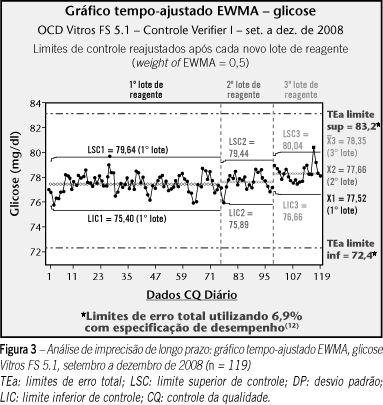
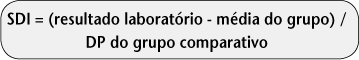
INTRODUCTION: Countless questions have been raised concerning quality of health services, mainly since the beginning of this decade. One approach that has been widely pursued as a theoretical basis for this issue is high reliability organization (HRO). In this paper we propose a new approach to analytical quality control (AQC), based on HRO principles and focused on patient safety improvement. OBJECTIVES: To propose a new approach to AQC system, aligned with HRO theory and focused on patient safety improvement. MATERIALS AND METHODS: In order to optimize the traditional AQC approach and make feasible the long term assessment of system stability, we propose a model based on evaluation of random and systematic error by means of advanced control charts. We used real data obtained from the routine to validate the simulated model (glucose, Vitros FS 5.1, OCD). RESULTS: The studied assay was evaluated in terms of long term performance and showed an adequate performance (4.1 sigma) for its diagnostic use. DISCUSSION: The proposed model suggests an alternative tool based on expertise already widely applied in statistical process control in order to control long-term stability of laboratory methods. CONCLUSION: The new proposed approach, which is a complement to the traditional quality control system and involves the sequential and associated use of different statistical tools, proved to be a valid and useful model for effective performance evaluation and its ongoing performance stability.
Process; Safety; Quality; Risk; High reliability organization; Control