Abstracts
OBJECTIVES: To report on the pharmacology, efficacy and safety of omalizumab , a new option for the treatment of asthma and allergic diseases and the first monoclonal anti-IgE antibody approved for clinical use. SOURCES: MEDLINE, a non-systematic search including reviews and original papers, chosen according to their relevance in the authors' opinion. SUMMARY OF THE FINDINGS: The paper emphasizes the central role IgE plays in allergic diseases and the biological rationale for its use, the evidence upon which the current recommendations for the use of anti-IgE in uncontrolled asthma are based and its possible future applications, in addition to the recommendation that in clinical practice doses must be adjusted for weight and serum IgE levels. Omalizumab was approved in Brazil for patients with severe uncontrolled asthma presenting with a positive skin prick test for one or more relevant aeroallergen, or IgE specific to a relevant allergen detected in serum, having a total IgE level of between 30 and 700 UI/mL. For the time being its use should be restricted to patients aged 12 years or more, but there are prospects that it will be licensed for use with children over 6 years old. CONCLUSIONS: Some severe asthma cases cannot be controlled with the regular treatment options aimed at preventing symptoms and exacerbations, and so require frequent or prolonged use of systemic corticosteroids. These patients may benefit from treatment with anti-IgE, after a meticulous reevaluation of possible reasons for the failure to control asthma.
Omalizumab; anti-IgE; asthma; severe asthma; allergic rhinitis; atopic dermatitis
OBJETIVO: Descrever as características farmacológicas, a eficácia e a segurança do omalizumabe, o primeiro anticorpo monoclonal anti-IgE aprovado para uso clínico, uma nova opção de tratamento da asma e das doenças alérgicas. FONTES DE DADOS: Pesquisa não sistemática na MEDLINE. Os principais artigos de revisão ou ensaios clínicos foram escolhidos com base em sua relevância segundo a opinião dos autores. SÍNTESE DOS DADOS: O artigo destaca o importante papel da IgE na patogênese da doença alérgica, a lógica biológica para o uso da anti-IgE, as evidências que definiram a sua indicação atual na asma não controlada e possíveis indicações futuras, bem como as recomendações para uso clínico com doses ajustadas pelo peso e níveis séricos de IgE. O omalizumabe foi aprovado para uso em pacientes com asma grave não controlada que apresentem teste cutâneo positivo a pelo menos um aeroalérgeno relevante ou que apresentem IgE sérica alérgeno-específica para alérgenos relevantes, e cujo nível de IgE total esteja entre 30 e 700 UI/mL. Por enquanto, o uso deve ser restrito a pacientes maiores de 12 anos, mas é possível que a droga seja aprovada para uso a partir dos 6 anos de idade. CONCLUSÕES: Em alguns pacientes, a asma grave não é controlada com as opções de tratamento disponíveis para prevenção de sintomas e exacerbações, exigindo o uso freqüente ou prolongado de corticosteróides sistêmicos. Esses pacientes poderiam se beneficiar de tratamento com anti-IgE depois de reavaliação meticulosa das possíveis razões para a falta de controle da sua asma.
Omalizumabe; anti-IgE; asma; asma grave; rinite alérgica; dermatite atópica
REVIEW ARTICLE
Anti-IgE monoclonal antibody for the treatment of asthma and other manifestations related to allergic diseases
Emanuel SarinhoI; Álvaro Antonio CruzII
IProfessor adjunto, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brasil
IIProfessor adjunto, Departamento de Clínica Médica, Faculdade de Medicina, Universidade Federal da Bahia (UFBA), Salvador, BA, Brasil
Correspondence Correspondence: Emanuel Sarinho Av. Parnamirim, 327/202 - Parnamirim CEP 52060-000 - Recife, PE - Brazil Tel.: +55 (81) 3441.4801/9994.1144 E-mail: emanuel.sarinho@gmail.com
ABSTRACT
OBJECTIVES: To report on the pharmacology, efficacy and safety of omalizumab , a new option for the treatment of asthma and allergic diseases and the first monoclonal anti-IgE antibody approved for clinical use.
SOURCES: MEDLINE, a non-systematic search including reviews and original papers, chosen according to their relevance in the authors' opinion.
SUMMARY OF THE FINDINGS: The paper emphasizes the central role IgE plays in allergic diseases and the biological rationale for its use, the evidence upon which the current recommendations for the use of anti-IgE in uncontrolled asthma are based and its possible future applications, in addition to the recommendation that in clinical practice doses must be adjusted for weight and serum IgE levels. Omalizumab was approved in Brazil for patients with severe uncontrolled asthma presenting with a positive skin prick test for one or more relevant aeroallergen, or IgE specific to a relevant allergen detected in serum, having a total IgE level of between 30 and 700 UI/mL. For the time being its use should be restricted to patients aged 12 years or more, but there are prospects that it will be licensed for use with children over 6 years old.
CONCLUSIONS: Some severe asthma cases cannot be controlled with the regular treatment options aimed at preventing symptoms and exacerbations, and so require frequent or prolonged use of systemic corticosteroids. These patients may benefit from treatment with anti-IgE, after a meticulous reevaluation of possible reasons for the failure to control asthma.
Key words: Omalizumab, anti-IgE, asthma, severe asthma, allergic rhinitis, atopic dermatitis.
Introduction
Immunoglobulin E (IgE) is the key molecule in immediate allergic reactions. It was identified in 1966, when Ishizaka confirmed that the reagin involved in anaphylactic reactions was, in fact, an immunoglobulin, IgE. The delay in making this discovery is due to the fact that IgE is a cytophilic antibody with serum concentrations so low that they were not detectable by the techniques available at the time.1,2
After allergen exposure, during the sensitization phase, IgE can be detected in serum soon after it is synthesized by plasmacytes, but the greatest concentration is to be found fixed in basophils and mast cells by high-affinity receptors (FceRI). These receptors capture IgE by means of the third domain of the heavy chain constant region (CH3). When contact with the antigen occurs once more, the effector phase begins, with the formation of a bridge that permits a bivalent bond between two immunoglobulin E molecules and the allergen. This process triggers cellular degranulation, with liberation of countless preformed mediators and cytokines.2
Even though IgE has been known about for more than 30 years, its natural function in biological terms remains a mystery. It is known that, in several types of helminthiasis, serum IgE titers are extremely elevated; however there are doubts whether this increase reflects a role in the defense against intestinal worms or whether it is simply an escape mechanism used by the parasites, originating from interleukin 4 overproduction (IL4) with a resultant polyclonal nonfunctional increase in IgE.3,4 Despite doubts about the true biological function of IgE in relation to the pathological process of the allergy, this immunoglobulin is recognized as the central inductive factor in the anaphylactic process. Therefore, neutralization or inhibition of IgE synthesis could be a rational option for the treatment of allergic diseases.1,2,5
One wish of researchers working on the subject has been to employ an anti-IgE, since there is an evident biological rationale for this: it would act before symptoms appear, providing a preventative action prior to the inflammatory process and a direct action against the target of the allergic process.2,6 As the concept of an anti-IgE matured, an antibody was synthesized in laboratory that has does not trigger the allergenic inflammatory process.7 The molecule synthesized was to exhibit high affinity for both serum IgE and recently-synthesized IgE, which is still fixed in the lymphocyte B membrane. On the other hand it had to be inactive with respect to IgE bonded to high-affinity receptors (FceRI) fixed in the surface of basophil and mast cell membranes, and also should not recognize IgE bonded to low-affinity receptors (FceRII or CD23), which are abundantly distributed across several different types of cell.8-11 Tests with laboratory animals and clinical trials corroborated these properties, which had been established as goals for the molecular engineering that resulted in the synthesis of omalizumab.9-11
Omalizumab, as a nonanaphylactic synthetic antibody, represents a new class of medicine for the treatment of allergies: monoclonal immunomodulators. Licensed for clinical use, omalizumab is an anti-IgE antibody that acts to inhibit IgE from bonding with the high-affinity receptor on mast cells and basophils right from the initial sensitization phase of the allergic response, in addition to blocking the immunoglobulin from binding with the low-affinity receptor in B lymphocytes and several other cell types.7,9-13
Those high-affinity receptors that are not occupied then reduce, by means of a feedback mechanism, their own synthesis, meaning that over time the mast cells become less available for IgE-mediated degranulation. Those B lymphocytes that have membrane IgE blocked by the synthetic antibody then exhibit an accelerated process of apoptosis (programmed cell death). Therefore, the objectives of omalizumab are to block IgE in lymphocyte membranes and capture free IgE in serum. These objectives are achieved with practically no risk of anaphylactic shock, since functional deactivation of both high and low-affinity receptors (CD23) takes place and it is these receptors that are responsible for the biological action of immunoglobulin E.
These effects have already been demonstrated experimentally by the reduction of total free IgE in serum, by reduced expression of high-affinity receptors in mast cells and the reduction of B lymphocytes expressing IgE in the cell membrane. Furthermore, the site where omalizumab bonds with the IgE molecule is exactly the point where IgE would bind with cell receptors.14,15
Blocking IgE with omalizumab in allergic patients with severe asthma that cannot be controlled with inhaled corticosteroids and long-acting bronchodilators can result in detectable improvements in clinical status.16,17
Characteristics of omalizumab - the first Anti-IgE in clinical use
Several types of anti-IgE antibody have been synthesized, but the one that has been licensed for clinical use is omalizumab, which was cloned in 1992.10
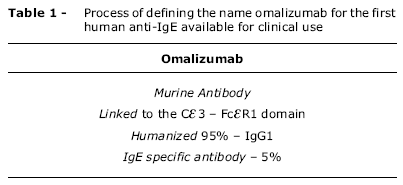
Table 1 explains didactically the reasons why the anti-IgE antibody available for clinical use is called omalizumab. Ninety-five percent of the molecule's structure is a humanized IG1 antibody. To this are joined 5% of a murine antibody which works as an epitope against the fraction of the IgE Ce3 domain, which is the part of human immunoglobulin that bonds with the high-affinity receptor of mast cells and basophils.18
Since omalizumab is directed against the Ce3 domain in the heavy chain constant region that exists in all IgE molecules, it binds nonspecifically to any and all soluble IgE that is present in the body, and this bond causes the formation of soluble compounds that are eliminated without activating the complement.11 Therefore, it can be stated that omalizumab is an immunoglobulin of the IgG1 isotype that functions as a nonspecific IgE blocker, with the following characteristics: it binds with IgE in serum, inhibiting IgE from bonding with high-affinity receptors, but does not act upon IgE that is already bonded to mast cells, which avoids degranulation and activation of the complement. When omalizumab bonds with free IgE, it forms small inert immunocompounds bereft of any biological action whatsoever, which do not fix the complement and are eliminated by the reticuloendothelial system.12-15
Although it bonds with IgE free in serum, omalizumab does not recognize IgA, IgG or IgM, and neither does it act on IgE that is already fixed in mast cells or basophils. There is, therefore, no risk of degranulation. There is a possibility that omalizumab, by means of a feedback mechanism, reduces production of high-affinity IgE receptors.14
During its preclinical phase, omalizumab was demonstrated to be safe, to have side effects comparable with placebo, and antibodies against anti-IgE were not produced.14 Pharmacokinetic studies with human beings demonstrated that there is no need to adjust dosage for sex, race or age in patients over 12 years old.19,20 After the product is administered, there is an increase in total serum IgE levels due to the delay in eliminating IgE-omalizumab immunocompounds. Nevertheless, total free IgE levels in serum drop dramatically in more than 96% of cases 1 hour after the medication is given. These levels are maintained with continued application of the product.10
In human beings, the product is very well tolerated by the majority of patients, permitting an accentuated drop in total free serum IgE concentration for 4 to 6 weeks, due to its extended half-life with a single subcutaneous injection. This is explained by the fact that anti-IgE is an immunoglobulin of the IgG1 isotype, which is eliminated slowly by the cells of the reticuloendothelial system in the hepatic sinusoids hepatic sinusoids.10,12,13,15 Clinical studies of prolonged use demonstrate that there is a reduction in the number of high-affinity receptors on the surface of basophils, in addition to reductions in expression of low-affinity receptors in other inflammatory cells.21
Certain precautions should be taken with patients on omalizumab. It should be stressed that the product is not indicated for acute episodes of asthma, and even when response to the product is good, untimely withdrawal of corticoids should be avoided. It is important to remember that the physician should employ the correct dose (0.016 mg/kg/level of total IgE in UI/mL), as recommended in Table 2, for the treatment to be effective. Among the countless variables studied, the weight of the patient and initial total IgE level were the most important factors for defining the omalizumab dose needed to achieve response to the treatment.10 It may take up to 12 to 16 weeks for the clinical effect of the medication to be observed. After this period, patients who do not exhibit improvement in symptoms and quality of life should seek another therapeutic option. Studies to evaluate omalizumab in patients under 12 years old are currently ongoing.10-12,15
The effect of omalizumab correlated with total free IgE dropping to below 50 ng/mL. The goal of treatment is to obtain an omalizumab dose, based on the level of total IgE, which is sufficient to reduce the level of total free IgE to around 25 ng/mL in more than 95% of the patients.10,21 omalizumab also improves the efficacy and safety of immunotherapy. It is possible that in the future, it will be used in association with that approach to promote improved clinical tolerance and advances in the time of this type of treatment, both for asthma and allergic rhinitis.12,22,23
Clinical use of omalizumab has raised certain questions that have not yet been completely elucidated. Although the reduction in total free IgE is followed by rapid improvement in some patients, in others clinical improvement is delayed, or, sometimes does not occur, even when total free IgE is reduced to zero.22-24
Use in severe asthma
Removal of IgE from circulation, blocking it from fixing to low and high-affinity receptors in mast cells and basophils, represents one more step in the treatment of asthma and other IgE-mediated diseases.10,20-22,24
Omalizumab was developed to treat allergic disease, and offers recognized efficacy for the treatment of moderate and severe asthma and for the treatment of seasonal or perennial treatment. However, the currently established recommendation is for severe cases of refractory asthma.12,15,22 omalizumab inhibits the early and late phases of the allergen-induced asthmatic and prevents the development of eosinophilia and bronchial hyperreactivity.12
Studies conducted on patients with moderate and severe asthma demonstrated a reduction in corticosteroid use and in asthma exacerbations when omalizumab was given intravenously.25-27 Other studies that used subcutaneous omalizumab at the Standard dose of 0.016 mg/kg per UI/mL of serum IgE every 4 weeks also demonstrated reductions in inhaled corticosteroid use, in symptoms and in the use of emergency medication.28-30 In three further studies, the frequency of a asthma exacerbations, defined as increase in inhaled corticosteroid dose or treatment with oral or intravenous corticosteroid, reduced significantly when compared with placebo groups. The same studies further reported reductions in the use of emergency medication and improved quality of life.31-33 omalizumab has consistently been related to improvements in severe uncontrolled asthma patients, both in terms of asthma symptoms, with improved tolerance of exposure to the environment, and also in terms of daily activity and emotional wellbeing. A study that used subcutaneous omalizumab with severe asthma sufferers on high dose inhaled corticosteroids associated with long acting bronchodilators (LABA), demonstrated reductions in episodes of exacerbation, severity of exacerbations and emergency room visits.34
Holgate et al.,24 in a double-blind, randomized, placebo controlled study, investigated patients with severe asthma, on high dose inhaled corticosteroids. In the group that used omalizumab there was a significant reduction in inhaled steroid use, in acute exacerbations, emergency bronchodilator consumption and evident improvements in quality of life.
The efficacy of omalizumab has also been investigated with patients suffering from severe asthma and on oral corticosteroids with anti-leukotrienes and LABA. Omalizumab significantly reduced deterioration, thus demonstrating that associating this monoclonal antibody to the standard treatment of patients with severe asthma and poor control of symptoms can be of real benefit.17
A clinical trial involving 1,405 patients with moderate and severe asthma demonstrated that anti-IgE reduced the rate of severe exacerbations and reduced emergency service usage and hospitalizations.35 According to a systematic Cochrane review, omalizumab was capable of exhibiting a reductive effect on steroid use, but that the advantage of that effect should be evaluated in terms of its cost/benefit ratio. Further studies into this aspect are needed, especially ones including pediatric patients.36
Currently, omalizumab is licensed in the United States for patients over 12 years old with persistent moderate or severe asthma who have a positive skin test or in vitro test for an aeroallergen, with poor control using inhaled steroids, and whose total IgE in serum is from 30 to 700 UI/mL.13,21,37 In the European Community and Brazil, approval is limited to the treatment of severe asthma.
Use for allergic rhinitis
It has been demonstrated that systemic treatment with omalizumab reduced symptoms and caused improved quality of life in patients with moderate/severe allergic rhinitis.38 Studies conducted by Plewako et al.39 with patients with seasonal allergic rhinitis demonstrated reduced eosinophil counts in blood and nasal mucosa during the pollen season. This data correlated with levels of free IgE. Chervinsky et al. studied40 patients with moderate/severe allergic rhinitis and demonstrated significant improvements in symptoms, with reduced antihistamine consumption for exacerbation control and improved quality of life.
Therefore, omalizumab has been shown to be effective for the reduction of symptoms and improvement of the quality of life of patients with perennial or seasonal allergic rhinitis, representing one more possibility in the therapeutic arsenal available to treat allergic rhinitis, either in isolation or associated with immunotherapy.41,42
Use with allergic skin diseases
Omalizumab has not yet been adequately evaluated for the treatment of allergic skin diseases. The majority of patients with atopic dermatitis have a family history of atopic disease and a good proportion of them exhibit elevated serum IgE levels and also positive immediate sensitivity or specific IgE in serum test for food or airborne antigens. Clinical and experimental data suggest that IgE is an active participant in the pathogenesis of atopic dermatitis, acute urticaria and angioedema and in some cases chronic urticaria with auto-antibodies.43 It has already been demonstrated that the IgE-mediated immunoresponse promotes the inflammatory process in skin. In atopic dermatitis, bonding between IgE and Langerhans cells causes liberation of Th2 cytokines with a consequent inflammatory process. There was improvement in recalcitrant atopic dermatitis lesions in patients given omalizumab, showing that this medication could be useful for atopic dermatitis.44,45 Nevertheless, these patients tend to have very high IgE levels, limiting use of omalizumab. Controlled trials are needed to establish the efficacy and a tolerability of omalizumab for atopic dermatitis and other allergic skin conditions.
Use for anaphylactic shock and food allergy
There have been no studies of the applicability of omalizumab in cases of IgE-mediated food intolerance reactions. However, studies using a different anti-IgE, administered experimentally to humans, found increased tolerance of peanuts in patients subject to anaphylactic reactions to the food, which could prevent severe reactions in cases of accidental ingestion.46 This could be an additional indication for omalizumab if the same property can be confirmed, since it is the anti-IgE that is approved for sale and use with human beings.
Safety of the medication in children
Despite the advances in diagnosis and therapy during recent years, the prevalence of asthma in children has increased significantly. In a double-blind, placebo controlled study of patients aged 6 to 12 years of age and on regular inhaled corticosteroids and short-action bronchodilators, omalizumab used at the standard dose of 0.016mg/kg/IgE(UI/mL) every 4 weeks led to a reduction in inhaled corticosteroid use and also in total free IgE in serum levels.47 The most common adverse events associated with omalizumab were headaches and upper airway infections, which occurred with comparable frequency among the children in the placebo group. Urticaria, generally not severe, also occurred. One patient exhibited severe urticaria, but a causal link with omalizumab was not confirmed. The study demonstrated the efficacy and tolerability of omalizumab in this group of patients, suggesting, therefore, that the drug could offer a new treatment option for juvenile allergic asthma.47 Research has demonstrated the beneficial effects of omalizumab in severe childhood asthma, with improvements in inflammatory mediators and in quality of life.48-50 Nevertheless, further studies are needed to increase the number of children observed.
Tolerability
The most frequently observed adverse events in studies with omalizumab were headaches, affecting from 1 to 10% of patients, and local reactions at the injection site, such as pain, edema, erythema and itching. Uncommon events, occurring in less than 1% of patients, were weight gain, postural hypotension, dizziness, somnolence, paresthesia, pharyngitis, paradoxical bronchospasm, nausea, diarrhea, dyspepsia and cutaneous effects such as reddening, urticaria, exanthema, itching, edema of lower members and photosensitization.51 The possibility of parasitic infections and anaphylactic shock were considered rare events, with frequencies of less than 1 in every 1,000 patients who used the product.52
Anaphylactic reactions that have been reported with omalizumab are rare and, in general, take place within the first 30 minutes after administration, preceded by urticaria and/or edema of the tongue or glottis. For this reason it is recommended that patients are kept under observation after administration of the medication. In cases of hypersensitivity reaction, therapeutic measures should be promptly taken and treatment with the product suspended. A study carried out by Cruz et al. in Brazil found that, among 139 children and young adults with a high risk of geo-helminthiasis followed for 1 year, although there was a tendency towards an increased number of helminthic infestations among those treated with omalizumab, the occurrence of clinical manifestations of adverse events related to the product was comparable with that observed for the placebo group, with the exception of local symptomology at the administration site.53
Conclusions
Asthma is a disease with multiple causes. For patients whose disease is predominantly mediated by IgE, there is a plausible biological rationale for believing that treatment with omalizumab will be beneficial. In practice, current indications are restricted to patients with severe asthma who do not respond well to the usual treatment agents, which makes the choice of candidates complex. Asthmatic patients whose conditions are predominantly allergic, with deterioration of pulmonary function parameters and history of frequent exacerbations, and who cannot be controlled with the treatment options available to prevent symptoms and exacerbations, making frequent or prolonged systemic corticosteroids necessary, could benefit from treatment with anti-IgE, after meticulous reevaluation of possible reasons for the failure to control their asthma.54
References
- 1. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459-65.
- 2. Infuhr D, Crameri R, Lamers R, Achatz G. Molecular and cellular targets of anti-IgE antibodies. Allergy. 2005;60:977-85.
- 3. Cooper PJ. Intestinal worms and human allergy. Parasite Immunol. 2004;26:455-67.
- 4. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites and the hygiene hypothesis. Science. 2002;296:490-4
- 5. Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005; 35:408-16.
- 6. Corry DB, Kheradmand F. Control of allergic airway inflammation through immunomodulation. J Allergy Clin Immunol. 2006;117(2 Suppl Mini-Primer):S461-4.
- 7. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184-90.
- 8. Berger WE. What is new in antiimmunoglobulin E asthma therapy. Allergy Asthma Proc. 2005;26:428-34.
- 9. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354: 2689-95.
- 10. Hochhaus G, Brookman L, Fox H, Johnson C, Matthews J, Ren S, Deniz Y. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003;19:491-8.
- 11. Soresi S, Togias A. Mechanisms of action of anti-immunoglobulin E therapy. Allergy Asthma Proc. 2006;27(2 Suppl 1):S15-23.
- 12. Brownell J, Casale TB. Anti-IgE therapy. Immunol Allergy Clin North Am. 2004;24:551-68.
- 13. Marcus P; Practice Management Committee, American College of Chest Physicians Incorporating anti-IgE (omalizumab) therapy into pulmonary medicine practice: practice management implications. Chest. 2006;129:466-74.
- 14. Wagelie-Steffen AL, Kavanaugh AF, Wasserman SI. Biologic therapies for the treatment of asthma. Clin Chest Med. 2006;27:133-47.
- 15. Clark J, Chiang D, Casale TB. Omalizumab in the treatment of allergic respiratory disease. J Asthma. 2006;43:87-93.
- 16. Huang YC, Leyko B, Frieri M. Effects of omalizumab and budesonide on markers of inflammation in human bronchial epithelial cells. Ann Allergy Asthma Immunol. 2005;95:443-51.
- 17. Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy: (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309-16
- 18. Rolinck-Werninghaus C, Wahn U, Hamelmann E. Anti-IgE therapy in allergic asthma. current drug targets. Inflamm Allergy. 2005;4:551-64.
- 19. Chang TW. The pharmacological basis of anti-IgE therapy. Nat Biotechnol. 2000;18:158-62.
- 20. Belliveau PP. Omalizumab: a monoclonal anti-IgE antibody. Med Gen Med. 2005;7:27.
- 21. Lanier BQ. Unanswered questions and warnings involving anti-immunoglobulin E therapy based on 2-year observation of clinical experience. Allergy Asthma Proc. 2005;26:435-9.
- 22. Clark J, Chiang D, Casale TB. Omalizumab in the treatment of allergic respiratory disease. J Asthma. 2006;43:87-93.
- 23. Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, et al; Immune Tolerance Network Group. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117:134-40.
- 24. Holgate ST, Holloway J, Wilson S, Howarth PH, Haitchi HM, Babu S, et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006;117:496-506.
- 25. Milgrom H, Fick RBJr, Su JQ, Reimann JD, Bush RK, Wautrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody rhuMAb-E25 Study Group. N Engl J Med. 1999;341:1966-73.
- 26. Jonkers RE, van der Zee JS. Anti-IgE and other new immunomodulation-based therapies for allergic asthma. Neth J Med 2005;63:121-8.
- 27. Buhl R. Omalizumab (Xolair®) improves quality of life in adult patients with allergic asthma: a review. Respir Med. 2003;97:123-9.
- 28. Soler M, Matz J, Townley R, Buhl H, O'Brien J, Fox H, et al. The IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254-61.
- 29. Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
- 30. Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59:701-8.
- 31. Buhl R, Hanf G, Soler M, Bensch G, Wolfe J, Everhard F, et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J. 2002;20:1088-94.
- 32. Lemanske RF, Nayak A, McAlary M, Everhard F, Fowler-Taylor, Gupta N. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics. 2002;110:E55.
- 33. Finn A, Gross G, van Bavel J, Lee T, Windom H, Everhard F, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111:278-84.
- 34. Niebauer K, Dewilde S, Fox-Rushby J, Revicki DA. Impact of omalizumab on quality-of-life outcomes in patients with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol. 2006;96:316-26.
- 35. Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. 2003;111:87-90.
- 36. Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti-IgE for chronic asthma in adults and children (Cochrane Review). In: The Cochrane Library, Issue 4, 2004.
- 37. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378-86.
- 38. Rolinck-Werninghaus C, Hamelmann E, Keil T, Kulig M, Koetz K, Gestner B, et al The co-seasonal application of anti-IgE after preseasonal specific immunotherapy decreases ocular and nasal symptom scores and rescue medication use in grass pollen allergic children. Allergy. 2004;59:973-9.
- 39. Plewako H, Arvidsson M, Petruson K, Oancea I, Holmberg K, Adelroth E, et al. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2002;110:68-71.
- 40. Chervinsky P, Casale T, Townley R, Triphaty I, Hedgecock S, Fowler-Taylor A, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91:160-7.
- 41. Poole JA, Matangkasombut P, Rosenwasser LJ. Targeting the IgE molecule in allergic and asthmatic diseases: review of the IgE molecule and clinical efficacy. J Allergy Clin Immunol. 2005;115:S376-85.
- 42. Hamelmann E, Rolinck-Werninghaus C, Wahn U. Is there a role for anti-IgE in combination with specific allergen immunotherapy? Curr Opin Allergy Clin Immunol. 2003;3:501-10.
- 43. Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G, Woisetschlager M, et al. Serum IgG antibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2006-12.
- 44. Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54:68-72
- 45. Vigo PG, Girgis KR, Pfuetze BL, Critchlow ME, Fisher J, Hussain I. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55:168-70
- 46. Leung DY, Sampson HA, Yunginger JW, Burks AW Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986-93
- 47. Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004;113:776-82.
- 48. Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003;91:182-8.
- 49. Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, Mc Alary M, at al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
- 50. Silkoff PE, Romero FA, Gupta N, Townley RG, Milgrom H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics. 2004;113:e308-12.
- 51. Holgate ST, Chuchalin AG, Hebert J, Lötvall J, Persson GB, Chung KF, et al. Omalizumab 011 International Study Group. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34:632-8.
- 52. Holgate ST, Holloway J, Wilson S, Howarth PH, Haitchi HM, Babu S, et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006;117:496-506.
- 53. Cooper PJ, Lima F, Sarinho EC, Ayre G, Martin C, Fox H, Cruz AA. Safety of Anti-IgE Therapy with Omalizumabe in Allergic Patients at Risk of Geohelminth Infection. J Allergy Clin Immunol. 2006;117:S9.
- 54. Cruz AA. Asma, Um Grande Desafio. Rio de Janeiro: Atheneu; 2004.
Correspondence:
Publication Dates
-
Publication in this collection
23 Feb 2007 -
Date of issue
Nov 2006