Abstracts
OBJECTIVE: To compare different levels of ablation in terms of the degree of patient satisfaction and extent of postoperative reflex sweating in sympathectomized patients. METHODS: A retrospective study involving 521 patients with primary hyperhidrosis, submitted to thoracic sympathectomy at the Monte Sinai Hospital and University Hospital of the Federal University of Juiz de Fora, from January of 2001 to December 2005. All patients were submitted to thermal ablation of the sympathetic stem and were divided into three groups: up to T2 (group I, n = 162); up to T3 (group II, n = 65); and up to T4 (group III, n = 294). RESULTS: Optimal postoperative control of palmar/axillary hyperhidrosis was achieved in, respectively, 94/82% of the patients of group I, 89/89% of those in group II and 80/80% of those in group III. Postoperative reflex sweating was observed in 67% of the patients in groups I and II, compared with 61.29% of those in group III. Severe reflex sweating occurred in 32% of the group I patients, 9% of the group II patients and 4% of the group III patients. CONCLUSION: Sympathectomy provided excellent patient satisfaction and a low incidence of complications. There was no significant difference between the levels of ablation in terms of reflex sweating, although the intensity of this complication decreased when lower levels of blockage, principally at the T4 level, were employed.
Sympathectomy; Thoracoscopy; Hyperhidrosis
OBJETIVO: Comparar grau de satisfação dos pacientes simpatectomizados e presença de sudorese reflexa, de acordo com diferentes níveis de ablação. MÉTODOS: Estudo retrospectivo de 521 pacientes com hiperidrose primária, submetidos à simpatectomia torácica no Hospital Monte Sinai e Hospital Universitário da Universidade Federal de Juiz de Fora - UFJF, de janeiro de 2001 a dezembro de 2005. Grupo I (n = 162): termoablação do tronco simpático, tendo T2 como nível mais cranial da ressecção, independentemente de outros níveis seccionados caudalmente. Grupo II (n = 65): termoablação do tronco simpático, tendo T3 como nível mais alto. Grupo III (n = 294): termoablação do tronco simpático, tendo T4 como nível mais alto. RESULTADOS: Pós-operatório ótimo no controle da hiperidrose palmar/axilar em, respectivamente, 94/82% dos pacientes do grupo I, 89/89% do grupo II e 80/80% do grupo III. Sudorese reflexa em 67% dos pacientes dos grupos I e II, caindo para 61,29% no grupo III devido à maior termoablação a partir de T4. Ocorreu sudorese reflexa severa em 32% dos pacientes do grupo I, 9% do grupo II e 4% do grupo III. CONCLUSÃO: A simpatectomia propiciou excelente grau de satisfação e baixo índice de complicações. Não houve diferença na incidência de sudorese reflexa com diferentes níveis seccionados; porém, a intensidade desta complicação se mostrou menor quando optamos por níveis de bloqueio mais baixos, principalmente T4.
Simpatectomia; Toracoscopia; Hiperidrose
ORIGINAL ARTICLE
Video-assisted thoracic sympathectomy in the treatment of primary hyperhidrosis: a retrospective study of 521 cases comparing different levels of ablation* * Study carried out at the Thoracic Surgery Department of the Monte Sinai Hospital and at the University Hospital of the Juiz de Fora Federal University, Juiz de Fora (MG) Brazil.
Jorge MontessiI; Edmílton Pereira de AlmeidaII; João Paulo VieiraIII; Marcus da Matta AbreuIV; Renato Lucas Passos de SouzaV; Oswaldo Victor Duarte MontessiVI
IPhD in Medicine in the area of Thoracic Surgery from the Juiz de Fora Federal University, Juiz de Fora (MG) Brazil
IIFull member of the Brazilian Thoracic Surgery Society. Juiz de Fora Federal University, Juiz de Fora (MG) Brazil
IIIFull Member of the Brazilian Thoracic Surgery Society. Juiz de Fora School of Medical and Health Sciences SUPREMA Juiz de Fora (MG) Brazil
IVThoracic Sugery Resident at the Juiz de Fora Federal University, Juiz de Fora (MG) Brazil
VFifth-year Medical Student at the Juiz de Fora Federal University, Juiz de Fora (MG) Brazil
VIThird-year Medical Student at the Juiz de Fora School of Medical and Health Sciences SUPREMA Juiz de Fora (MG) Brazil
Correspondence to Correspondence to: Jorge Montessi Centro de Estudos Hospital Monte Sinai Rua Vicente Beguelli, 315, Dom Bosco CEP 36035-550, Juiz de Fora, MG, Brasil Phone 55 32 3239-4194 Fax 55 32 3239-4500 E-mail: jmontessi@terra.com.br
ABSTRACT
OBJECTIVE: To compare different levels of ablation in terms of the degree of patient satisfaction and extent of postoperative reflex sweating in sympathectomized patients.
METHODS: A retrospective study involving 521 patients with primary hyperhidrosis, submitted to thoracic sympathectomy at the Monte Sinai Hospital and University Hospital of the Federal University of Juiz de Fora, from January of 2001 to December 2005. All patients were submitted to thermal ablation of the sympathetic stem and were divided into three groups: up to T2 (group I, n = 162); up to T3 (group II, n = 65); and up to T4 (group III, n = 294).
RESULTS: Optimal postoperative control of palmar/axillary hyperhidrosis was achieved in, respectively, 94/82% of the patients of group I, 89/89% of those in group II and 80/80% of those in group III. Postoperative reflex sweating was observed in 67% of the patients in groups I and II, compared with 61.29% of those in group III. Severe reflex sweating occurred in 32% of the group I patients, 9% of the group II patients and 4% of the group III patients.
CONCLUSION: Sympathectomy provided excellent patient satisfaction and a low incidence of complications. There was no significant difference between the levels of ablation in terms of reflex sweating, although the intensity of this complication decreased when lower levels of blockage, principally at the T4 level, were employed.
Keywords: Sympathectomy; Thoracoscopy; Hyperhidrosis.
Introduction
Primary hyperhidrosis, also known as essential hyperhidrosis, is a condition characterized by excessive and uncontrollable sweating for no apparent reason.(1) This common disease is little understood and is frequently worsened by emotional stress or psychological factors. Hyperhidrosis is preferably located in the armpits, the palms of the hands, plants of the feet, and face. It is estimated that it affects from 0.6 to 1.0% of the population, generally young adults.(1) In 30 to 50% of cases, there is a positive family history.(1) Localized, moderate to severe focal hyperhidrosis is associated with considerable morbidity, including maceration of the skin, as well as secondary bacterial and fungal skin infections. However, the principal characteristic of this disease is the intense discomfort of the patients, which affects their social, affective, and professional life. Primary hyperhidrosis, in general, manifests in early adolescence or even in childhood, does not occur during sleep, and can be worsened by emotional stimuli.(1) There is evidence that primary palmar hyperhidrosis is a hereditary disorder.(2)
Excessive sweating on the palms of the hands is a condition that is mostly inconvenient in professional and social meetings. Axillary hyperhidrosis is a common and distressing condition. The fact that such sweating is not socially accepted, the discomfort caused by constant wetness, and the odor, as well as the stained clothes, significantly decrease quality of life.(3)
Clinical treatment can be topical, electrical, or systemic. In mild cases of localized hyperhidrosis, the use of absorbent talc and 20% aluminum chloride can be sufficient. Iontophoresis,(4) when properly administered, is a simple, sometimes effective, safe and affordable method of treating mild to moderate primary hyperhidrosis. The use of systemic medication can also be indicated. However, its efficacy is debatable, and the rates of side effects are high. Another option is the use of botulinum toxin type A. Although it has innumerable advantages, its high cost and the temporary nature of its effect make it impractical for most patients.(5)
In most cases, there are no treatments that can resolve this condition satisfactorily, and surgical procedures become necessary. One treatment option involves the removal of the eccrine and apocrine glands from the axillary region. Various techniques have been proposed, all of which present a high index of complications.(6-8) Liposuction of sweat glands is another method. However, complications such as aesthetically unappealing scars, bleeding, slow recovery, and tissue necrosis, and well as infection, limit its use.(9)
Other topical methods of treating localized hyperhidrosis are expensive, have limited efficacy, and provide only transitory benefit. This, together with the increasingly greater availability of video-assisted thoracic surgery, have contributed decisively to the establishment of sympathectomy as the current gold standard in the definitive treatment of severe cases of palmar and axillary hyperhidrosis, presenting over 98% effectiveness and a high rate of patient satisfaction.(10) The first sympathectomy by thoracoscopy was performed in 1951.(11) Since then, different levels of the sympathetic trunk have been approached in the treatment of excessive sweating. The evolution of video-assisted thoracoscopic techniques have allowed thoracic sympathectomy to be performed quite safely, with good results and minimal morbidity.
The complications related to the manipulation of the pleural cavity, such as hemothorax, pneumothorax, wall infection, and pulmonary decompression-induced edema, have been occasionally described, although the incidence has always been lower than 1%.(10) Some difficulties, such as those caused by pleural adherences and the need for a second sympathectomy (less than 3%), have been reported, although all such cases were attributable to some anatomic variant.(10) Without a doubt, the most significant complication of sympathectomy is reflex sweating. In most studies, reflex sweating has been observed in the majority of patients.(12-18)
Method
Between January of 2001 and December of 2005, 521 patients with primary hyperhidrosis were submitted to video-assisted thoracic sympathectomy (thermal ablation) at the Monte Sinai Hospital and at the University Hospital of the Juiz de Fora Federal University. Ages ranged from 9 to 69 years. Females predominated, 336 (64.49%) of the patients being women. The predominant age bracket was that from 20 to 29 years, with 267 patients (51.24%), followed by that from 10 to 19 years, with 132 patients (25.33%). The most common clinical manifestation of hyperhidrosis was palmoplantar-axillary sweating (in 34.7%), followed by palmoplantar sweating (in 28.4%), and axillary sweating (in 19%). The patients were divided into three groups based on the initial cranial level (ganglion) of the sympathetic trunk at which the ablation began, regardless of the additional levels sectioned caudally: group I, consisting of patients undergoing ablation at T2; group II, consisting of patients undergoing ablation at T3; and group III, consisting of patients undergoing ablation at T4. We designated T2 thermal ablation 'sympathicotomy performed over the second costal arch', and so on consecutively. Cranial and caudal isolation of the ganglia was unnecessary in our series. The clinical evolution was evaluated through outpatient monitoring and phone calls. The patients were asked simple questions related to the resolution of the symptoms, satisfaction with the final result of the surgical procedure, and the occurrence/intensity of reflex sweating. The data were entered into a program created for the Statistical Analysis System reader. The statistical analyses were performed using the chi-square test, and the level of statistical significance was set at p < 0.05.
Results
The total number of patients submitted to video-assisted thoracic sympathectomy at our facility was 521: 162 (31.09%) in group I; 65 (12.47%) in group II; and 294 (56.42%) in group III. In groups I and III, 63% of the patients were female, compared to 62% in group II. Palmar hyperhidrosis was reported by 90% of the patients in group I, 70.5% of those in group II, and 61% of those in group III. Axillary hyperhidrosis was reported by 59.5% in group I, 72.3% in group II, and 77.4% in group III.
Initially, we used T2 and T3 ablations in the treatment of palmar and axillary hyperhidrosis. Based on relevant publications,(19) we began to perform T4 ablations, and observed a reduction in the incidence of reflex hyperhidrosis (67% in groups I and II vs. 61.29% in group III, p < 0.05). (Figure 1)
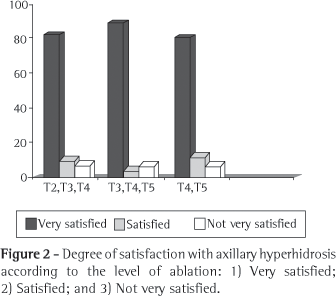
Severe reflex sweating occurred in 32% of the patients in group I, 9% of those in group II, and 4% of those in group III (p < 0.05). Moderate sweating occurred in 18% of the patients in group I, 31% of the patients in group II, and in 36% of the patients in group III. The mild form occurred in 50% of those in group I and 60% of those in groups II and III. Patients who reported any degree of reflex hyperhidrosis were asked to characterize the symptom using these categories. (Figure 2)
The incidence of postoperative complications was 3.07%, which was not statistically significant. Pneumothorax occurred in 12 cases (2.3%). In 9 of those cases, water-sealed thoracic drainage was used, remaining in place for less than 12 h. In the remaining cases, we opted for a conservative procedure. Transitory Claude Bernard-Horner syndrome was observed in 2 patients (0.38%) who were submitted to T2 ablation. However, it reversed within one week after the surgical procedure. Pleural effusion was also observed as a complication in 2 cases (0.38%), but the problem was resolved through thoracentesis.
Our method resulted in unilateral failure in 6 cases (1.15%), and 4 patients underwent a second operation. There were 2 patients who declined to be submitted to a second surgical procedure.
Regarding the degree of satisfaction indicated by the patients, 85% stated that they were 'very satisfied', stated that they were 'satisfied', and 3% stated that they were 'not very satisfied' or 'completely dissatisfied'. This classification regarding the satisfaction with the procedure was obtained spontaneously during a clinical interview with the patients and, when this did not occur, we offered the patients four options to choose from so that they could classify the final result of the surgical procedure. Only 1% of the patients regretted having undergone the operation.
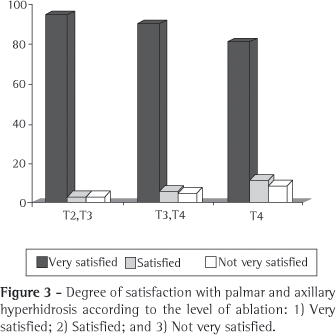
Regarding the degree of satisfaction by symptom site, 94% of the group I patients with palmar hyperhidrosis reported that the result of the sympathectomy was very good, and 3% considered the result good. Of the group II patients with palmar hyperhidrosis, 89% considered the result very good, and 6% stated that it was good. Of the group III patients with palmar hyperhidrosis, 80% considered the result very good, and 12% considered it good. Among the patients with axillary hyperhidrosis, the result of the sympathectomy was considered 'very good' by 82% of those in group I, 89% of those in group II, and 80% of those in group III, whereas the result was considered 'good' by 10% of the patients in group I, 5% of those in group II, and 12% of those in group III (Figures 3 and 4).
Discussion
Hyperhidrosis can be primary (idiopathic) or secondary. Secondary hyperhidrosis can be caused by a variety of diseases or, occasionally, by the use of certain drugs, such as tricyclic antidepressants and venlafaxine. Regardless of type or cause, it is a socially embarrassing and functionally incapacitating condition.(20)
The efficacy of the sympathectomy in the control of palmar and axillary hyperhidrosis has been known for many decades. The complexity of the procedure (when cervical access is used), together with its morbidity when performed in conjunction with two axillary thoracotomies, has, until recently, restricted its use to selected cases.
The first sympathectomy for the treatment of hyperhidrosis was performed in 1920.21) Anterior cervical access was used. There was a great advance in the technique in 1942, when it was demonstrated that it was unnecessary to sacrifice the stellate ganglion in order to denervate the upper limb.(22) This fact is of great relevance, since it practically eliminated one of the most feared complications in thoracic sympathectomy, Claude Bernard-Horner syndrome.
In 1949, another author described the axillary approach, and, in 1954, the same author reported a series of 26 operated patients, emphasizing the advantages of this approach when performing an open sympathectomy.(23) The minimally invasive approach to the sympathetic trunk started in 1942,(15) when the endoscopic sympathectomy was described, with local anesthesia, in semi-sitting position, with two entry portals.
In 1950,(11) the endoscopic method was demonstrated in 500 patients, increasing its popularity. With a few variations, this is the method currently used in most health care facilities.
The era of video-assisted surgery began in the 1980s, when the first video-assisted sympathectomy was performed. In 1996, a survey was conducted in various hospitals throughout China.(24) Data regarding a total of 9988 operated patients were published. This study resulted in a worldwide explosion in the use of this surgical technique, since it proved that the procedure was feasible in semi-sitting position, easily allowing the bilateral procedure, without the need for selective intubation, or even for insufflation of carbon dioxide in the pleural cavity, in most cases. The authors also reported a low rate of immediate complications and a high rate of success.(24) However, there was a high prevalence of reflex hyperhidrosis in T2 and T3 ablations.(24)
Reflex sweating is the most common post-sympathectomy complication, generally occurring in the trunk and lower limbs. Reflex plantar sweating is rare. This complication accounts for the cases of dissatisfaction with the surgical procedure, creating great interest among surgeons in discovering means to decrease or eliminate the occurrence of this undesirable event.(14,17,24) Reflex sweating was long thought to be related to the number of resected lymph node stations, motivating various authors to perform sympathectomies at a single ganglion level.(12,17,24) A survey involving nearly 10,000 level T2sympathectomized patients failed to demonstrate a decrease in the prevalence of this procedure.(24)
In this context, a study carried out in The Netherlands, comprising a series of 14 patients with T3 ablation for treatment of palmar hyperhidrosis, revealed a success equivalent to that of T2-T3 ablation, although without reflex hyperhidrosis.(17)
A randomized study involving 171 patients compared sympathectomy at T3-T4, T4, and T4T5 for the treatment of hyperhidrosis and axillary bromhidrosis. A 70% reduction in reflex hyperhidrosis was reported in the T3-T4 group, compared to 29% in T4 and T4-T5 groups, demonstrating the importance of T3 in the prevalence of reflex sweating. The need for a T4 and T5 ablation for the treatment of axillary hyperhidrosis became evident as well.(14)
Even with the evolution of the technique, reflex sweating remained an inconvenience of sympathectomy in a significant number of patients, although only a minority of the patients were dissatisfied with the surgical procedure.(12-15) The idea of clipping the sympathetic trunk was designed to replace thermal ablation, with the intention of making the operation reversible, in case the patient was dissatisfied with the final result of the procedure. However, the number of reversals is very low in most series. It is now also known that this reversal should occur within approximately 30 days after surgery, since, after this period, the success of the reversal is limited to approximately 25% of the patients.(25)
The great merit of clipping was an accidental discovery.(26) In a trial with 165 patients submitted to the clipping of the sympathetic trunk, 1 patient presented severe compensatory reflex sweating on one side only. Radiologic control revealed that the sweating occurred only on the side where the clip had been correctly placed, that is, at T2. However, when the clip was inadvertently placed at a lower level (T3), there was no reflex sweating. The control of palmar hyperhidrosis, however, was equal on both sides. Since then, those authors have operated on 100 consecutive patients with section of the station at T3 and have not again encountered severe reflex sweating. Subsequently, they began to amputate the station at T4 level and reflex sweating became even milder. However, in some cases, with the section at T4, the palmar hyperhidrosis was not controlled, and some patients required a second surgical procedure in order to section the station at a more cranial level. They found that none of the patients submitted to bilateral lumbar sympathectomy reported reflex sweating, and suggested an explanation for the phenomenon: the fact that the autonomous nervous system acts by mechanisms of positive or negative hypothalamic feedback. More surprising was the demonstration that T4 ablation controls palmar hyperhidrosis, whereas T4-T5 ablation controls palmar and axillary hyperhidrosis, as well as bromhidrosis.(19)
At our facility, we began to perform T4-T5 ablation to control the axillary form based on relevant studies, especially one that was published in 2001.(19) As a result of this change, the incidence of reflex sweating dropped from 67 to 61.29%. Of the patients submitted to thermal ablation at T2, 32% presented severe reflex sweating, compared with 6% of those not submitted to thermal ablation at T2.
The degree of postoperative patient satisfaction is intimately related to the development of reflex sweating and to failure of the technique. A rapid review of the literature on reflex sweating revealed controversial findings, some authors reporting a prevalence of approximately 5% and others reporting a prevalence of approximately 85%.(27,28) Such variations result in the lack of a clear definition of the condition, as previously mentioned by other authors.(28,29)
Various authors, using sophisticated methods for the quantification of reflex sweating, such as gravimetry and sudometry, have found significant discrepancies between the discomfort reported and the degree of sweating effectively quantified.(27,30) The intensity of reflex sweating is directly related to ambient temperature, humidity, and physical activity, being least tolerated in hot, humid climates. In addition, the same patient can quantify the intensity of sweating in a quite different manner over a period of just a few days. In the present study, we adopted a semiquantitative method frequently used in the literature, which classifies reflex sweating as mild, moderate, or intense, taking the impression of the patient into account.
Bilateral video-assisted thoracic sympathectomy constitutes a simple intervention that presents low morbidity for the definitive treatment of primary craniofacial, palmar and axillary hyperhidrosis.
Reflex sweating is the most pronounced side effect of sympathectomy, and is usually well tolerated, having little influence on the result of the surgery. In some cases, however, it can become a serious complication and, therefore, a reason for regret on the part of the patients.
The greatest advance that has recently been made was the identification of the second sympathetic ganglion as the probable center of reflex sweating, and its preservation is the safest means of preventing the condition without compromising the effective control of palmar and axillary hyperhidrosis. The second ganglion being preserved, some degree of reflex sweating can still be observed. However, such sweating is generally mild, tolerable, and transitory. Therefore, modifications in the level of sympathectomy have been considered, principally that of avoiding T2 thermal ablation.
After a review of the literature, we opted for a T3 thermal ablation for the control of craniofacial hyperhidrosis, T4 for the cases of palmar complaint, and T4-T5 for axillary hyperhidrosis.
References
Submitted: 2 May 2006. Accepted, after review: 4 September 2006.
- 1. Stolman LP. Treatment of hyperhidrosis. Dermatol Clin. 1998;16(4):863-9.
- 2. Ro KM, Cantor RM, Lange KL, Ahn SS. Palmar hyperhidrosis: evidence of genetic transmission. J Vasc Surg. 2002;35(2):382-6.
- 3. Amir M, Arish A, Weinstein Y, Pfeffer M, Levy Y. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci. 2000;37(1):25-31.
- 4. Holzle E, Alberti N. Long-term efficacy and side effects of tap water iontophoresis of palmoplantar hyperhidrosisthe usefulness of home therapy. Dermatologica. 1987;175(3):126-35.
- 5. Hexsel AM, Hexsel CL. Toxina Botulínica no Tratamento das Hiperidroses. In: Almeida ART, Hexsel DM, editors. Hiperidrose e Toxina Botulínica. São Paulo: Know-how Editorial; 2003. p. 145-227.
- 6. Skoog T, Thyresson N. Hyperhidrosis of the axillae. A method of surgical treatment. Acta Chir Scand. 1962;(124):531-8.
- 7. Sabatier H, Picaud AJ. The surgical treatment of axillary hyperhidrosis. J Dermatol Surg. 1976;2(4):331-2.
- 8. Eldh J, Fogdestam I. Surgical treatment of Hyperhidrosis axillae. Scand J Plast Reconstr Surg. 1976;10(3):227-9.
- 9. Swinehart JM. Treatment of axillary hyperhidrosis: combination of the starch-iodine test with the tumescent liposuction technique. Dermatol Surg. 2000;26(4):392-6.
- 10. Chung IH, Oh CS, Koh KS, Kim HJ, Paik HC, Lee DY. Anatomic variations of the T2 nerve root (including the nerve of Kuntz) and their implications for sympathectomy. J Thorac Cardiovasc Surg. 2002;123(3):498-501.
- 11. Kux E. The endoscopic approach to the vegetative nervous system and its therapeutic possibilities; especially in duodenal ulcer, angina pectoris, hypertension and diabetes. Dis Chest. 1951;20(2):139-47.
- 12. Drott C, Gothberg G, Claes G. Endoscopic procedures of the upper-thoracic sympathetic chain. A review. Arch Surg. 1993;128(2):237-41.
- 13. Gossot D, Toledo L, Fritsch S, Célérier M. Thoracoscopic sympathectomy for upper limb hyperhidrosis: Looking for the right operation. Ann Thorac Surg. 1997;64(4):975-8.
- 14. Hsu CP, Shia SE, Hsia JY, Chuang CY, Chen CY. Experiences in thoracoscopic sympathectomy for axillary hyperhidrosis and osmidrosis: focusing on the extent of sympathectomy. Arch Surg. 2001;136(10):1117-8.
- 15. Kux M. Thoracic Endoscopic sympathectomy in palmar and axillary hyperhidrosis. Arch Surg. 1978;113(3):264-6.
- 16. Reisfeld R, Nguyen R, Pnini A. Endoscopic thoracic sympathectomy for hyperhidrosis: Experience with both cauterization and clamping methods. Surg Laparosc Endosc Percutan Tech. 2002;12(4):255-7.
- 17. Riet MV, Smet AA, Kuiken H, Kazemier G, Bonjer HJ. Prevention of compensatory hyperhidrosis after thoracoscopic sympathectomy for hyperhidrosis. Surg Endosc. 2001;15(10):1159-62.
- 18. Zacher J, Imhof M, Huber ER, Plas EG, Herbst F, Jaskesz R, et al. Video assistance reduces complication rate of thoracoscopic sympathicotomy for hyperhidrosis. Ann Thorac Surg. 1999;68(4):1177-81.
- 19. Lin CC, Telaranta T. Lin-Telaranta classification: the importance of different procedures for different indications in sympathetic surgery. Ann Chir et Gynaecol. 2001;90(3):161-6.
- 20. Almeida EP, Montessi J, Vieira JP, Marsico GA. Simpatectomia Torácica. HU Revista. 2004;30(1):23-6.
- 21. Mack M. Thoracoscopy. In: Pearson FG, Cooper JD, Deslauriers J, Ginsberg RJ, Hiebert C, Patterson GA, Urschel HC, editors. Thoracic Surgery. 1st ed. New York: Churchill Livingstone; 1995. p. 1488-509.
- 22. Hyndman OR, Wolking J. Sympathectomy of the upper extremity: evidence that only the second dorsal ganglion need be removed for complete sympathectomy. Arch Surg. 1942;(45):145-55.
- 23. Atkins HJ. Sympathectomy by the axillary approach. Lancet. 1954;266(6811):538-9.
- 24. Kao MC, Lin JY, Chen YL, Hsieh CS, Cheng LCJ, Huang SJ. Minimally invasive surgery: video endoscopic thoracic sympathectomy for palmar hyperhidrosis. Ann Acad Med Singapore. 1996;25(5):673-8.
- 25. Lin TS. Endoscopic clipping in video-assisted thoracoscopic sympathetic blockade for axillary hyperhidrosis. An analysis of 26 cases. Surg Endosc. 2001;15(2):126-8.
- 26. Lin CC, Wu HH. Endoscopic t4-sympathetic block by clamping (ESB4) in treatment of hyperhidrosis palmaris et axillarisexperiences of 165 cases. Ann Chir Gynaecol. 2001;90(3):167-9.
- 27. Gossot D, Kabiri H, Caliandro R, Debrosse D, Girard P, Grunenwald D. Early complications of thoracic endoscopic sympathectomy: a prospective study of 940 procedures. Ann Thorac Surg. 2001;71(4):116-9.
- 28. Leão LEV, Oliveira R, Szule R, Mari JJ, Crotti PL, Gonçalves JJ. Role of video-assisted thoracoscopic sympathectomy in the treatment of primary hyperhidrosis. São Paulo Med J. 2003;121(5):191-7.
- 29. Gossot D, Galetta D, Pascal A, Debrosse D, Caliandro R, Girard P, et al. Long-term results of endoscopic thoracic sympathectomy for upper limb hyperhidrosis. Ann Thorac Surg. 2003;75(4):1075-9.
- 30. Hashmonai M, Kopelman D, Assalia A. The treatment of primary palmar hyperhidrosis: a review. Surg Today. 2000;30(3):211-8.
Publication Dates
-
Publication in this collection
25 Sept 2007 -
Date of issue
June 2007
History
-
Received
02 May 2006 -
Accepted
04 Sept 2006