Abstracts
OBJECTIVE: To report the long-term follow-up of the first living-donor lobar lung transplantation performed in Latin America. DESCRIPTION: The patient was a 12-year-old boy with post-infectious obliterative bronchiolitis with end-stage pulmonary disease. He was on continuous oxygen support, presenting with dyspnea even during minimal activity. He underwent bilateral lobar lung transplantation with living donors. The procedure was performed with the left and right lower lobes of two different related donors. In the second side cardiopulmonary bypass was required. The transplant was uneventful, and the patient was extubated after 14 hours and discharged with 44 days, after resolution of infectious, immunological and drug-related complications. After 12 years of follow-up, he presents with adequate lung function and has resumed his habitual activities. COMMENTS: Living-donor lobar lung transplantation is a complex procedure feasible for the treatment of selected pediatric end-stage pulmonary disease. This particular population might benefit from this approach since the availability of pediatric donors is very scarce and the clinical course of pediatric advanced pulmonary disease may be unpredictable.
Lung transplantation; bronchiolitis obliterans; living donors; pediatrics
OBJETIVO: Apresentar o acompanhamento a longo prazo do primeiro caso de transplante pulmonar intervivos realizado na América Latina. DESCRIÇÃO: Paciente do sexo masculino, com 12 anos de idade, portador de bronquiolite obliterante com doença pulmonar avançada. Fazia uso de oxigênio domiciliar contínuo, com dispneia aos mínimos esforços. Foi submetido a transplante pulmonar bilateral com doadores vivos. A cirurgia foi realizada utilizando os lobos inferiores esquerdo e direito de dois doadores diferentes e com grau de parentesco com o receptor. No segundo lado (direito), foi necessário emprego de circulação extracorpórea. O transplante não teve intercorrências, e o paciente foi extubado com 14 horas de pós-operatório; com 44 dias, recebeu alta hospitalar, após a resolução de complicações infecciosas, imunológicas e medicamentosas. Após 12 anos de seguimento, encontra-se com função pulmonar preservada e desempenha normalmente suas atividades. COMENTÁRIOS: O transplante pulmonar intervivos é um procedimento de alta complexidade que pode contribuir para o tratamento de algumas pneumopatias na infância. Essa população se beneficia dessa abordagem, uma vez que a disponibilidade de doadores pediátricos é muito rara, e as pneumopatias pediátricas tendem a seguir um curso imprevisível.
Transplante de pulmão; bronquiolite obliterante; doadores vivos; pediatria
ORIGINAL ARTICLE
Twelve-year survival of the first living-donor pediatric lung transplantation in Brazil
Tiago Noguchi MachucaI; Luzielio Alves Sidney FilhoII; Sadi Marcelo SchioIII; Spencer Marcantonio CamargoII; José Carlos FelicettiI; José de Jesus Peixoto CamargoIV
IPhD, Ciências Pneumológicas, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
IIPhD candidate, Ciências Pneumológicas, UFRGS, Porto Alegre, RS, Brazil.
IIISpecialist in Cardiologia. Clinical coordinator, Grupo de Transplante Pulmonar, Santa Casa de Porto Alegre, Porto Alegre, RS, Brazil.
IVPhD candidate, Ciências Pneumológicas, UFRGS, Porto Alegre, RS, Brazil. Coordinator, Grupo de Transplante Pulmonar, Santa Casa de Porto Alegre, Porto Alegre, RS, Brazil.
ABSTRACT
OBJECTIVE: To report the long-term follow-up of the first living-donor lobar lung transplantation performed in Latin America.
DESCRIPTION: The patient was a 12-year-old boy with post-infectious obliterative bronchiolitis with end-stage pulmonary disease. He was on continuous oxygen support, presenting with dyspnea even during minimal activity. He underwent bilateral lobar lung transplantation with living donors. The procedure was performed with the left and right lower lobes of two different related donors. In the second side cardiopulmonary bypass was required. The transplant was uneventful, and the patient was extubated after 14 hours and discharged with 44 days, after resolution of infectious, immunological and drug-related complications. After 12 years of follow-up, he presents with adequate lung function and has resumed his habitual activities.
COMMENTS: Living-donor lobar lung transplantation is a complex procedure feasible for the treatment of selected pediatric end-stage pulmonary disease. This particular population might benefit from this approach since the availability of pediatric donors is very scarce and the clinical course of pediatric advanced pulmonary disease may be unpredictable.
Keywords: Lung transplantation, bronchiolitis obliterans, living donors, pediatrics.
Introduction
Since 1983, when the Toronto Lung Transplant Group reported the first successful lung transplantation, several advances have consolidated this procedure as a treatment modality for patients with end-stage pulmonary disease.
1 However, the increase in the waiting lists for a new organ was not accompanied by an increase in the number of available donors. One of the alternatives found by the group of University of Southern California led by Vaughn Starnes, directed at a population with difficulties in receiving organs from cadavers (children needing bilateral transplantation), was performing a transplant with lobes from living donors in 1992.
2 Starnes' achievement was followed by that of a group by Hiroshi Date, from Okayama University, which conducted a living-donor lobar transplantation in a patient with ciliary dyskinesia in 1998.
3 Afterwards, following the pioneering example in lung transplantation with cadaveric donors, our group performed the first living-donor lung transplantation in Latin America, whose report is the aim of the present paper.
Case description
The patient, a 12-year-old boy with advanced pulmonary disease due to obliterative bronchiolitis, was admitted for pre-lung transplant evaluation. On that occasion, he was dependent on oxygen, with a forced expiratory volume in 1 second (FEV1) of 150 mL (12% of the predicted value) and a forced vital capacity (FVC) of 230 mL (17% of the predicted value). The 6-minute walk test was not performed due to lack of clinical conditions (Figure 1). There was no colonization of the lower airways. Two related donors were selected based on blood compatibility and lobe size. Donors underwent fibrobronchoscopy, evidencing anatomically normal lower airways.
Subsequently, the bilateral lung transplantation was conducted by sequential posterolateral thoracotomy. Orotracheal intubation was performed with Carlens' tube, and blood pressure and pulmonary artery pressure were monitored invasively. Because perfusion scintigraphy showed homogeneous disease (right perfusion 49%), we chose to begin from the left side due to the fact that cannulation for cardiopulmonary bypass (CPB) was easier in the right side (the second side shows a higher risk of requirement for CPB). The first donor underwent left lower lobectomy, with lobe preparation on a special table with retrograde and antegrade perfusion with 4 L of Perfadex and ambient air manual ventilation. After the recipient underwent left pneumonectomy, the implantation went on with mouth to mouth bronchial anastomosis, with a continuous suture in the membranous wall and simple stitches of 4-0 polydioxanone in the cartilaginous wall, followed by arterial anastomosis with a continuous 5-0 polypropylene suture and venoatrial anastomosis with a continuous 4-0 polypropylene suture. The reperfusion was performed after 50 minutes of ischemia by slowly opening the pulmonary artery clamp and manually ventilating the lobe. A similar technique was performed in the right side, using the lower lobe of the second donor, with ischemia time of 55 minutes. However, in this side, CPB was required due to respiratory instability, with cannulation of the aorta and right atrium. CPB time was 54 minutes. Fibrobronchoscopy immediately after surgery showed permeable anastomoses with excellent appearance.
In the intensive care unit (ICU), he required vasoactive drugs (dopamine) for 18 hours and was extubated 14 hours after admission. The post-surgery period was marked by two episodes of acute rejection, the first of which (17 days after surgery) was treated with pulse therapy and the second (33 days after surgery) also with pulse therapy, together with a change in the immunosuppression scheme by replacing azathioprine with mycophenolate mofetil. Moreover, he presented with respiratory infection, with the isolation of Stenotrophomonas maltophilia in bronchial washing (12 days after surgery) and treatment with sulfamethoxazole-trimethoprim. Another post-surgery complication was neurotoxicity due to cyclosporine, with important symptoms compatible with peripheral neuropathy and prompt improvement after transient suspension of the drug. After 33 days he was discharged from the ICU and after 44 days was discharged from hospital in good general state and with no oxygen requirement.
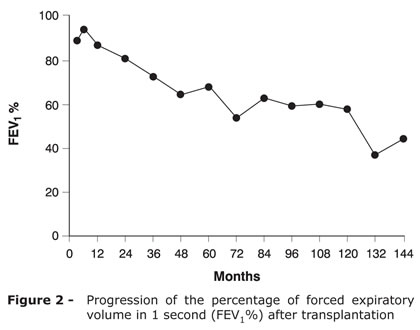
Figure 2 shows the evolution of pulmonary function testing. At present, 12 years after surgery, the patient has resumed his habitual activities with no restrictions (Figure 3) and has kept the immunosuppresion scheme with cyclosporine, mycophenolate mofetil, and prednisone.
Discussion
The idea of removing an organ from healthy individuals, putting them at potentially life-threatening risk, with the purpose of compensating for an organ failure of another person, raises questions per se. But, when the procedure involves not only one but two healthy people, controversy is much greater, as the potential risk is often extended to a whole family. However, the advances in and the acceptance of lung transplantation as an effective therapeutics for end-stage pulmonary disease left an important population helpless: that of patients of short stature and who need to receive two grafts and are not capable of surviving for a long period waiting for a cadaveric donor. Due to the difficulty in finding a suitable donor for this group and to the peculiar feature that recipients usually suffers from diseases with unpredictable course, such as cystic fibrosis, the creation of a pool of readily available donors was imperative. In order to fill this important gap, the first living-donor lung transplantations were reported in 1992.2 Subsequently, the diffusion of the encouraging results in the mid-term by the same group has led this transplantation modality to be considered in clinical practice.4 Following this trend, another centers worldwide also began to perform living-donor transplantations, showing similar results.
With the experience obtained, the important questioning on the possibility of two lobes providing an equivalent function to that of a lung was answered. When patients who underwent living-donor lobar transplantation were compared with those who received lungs from a cadaveric donor, the increase in total lung capacity up to 12 months after transplantation was similar.5 However, observing the decrease in carbon monoxide diffusion, this was more significant in the first group, suggesting that the gain in lung function is at the expense of alveolar dilatation. Besides that, the analysis of the experience in adults showed that, although FVC and FEV1 were lower in the living-donor group from the 1st to the 3rd month after transplantation, these values improved and are equivalent to those of patients who received a lung from a cadaveric donor from the sixth month on.6
Regarding long-term results, recent studies showed a very encouraging scenario. In the series of Date et al., including 30 patients, hospital survival was 100%, with all patients alive at the moment of publication.7 It should be highlighted that this series is exceptional, due to the peculiarities of the Japanese population, which has a low incidence of cystic fibrosis and presents pulmonary arterial hypertension and interstitial fibrosis as the main indications for transplantation. As in our case, average ICU and hospital stay tend to be long (23 and 64 days respectively in their study).
In the series of Sweet et al., survival at 1 and 3 years was 60 and 48%, respectively.8 Despite a worse survival compared with cadaveric donor recipients (mostly due to infectious complications), evolution time with no obliterative bronchiolitis was significantly lower (92 vs. 75% at 1 year and 85 vs. 53% at 3 years). Corroborating this finding, our patient is free of obliterative bronchiolitis 12 years after transplantation.
In the largest series, with 138 cases (90 adults and 48 children), cystic fibrosis was the indication for transplantation in 86% of them. Survival at 1, 3 and 5 years were 70, 54 and 45%, respectively. In this study, the authors emphasized that 67% of the patients were hospitalized at the moment of transplant indication, and 20% depended on mechanical ventilation. Moreover, the cause of death in the first 30 days and in the first year was the same: infection (mainly by Pseudomonas sp., Staphylococcus sp., and Aspergillus sp.). After the first year, obliterative bronchiolitis joins infection as the main cause of death.9
Another important issue concerns donor morbidity. It is known that lung lobectomy, even in healthy patients, is not free of risks. In an assessment of 253 donors, Bowdish et al. found 19.8% of morbidity and no deaths.10 In a recent publication, our group reported complications in 32 donors, with 31.25% of morbidity and zero mortality.11 Again, it was emphasized the crucial need of balancing the risks of the procedure (for the three patients) with its potential benefits. Other very important data to be considered at the moment of indication for living-donor transplantation are the current course of the pneumopathy and the real chance of finding a compatible cadaveric donor, in case the patient is included in the waiting list.
In summary, we highlight the importance of considering living-donor lung transplantation in well selected cases. This transplant modality may represent the only alternative in pediatric patients with end-stage pneumopathies. The results are encouraging when considering the severity of the study population. Additionally, it is possible to obtain long-term survival with quality of life.
References
- 1. Unilateral lung transplantation for pulmonary fibrosis. Toronto Lung Transplant Group. N Engl J Med. 1986;314:1140-5.
- 2. Starnes VA, Lewiston NJ, Luikart H, Theodore J, Stinson EB, Shumway NE. Current trends in lung transplantation. Lobar transplantation and expanded use of single lungs. J Thorac Cardiovasc Surg. 1992;104:1060-6.
- 3. Date H, Yamamoto H, Yamashita M, Aoe M, Kubo K, Shimizu N. One year follow-up of the first bilateral living-donor lobar lung transplantation in Japan. Jpn J Thorac Cardiovasc Surg. 2000;48:648-51.
- 4. Starnes VA, Barr ML, Cohen RG, Hagen JA, Wells WJ, Horn MV, et al. Living-donor lobar lung transplantation experience: intermediate results. J Thorac Cardiovasc Surg. 1996;112:1284-91.
- 5. Sritippayawan S, Keens TG, Horn MV, MacLaughlin EF, Barr ML, Starnes VA, et al. Does lung growth occur when mature lobes are transplanted into children? Pediatr Transplant. 2002;6:500-4.
- 6. Bowdish ME, Pessotto R, Barbers RG, Schenkel FA, Starnes VA, Barr ML. Long-term pulmonary function after living-donor lobar lung transplantation in adults. Ann Thorac Surg. 2005;79:418-25.
- 7. Date H, Aoe M, Sano Y, Nagahiro I, Miyaji K, Goto K, et al. Improved survival after living-donor lobar lung transplantation. J Thorac Cardiovasc Surg. 2004;128:933-40.
- 8. Sweet SC, de la Morena MT, Schuler PM, Patterson GA, Meyers BF, Schuller D, et al. Single center comparison of pediatric living donor lobar and cadaveric lung transplant. J Heart Lung Transplant. 2003;22:S145-6.
- 9. Barr ML, Schenkel FA, Bowdish ME, Starnes VA. Living donor lobar lung transplantation: current status and future directions. Transplant Proc. 2005;37:3983-6.
- 10. Bowdish ME, Barr ML, Schenkel FA, Woo MS, Bremner RM, Horn MV, et al. A decade of living lobar lung transplantation: perioperative complications after 253 donor lobectomies. Am J Transplant. 2004;4:1283-8.
- 11. Camargo SM, Camargo J de J, Schio SM, Sánchez LB, Felicetti JC, Moreira J da S, et al. Complications related to lobectomy in living lobar lung transplant donors. J Bras Pneumol. 2008;34:256-63.
Publication Dates
-
Publication in this collection
08 Nov 2012 -
Date of issue
Oct 2012
History
-
Received
01 Mar 2012 -
Accepted
24 May 2012