, 75182.
-
4Dupont, J.; Pfeffer, M.; Palladacycles - Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, 2008; Rosa, G. R.; Rosa, C. H.; Rominger, F.; Monteiro, A. L.; Dupont, J.; Inorg. Chim. Acta 2006, 359, 1947; Alonso, D. A.; Nájera, C.; Chem. Soc. Rev 2010, 39, 2891.
-
5Li, Y.; Liu, G.; Cao, C.; Wang, S.; Li, Y.; Pang, G.; Shi, Y.; Tetrahedron 2013, 69, 6241; Tang, X.; Huang, Y. T.; Liu, H.; Liu, R. Z.; Shen, D. S.; Liu, N.; Liu, F. S.; J. Organomet. Chem. 2013, 729, 95.
-
6Puthiaraj, P.; Ahn, W. S.; Catal. Commun. 2015, 65, 91.
-
7Yılmaz, Ü.; Küçükbay, H.; Deniz, S.; Şireci, N.; Molecules 2013, 18, 2501.
-
8Azua, A.; Mata, J. A.; Heymes, P.; Peris, E.; Lamaty, F.; Martinez, J.; Colacino, E.; Adv. Synth. Catal. 2013, 355, 1107.
-
9Hariprasad, E.; Radhakrishnan, T. P.; ACS Catal. 2012, 2, 1179.
-
10Faria, V. W.; Oliveira, D. G. M.; Kurz, M. H. S.; Gonçalves, F. F.; Scheeren, C. W.; Rosa, G. R.; RSC Adv. 2014, 4, 13446.
-
11Mahdavi, H.; Zirakzadeh, A.; Amani, J.; React. Funct. Polym. 2007, 67, 716.
-
12Tamami, B.; Ghasemi, S.; J. Mol. Catal. A: Chem. 2010, 322, 98.
-
13Soares, B. M.; Vieira, A. A.; Lemões, J. S.; Santos, C. M. M.; Mesko, M. F.; Primel, E. G.; D'Oca, M. G. M.; Duarte, F. A.; Bioresour. Technol. 2012, 110, 730.
-
14Protti, S.; Fagnoni, M.; Mella, M.; Albini, A.; J. Org. Chem. 2004, 69, 3465.
-
15Riggleman, S.; DeShong, P.; J. Org. Chem. 2003, 68, 8106.
-
16Inamoto, K.; Kuroda, J. I.; Sakamoto, T.; Hiroya, K.; Synthesis 2007, 18, 2853.
-
17Xiao, J.; Lu, Z.; Li, Y.; Ind. Eng. Chem. Res. 2015, 54, 790.
Publication Dates
-
Publication in this collection
Apr 2016
History
-
Received
24 Sept 2015 -
Accepted
27 Nov 2015