Abstracts
OBJECTIVE: To describe lung injuries in autopsied pediatric patients (neonates through 15 years old) subjected or not to total parenteral nutrition, in an intensive care unit. METHODS: Sections from six paraffin-embedded lung fragments, from 114 children were studied by routine staining. Demographic, clinical and therapeutic data were retrieved from the records. Statistical analysis was performed using Statistical Package for the Social Sciences. RESULTS: The 114 patients were divided in two groups: 50 who were treated with total parenteral nutrition with lipid emulsion and 64 who did not receive total parenteral nutrition. The two groups did not differ in gender (p = 0.654), age (p = 0.682) or body weight (p = 0.175), but duration of hospital stay (p = 0.000), prematurity (p = 0.008) and treatment with blood products (p=0.009) were all higher in the group treated with total parenteral nutrition. All patients received mechanical ventilation during hospitalization. Chi-square comparisons showed that diffuse alveolar injury (p=0.022), pulmonary fibrosis (p = 0.019), pneumocyte hyperplasia (p = 0.004), microthromboembolism (p = 0.047) and thrombophlebitis (0.033) all exhibited a significant relationship with total parenteral nutrition. However, a multivariate analysis by logistic regression, taking into account prematurity and duration of hospital stay, demonstrated that total parenteral nutrition was an independent factor only with respect of pulmonary fibrosis. CONCLUSION: Although lung injuries were significantly more frequent in children who had received total parenteral nutrition, it was impossible to conclude that the lipid infusion had a direct relationship with these injuries, because prematurity and duration of hospital stay were significant co-factors.
Parenteral nutrition; parenteral; lung; pathology
OBJETIVO: Descrever as lesões pulmonares em uma série de necropsias de pacientes com idade de até 15 anos, falecidos em unidade de terapia intensiva, submetidos ou não à nutrição parenteral total. MÉTODOS: Seis fragmentos de cada pulmão de 114 crianças foram corados por métodos de rotina. Dos prontuários foram obtidas informações referentes aos dados demográficos, clínicos e de terapêutica. Para a análise estatística, foi utilizado o Programa Statistical Package for the Social Sciences. RESULTADOS: Os 114 pacientes foram separados em dois grupos: 50 foram tratados com NPT contendo emulsão de lipídios e os 64 restantes, sem nutrição parenteral total. Os grupos eram semelhantes em relação ao sexo (p = 0,654), à idade (p = 0,682) e ao peso (p = 0,175), e apresentavam diferenças significativas no que tange às seguintes variáveis: tempo de internação (p = 0,000), prematuridade (p = 0,008) e tratamento com hemoderivados (p = 0,009). Todos foram submetidos à ventilação mecânica durante o período de internação. Na análise univariada, as lesões relacionadas à nutrição parenteral total foram: dano alveolar difuso (p = 0,022), fibrose pulmonar (p = 0,019), hiperplasia de pneumócitos (p = 0,004), microtromboembolismo (p = 0,047) e tromboflebite (p = 0,033). A análise multivariada, levando em consideração a prematuridade, o tempo de internação e a idade, mostrou que apenas a fibrose estava relacionada, de modo independente, ao uso da nutrição parenteral total. CONCLUSÃO: Embora as lesões pulmonares tenham sido mais freqüentes em pacientes tratados com nutrição parenteral total, não foi possível concluir que essa tenha sido diretamente responsável pela origem das lesões, tendo em vista que co-fatores como prematuridade e tempo de internação influenciaram significativamente no seu aparecimento.
Nutrição parenteral; pulmão; patologia
ORIGINAL ARTICLES
Pulmonary lesions and total parenteral nutrition in children admitted to a pediatric intensive care unit
Valmin Ramos-SilvaI; Jane S. CastelloII; Luciene L. da MottaII; Fausto E. L. PereiraIII; Norma S. OliveiraIV; Joel A. LamounierV
IPhD, Graduate Program in Children and Adolescent Healthcare (UFMG). Professor, Department of Pediatrics, Medicine School of Escola Superior de Ciências, Santa Casa de Misericórdia of Vitória, Espírito Santo, Brazil (EMESCAM). General Coordinator of the Pediatrics Residency Program, Hospital Infantil Nossa Senhora da Glória (HINSG), Vitória, ES, Brazil
IIPathologist, Pathologic Anatomy Laboratory, HINSG, Vitória, ES, Brazil
IIIPhD, Coordinator of the Graduate Program in Infectious Diseases, Universidade Federal do Espírito Santo, Vitória, ES, Brazil
IVPhD, Professor, Medicine School (EMESCAM). Scientific Coordinator of the Pediatrics Residency Program, HINSG, Vitória, ES, Brazil
VPhD, Graduate Program in Children and Adolescent Healthcare (UFMG). Professor, Pediatrics Department, Medicine School (UFMG), Belo Horizonte, MG, Brazil
Correspondence Correspondence to Valmin Ramos-Silva Rua Ulisses Sarmento, 362/20-BL6, Praia de Santa Helena CEP 29052-320 Vitória, ES, Brazil Tel.: +55 (27) 3345.5491 Fax.: +55 (27) 3315.1666 E-mail: valminramos@terra.com.br
ABSTRACT
OBJECTIVE: To describe lung injuries in autopsied pediatric patients (neonates through 15 years old) subjected or not to total parenteral nutrition, in an intensive care unit.
METHODS: Sections from six paraffin-embedded lung fragments, from 114 children were studied by routine staining. Demographic, clinical and therapeutic data were retrieved from the records. Statistical analysis was performed using Statistical Package for the Social Sciences.
RESULTS: The 114 patients were divided in two groups: 50 who were treated with total parenteral nutrition with lipid emulsion and 64 who did not receive total parenteral nutrition. The two groups did not differ in gender (p = 0.654), age (p = 0.682) or body weight (p = 0.175), but duration of hospital stay (p = 0.000), prematurity (p = 0.008) and treatment with blood products (p = 0.009) were all higher in the group treated with total parenteral nutrition. All patients received mechanical ventilation during hospitalization. Chi-square comparisons showed that diffuse alveolar injury (p = 0.022), pulmonary fibrosis (p = 0.019), pneumocyte hyperplasia (p = 0.004), microthromboembolism (p = 0.047) and thrombophlebitis (0.033) all exhibited a significant relationship with total parenteral nutrition. However, a multivariate analysis by logistic regression, taking into account prematurity and duration of hospital stay, demonstrated that total parenteral nutrition was an independent factor only with respect of pulmonary fibrosis.
CONCLUSION: Although lung injuries were significantly more frequent in children who had received total parenteral nutrition, it was impossible to conclude that the lipid infusion had a direct relationship with these injuries, because prematurity and duration of hospital stay were significant co-factors.
Key words: Parenteral nutrition, parenteral, lung, pathology.
Introduction
Several studies have suggested that the intravenous fat emulsions used for total parenteral nutrition (TPN) are associated with the genesis of some of the lung injuries suffered by critical patients, with emboli of fat in the lumen of arteries1-3 and capillaries1,2,4-7 being reported in addition to deposits in the cytoplasm of alveolar macrophages,1,2,4,7-9 alveolar epithelial cells,9 septal macrophages, bronchial cartilage chondrocytes and other interstitial cells.10
Injuries described as associated with or consequent on these fatty emboli and deposits, include nodular lesions,11 pulmonary infarct,8 granulomatous inflammatory reaction in the lumen and wall of pulmonary artery branchings,12-14 pulmonary hypertensive vascular disease with hypertrophy of small arteries and proliferation of foamy cells creating saliencies into the vascular lumen.2
A review of the literature shows that the majority of papers consists of reports of lung injuries after TPN lipid infusion, and of samples predominantly made up of newborns. Additionally, the number of cases in each of these observations was low and comparison groups were not included. Attention is drawn to the absence of systematic studies of the occurrence of lung injuries and their possible association with TPN lipid infusions. In this article the lung injuries observed in a series of autopsies of patients who died in an Intensive Care Unit will be described. The patients were aged up to 15 years and a group that received lipid infusion during total parenteral nutrition will be described together with a group that did not.
Methods
Study sample
Three hundred and one (25.7%) of the 1,172 children admitted to the Pediatric Intensive Care Unit at the Hospital Infantil Nossa Senhora da Glória in Vitória, Espírito Santo (Brazil) between January 1998 and December 2001 suffered fatal outcomes. In 114 (37.9%) of these cases permission was granted by parents for autopsy, which was performed within 12 hours of death. Of the autopsied patients, 50 had been on parenteral nutrition with lipid infusion and 64 did not receive parenteral nutrition as part of their therapy while in hospital. The study included all autopsies with no exclusion criteria.
Total parenteral nutrition
The solution contained a lipid emulsion at 20%, amino acids at 10% and micronutrients, administered intravenously in Y, continuously for 24 hours, by infusion pump, together with venous liquids composed of glucose solution, sodium chloride at 20%, potassium chloride at 10% and potassium phosphate at 10%. Calcium gluconate at 10% and magnesium sulphate were administered separately in bolus intravenously. In order to reduce the effect of lipid peroxidation by light, photosensitive equipment was used. Maximum lipid infusion for all patients was 0.17 g per kilo per hour and heparin was not added to the total parenteral nutrition solution.
The lipid emulsion used for TPN (Lipofundin®, B. Braun Melsungen AG, Germany) was composed of soy oil, long-chain triglycerides (LCT), with predominantly unsaturated fatty acids, medium-chain triglycerides (TCM) primarily caprylic acid (60%) and capric acid (40%), lecithin from eggs, glycerol, sodium oleate, a-tocopherol, linoleic acid and a-linoleic acid. The initial lipid dose was 0.5 g per kilo per day with daily increases of 1 g/kg up to a maximum of 3.5 g/kg. The laboratory test results for cholesterol and triglycerides remained within limits for normality throughout the procedure in all cases. In certain cases it was necessary to infuse the parenteral nutrition via the same line as other medications. Ninety-four children had a central venous catheter inserted.
Autopsy
Autopsies were performed at the Pathological Anatomy Laboratory of the Hospital Infantil Nossa Senhora da Glória, Vitória (ES). All were complete and for each patient, in addition to anatomopathological diagnoses, information was recorded on lung weight, characteristics and number of pulmonary lesions and presence or absence of deep venous catheter and its complications.
Six lung fragments were removed from each patient, including samples from the central portion, the parahilar area and the peripheral area. The fragments were paraffin embedded and stained with hematoxylin and eosin and, when necessary, with special methods (Gomori trichrome staining and PAS). The histopathological analysis of these fragments was performed at the Pathology Laboratory of the Hospital Infantil Nossa Senhora da Glória. The histopathological analysis procedure was reviewed by two investigators, one of them from the Infectious Diseases Research Center at the Universidade Federal do Espírito Santo.
Demographic and clinical patient data
Information was obtained from medical records on age, sex, weight, length of hospital stay, treatment and clinical diagnosis. Treatment details recorded were related to the infusion of vasoactive drugs, use of blood products and the lipid emulsion dosage. In order to calculate the lipid dose the TPN solution infusion start and end times were taken from prescription and nursing records. Since all of the patients had had more than one clinical diagnosis, the principal diagnoses were considered or the diagnoses that included other diseases. The most common were: sepsis, heart failure, acute kidney failure, asphyxia and prematurity. The diagnosis of sepsis was based on the criteria proposed by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (ACCP/SCCM, 1992),15 and modified by Hayden16 and maintained by Levy et al.17 For diagnoses of congestive heart failure acute kidney failure criteria described by Bernstein18 and Bergstein, respectively, were used.19 Perinatal asphyxia was defined using the score proposed by Apgar20 and the gestational ages of the newborns, appearing on all medical records for this sample patients, was assessed by the New Ballard Score.21
Statistical analysis
For the statistical analysis, the Statistical Package for the Social Sciences (SPSS 11.0 Inc. Chicago, IL, USA) software package was employed. Differences in proportions (frequencies) were calculated using the chi-square statistic, with correction for continuity if applicable. Continuous variables were compared with the Kolmogorov-Smirnov test and the test of adequate comparison was applied depending on type of variable distribution. Values were considered significant at p < 0.05, with all tests being two-tailed. Multivariate analysis by logistic regression was employed to test the inter-relation of co-factors with TPN in the origin of the lung injuries observed.
The study was approved by the Committee for Ethics in Research at the Universidade Federal de Minas Gerais, hearing ETIC 116/03.
Results
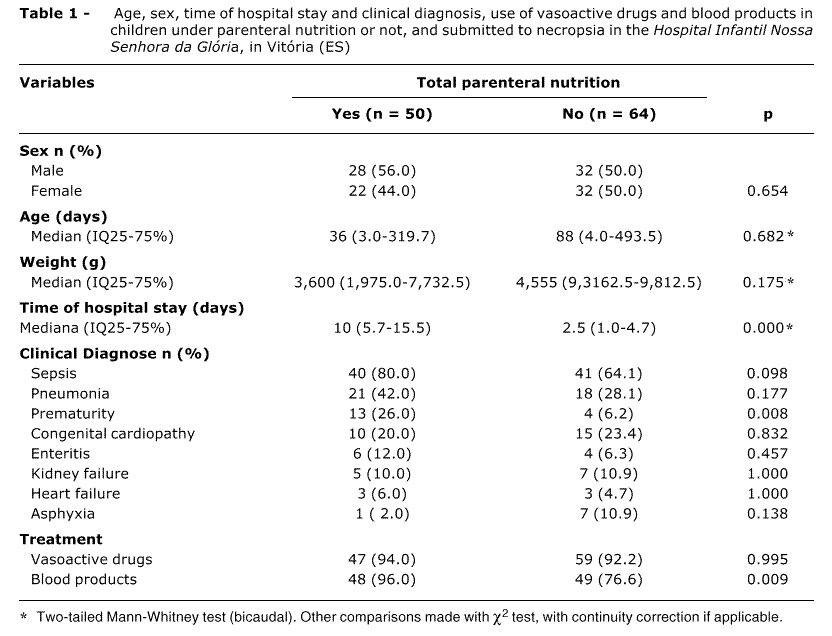
Data on age, sex, length of hospital stay, body weight, clinical diagnosis, use of vasoactive drugs and blood products for the 114 children, subjected or not to TPN, are summed up in Table 1. The groups are similar with respect of these variables, with the exception of length of hospital stay and prematurity.
All of the patients had required mechanical pulmonary ventilation with inspired oxygen fractions above 60% in order to maintain oxyhemoglobin saturation above 90%.
Lipid doses varied from 0.5 to 3.5 g/kg/day (mean: 1.95; SD: 0.89 g/kg/day; median: 2 g/kg/day) and TPN duration varied from 1 to 34 days (mean: 7.37; SD: 7.46 days; median: 5 days).
The main macroscopic injuries observed (pneumothorax, hydrothorax, pleural petechiae, serous secretion in the bronchi, ulcers in bronchial mucosa, infarcts and collapsed areas) did not differ significantly with respect of TPN use.
The principle microscopic injuries are listed in table 2, split by TPN use. Figure 1 shows some of the most relevant microscopic features of the injuries observed.
Statistically significant relationships with infusion of total parenteral nutrition were observed with interstitial pulmonary lesions compatible with diffuse alveolar damage (p = 0.022), pneumocyte hyperplasia (p = 0.004) and septal pulmonary fibrosis (p = 0.019). Acute respiratory distress syndrome (ARDS) was more common in the group that received parenteral nutrition, and the difference was at the limit of statistical significance (p = 0.053). Statistically significant relationships between TPN and vascular damage was observed for microthromboembolism (p = 0.047) and thrombophlebitis (p = 0.03). However, when a multivariate analysis by logistic regression was performed taking into account prematurity and length of hospital stay, which differed significantly between the two groups, it was shown that TPN was an independent factor only for fibrosis (Table 3).
Thrombotic damage had characteristics of recent or past formation with intravascular recanalization. Diffuse alveolar damage had identical histological characteristics to that observed in premature newborns with hyaline membrane disease or in patients who have developed acute respiratory distress syndrome. In some cases alveolar damage was focal and restricted to certain isolated areas.
In certain patients unidentified foreign bodies were observed in the intravascular space. Many vessels exhibited intraluminal or intramural calcification or this was observed adhering to the wall of the blood vessel. Granulomas with multinucleated giant cells were observed in vessel walls, close to foreign bodies and had a statistically significant relation with the period of time for which lipid emulsions were used infused in the total parenteral nutrition (p = 0.01). In five patients septic thrombophlebitis and thromboembolism were observed, caused by Candida or Staphylococcus aureus.
Discussion
Among the 114 patients in the study sample, the TPN group was made up of 22 newborns and 28 children (babies, toddlers, schoolchildren and adolescents). This is a numerically significant sample and, in addition to newborns, it contains a large number of older children. The group of 64 children who had not been given TPN included 25 newborns and 39 children (babies, toddlers, schoolchildren and adolescents). In this respect the two samples are comparable, with the same being true for age, sex, weight and clinical diagnoses. However, the samples exhibited significant differences with respect of length of hospital stay and the presence of prematurity, more common among the TPN group. These differences may constitute a bias when the results are analyzed. This does not invalidate the sample, but requires caution in analyzing certain comparisons between the results observed, even after the multivariate analysis by logistic regression has been performed taking account of those variables with significant differences between the groups.
The frequency of lung injuries observed macroscopically did not have any significant difference between the TPN and no TPN groups. This observation is to be expected since the injuries observed were those that are found in critical patients with systemic inflammatory syndromes. The lipid infusion does not appear to cause any injuries with specific macroscopic characteristics. Harman & Ragaz22, in experiments with rabbits subjected to severe dehydration reported: petechial hemorrhages, hemorrhagic suffusions in the pleura and pulmonary parenchyma, septal pulmonary fibrosis and atelectasis after infusion of homologous fats. These injuries are observed in the lungs of patients with ARDS, especially if the patient remains in the intensive care unit for a long time.
The microscopic alterations observed were those described in classic ARDS, which accompanies shock and systemic inflammation. Nevertheless, certain injuries were significantly more common in the TPN group when the comparison took account only of the use of TPN. Nevertheless, it became obvious by means of multivariate analysis that prematurity and length of hospital stay were important factors in the origin of these injuries. Only fibrosis was independently related to TPN.
The use of mechanical pulmonary ventilation and of oxygen at concentrations above 60% for ventilation are factors that aggravate these injuries.23,24 However, all of the patients had received these treatments when in hospital and the medical records did not contain the ventilation period before the children were transferred to the unit. As length of hospital stay was significantly greater for the TPN group and since all patients were put on mechanical ventilation while in the unit, without doubt the TPN group were ventilated for longer even without knowing the duration before admission.
The prevalence of microthromboembolism was 36.8% and significantly greater in the group that had received total parenteral nutrition (48%; p = 0.047). This is probably the first report on the prevalence of pulmonary microthromboembolism in children admitted to an Intensive Care Unit. The elevated prevalence of pulmonary microthromboembolism in this sample could be related to : (a) the clinical condition that led to the child receiving intensive care, especially sepsis; (b) interactions between drugs and parenteral nutrition, encouraging lipid precipitation; (c) trauma from external heart massage, leading to embolization of bone marrow; and (d) the presence of deep venous catheters.
It is probable that sepsis was not a significant factor in the frequency of thromboembolism among the patients given TPN. In fact the frequency of thromboembolism for these patients did not differ significantly between the subsets with or without sepsis (data not shown).
Septic emboli with bacterial or fungal colonization (Candida) were observed in five patients, all in the TPN group. The endothelial lesions induced by lipids infused with the parenteral nutrition can encourage both thrombosis and colonization by microorganisms.
The formation of fatty microemboli may encourage the formation of thrombi, which may explain, in part, the greater frequency of microthromboembolism observed in the patients who had received TPN. In vivo experimental studies of perfused rat lungs have demonstrated such endothelial damage induced by the intravenous infusion of lipids.25,26 Another study has demonstrated an increase in thromboxane production in the lungs of patients who received lipid infusion in TPN, which favors thrombosis.27
There are other aspects of the present study that should also be taken into account. All of the patients were submitted to treatment with multiple drugs with the potential for interaction with the lipids of the infusion encouraging their aggregation and thrombotic potential.28 Cardiac massage was used with all patients and is also an important factor in the genesis of emboli represented by fragments of myeloid tissue.29 It was not possible to characterize the presence of bone marrow embolus in the lung fragments examined from the thromboembolism cases.
Intravenous catheters are considered an important risk factor for pulmonary embolism.30,31 In the sample studied, the frequency of thromboembolism was not significantly related with the use of deep venous catheters, even in the TPN group (thromboembolism present in 35/94 with venous catheter and 7/20 without venous catheter; p = 0.533).
Intrapulmonary megakaryocytes were common (15.8% of 114 autopsies), but there was no significant difference between the TPN and no TPN groups (p = 0.838). These cells are found in normal lungs and intrapulmonary migration may have occurred after an insult.32 They can also be found in association with microthromboembolism, ARDS and disseminated intravascular coagulation.33 The statistical analysis showed that these associations were not significant in the present study.
In conclusion, the observations made of the study sample demonstrate that although certain lung injuries were more frequent in the group treated with total parenteral nutrition, the multivariate analysis showed that TPN, in isolation, does not appear to have a direct relation with these injuries.
References
Manuscript received Aug 25 2004, accepted for publication Nov 24 2004.
- 1. Barson AJ, Chiswick ML, Doing CM. Fat embolism in infancy alters intravenous fat infusions. Arch Dis Child. 1978;53:218-23.
- 2. Dahms BB, Halpin TC. Pulmonary arterial lipid deposit in newborn infants receiving intravenous lipid infusion. J Pediatr. 1980;97:800-05.
- 3. Hulman G, Levene MI. Intralipid microemboli. Arch Dis Child. 1986;61:702-03.
- 4. Friedman Z, Marks KH, Maisels MJ, Thorson R, Naeye R. Effect of parenteral fat emulsion on the pulmonary and reticuloendotelial systems in the newborn infant. Pediatrics. 1978;61:694-98.
- 5. Hertel J, Tygstrup I, Andersen GE. Intravascular fat accumulation after Intralipid infusion in the low-birth-weight infant. J Pediatr. 1982;100:975-76.
- 6. Levene MI, Batisti O, Wigglesworth JS, Desai R, Meek JH, Bulusu S, Hughes E. A prospective study of intrapulmonary fat accumulation in the newborn lung following intralipid infusion. Acta Paediatr Scand. 1984;73:454-60.
- 7. Puntis JW, Rushton DI. Pulmonary intravascular lipid in neonatal necropsy specimens. Arch Dis Child. 1991;66:26-28.
- 8. Levene MI, Wigglesworth JS, Desai R. Pulmonary fat accumulation after intralipid infusion in the preterm infant. Lancet. 1980;18:815-19.
- 9. Schröder H, Paust H, Schmidt R. Pulmonary fat embolism after intralipid therapy a post-mortem artifact?. Acta Paediatr Scand. 1984;73:461-64.
- 10. Shulman RJ, Langston C, Schanler RJ. Pulmonary vascular lipid deposition after administration of intravenous fat to infants. Pediatrics. 1987;79:99-102.
- 11. Landry BA, Melhem RE. Pulmonary nodules secondary to total parenteral alimentation. Pediatr Radiol. 1989;19:456-57.
- 12. Nikiforov IuE. [A rare complication of parenteral fat nutrition in a child.] Arkh Patol. 1990;52:56-9.
- 13. Puntis JW, Wilkins KM, Ball PA, Rushton DI, Booth IW. Hazards of parenteral treatment: do particles count? Arch Dis Child. 1992;67:1475-77.
- 14. Aksnes TA, Foerster A, Hovig T, Schmidith H, Nordstran K. Development of granulomas and vascular fibrocellular proliferations in the lungs of pigs receiving long-term lipid-based parenteral nutrition. APMIS. 1994;102:623-32.
- 15. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-74.
- 16. Hayden WR. Sepsis terminology in pediatrics. J Pediatr. 1994;124:657-58.
- 17. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001, SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Int Care Med. 2003;31:1250-56.
- 18. Bergstein JM. Acute renal failure. In Behrman RE, Kliegman RM, Arvin, AM. Nelson textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996. p. 1515-22.
- 19. Bernstein D. Congestive heart failure. In Behrman RE, Kliegman RM, Arvin, AM. Nelson textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996. p. 1359-63.
- 20. Apgar, V. The newborn scoring system. Pediatr Clin North Am. 1966;13:645-50.
- 21. Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. J Pediatr. 1991;119:417-23.
- 22. Harman J, Ragaz FJ. The pathogenesis of experimental fat embolism. Am J Path. 1950;26:551-63.
- 23. Yazdy AM, Tomashefski JF, Yagan R, Kleinerman J. Regional Alveolar Damage (RAD). A localized counterpart of diffuse alveolar damage. Am J Clin Pathol. 1989;92:10-15.
- 24. Sevit S. Diffuse and focal oxygen pneumonitis, a preliminary report on the threshold of pulmonary oxygen toxicity in man. J Clin Pathol. 1974;27:21-30.
- 25. Aksnes J, Eide TJ, Nordstrand K. Pulmonary intravascular macrophages appear in rats after long-term administration of lipid emulsion and amino acid solution. APMIS. 1998;106:687-692.
- 26. Cukier C. Waitzberg, Logullo AF. Bacchi CE, Travassos VH, Torrinhas RSM, Soares SRC, Saldiva PH, Oliveira TS, Heymsfield S. Lipid and lipid-free total parenteral nutrition: Differential effects on macrophage phagocytosis in rats. Nutrition. 1999;15:885-89.
- 27. Lekka ME, Liokatis S, Nathanail C, Galani V, Nakos G. The impact of intravenous fat emulsion administration in acute lung injury. Am J Resp Crit Car Med. 2004;169:639-44.
- 28. Restler CS. Preparo e compatibilidade de drogas na terapia nutricional parenteral. In Waitzberg DL. Nutrição oral, enteral e parenteral na prática clínica. 3Ş ed. São Paulo: Atheneu; 2000. p. 875-95.
- 29. Hulman G. The pathogenesis of fat embolism. J Pathol. 1995;176:3-9.
- 30. Findling R, Lipper B. Femoral vein pulmonary artery catheterization in the intensive care unit. Chest. 1994;105:874-77.
- 31. Krafte-Jacobs B, Sivit CJ, Mejia R, Pollack MM. Catheter-related thrombosis in critically ill children: Comparison of catheters with and with heparin bonding. J Pediatr. 1995;126:50-54.
- 32. Dehring DJ, Wismar BL. Intravascular macrophages in pulmonary capillaries of humans. Am Rev Resp Dis. 1989;139:1027-29.
- 33. Wells S, Sissons M, Hasleton PS. Quantitation of pulmonary megakaryocytes and fibrin thrombi in patients dying from burns. Histopathology. 1984;8:517-27.
Correspondence to
Publication Dates
-
Publication in this collection
30 July 2005 -
Date of issue
Apr 2005
History
-
Accepted
24 Nov 2004 -
Received
25 Aug 2004