ABSTRACT:
Agoutis are small-sized wild animals whose body weight can reach up to 4kg, and are found throughout Brazil. They are considered important seed dispersers, especially for big trees and there are species that rely almost exclusively on these animals for their territorial distribution. The objective of the present study was B scan and Doppler ultrasound characterization of the abdominal organs of healthy agoutis reared in captivity. Fifteen agoutis, chemically restrained, were used from the Nucleus for Wild Animal Studies and Conservation (Núcleo de Estudos e Preservação de Animais Silvestres - NEPAS), CCA-UFPI, submitted to B scan and Doppler ultrasound examination. The urinary bladder wall was hyperechogenic, thin, smooth and regular throughout its anatomic path, with 0.09±0.03cm mean thickness. The kidneys showed fine and homogeneous echotexture, preserved global echogenicity, hyperechogenic in relation to the spleen and isoechogenic or discreetly hyperechogenic in relation to the liver. The spectral Doppler trace showed systolic and diastolic peaks, wide and thread-like, with low flow resistance and a continuous and full diastolic portion that decreased gradually during the diastole (75.83±1.42cm/s, for the right kidney and 80.43±1.22cm/s, for the left kidney). The right adrenal gland was 0.61-1.18cm long and 0.17-0.32cm in diameter, while the left adrenal gland was 0.62-1.16 long with 0.14-0.25cm diameter. The agouti spleen was filiform in shape, with pointed poles and 1.02±0.18cm in diameter. The agouti liver occupied all the abdominal cavity cranial space in direct contact with the diaphragm. The intrahepatic vascular flow allowed individualization of the portal vein (PV) and hepatic vein (HV). The portal veins were distinguished from the hepatic veins mainly by their wall echogenic pattern. The pancreas was 0.51±0.1 cm thick and the pancreatic duct measured 0.12±0.02cm. The stomach was placed to the left the spleen and to the right of the proximal intestine and the transversal colon and the walls were 0.16±0.05cm thick. The abdominal aorta was 0.43±0.04cm in diameter and showed 95.2±2.16cm/s vascular flow. This study characterized agouti organs and abdominal blood vessels by B scan and Doppler ultrasound, that permitted definition of the size, shape, position, echogenicity and echotexture of the anatomic constituents and established reference values for the vascular network and blood flow in the species.
INDEX TERMS:
Abdominal B-mode; Doppler ultrasonography; agouti; Dasyprocta prymnolopha; ultrasound; morphology; hemodynamic; Hystricomorpha
RESUMO:
As cutias são animais silvestres de pequeno porte, cujo peso corpóreo pode chegar até 4kg, e existem em todo território brasileiro. São considerados importantes dispersores de sementes, especialmente para árvores de grande porte, existindo espécies que dependem quase que exclusivamente destas para sua distribuição territorial. Este trabalho teve por objetivo a caracterização ultrassonográfica modo B e Doppler dos órgãos abdominais de cutias hígidas criadas em cativeiro. Foram utilizadas 15 cutias, contidas quimicamente, oriundas do Núcleo de Estudos e Preservação de Animais Silvestres - NEPAS, CCA-UFPI, submetidas a exame ultrassonográfico em modo B e Doppler. A parede da vesícula urinária presentou-se hiperecogênica, fina, lisa e regular em todo seu trajeto anatômico, com espessura média de 0,09±0,03cm. Os rins demonstraram ecotextura fina e homogênea, ecogenicidade global preservada, hipoecogênico em relação ao baço e isoecogênico ou discretamente hipoecogênico em relação ao fígado. O traçado em Doppler espectral mostrou pico sistólico e diastólico, amplo e afilado, exibindo baixa resistência de fluxo, com uma porção diastólica contínua e cheia, que diminui gradativamente no decorrer da diástole (75,83±1,42cm/s para o rim direito e 80,43±1,22 cm/s para o esquerdo. A adrenal direita apresentou uma variação de comprimento entre 0,61 a 1,18cm e diâmetro variando entre 0,17 a 0,32cm, enquanto a adrenal esquerda evidenciou comprimento de 0,62 a 1,16 e diâmetro de 0,14 a 0,25cm. O baço das cutias mostrou formato filiforme, com polos pontiagudos e diâmetro de 1,02±0,18cm. O fígado da cutia ocupa todo o espaço cranial da cavidade abdominal, em contato direto com o diafragma. O fluxo vascular intrahepático permitiu individualizar as veias porta (VP) e veias hepáticas (VH). As veias porta foram distinguidas, particularmente pelo padrão ecogênico de suas paredes, quando comparadas com as veias hepáticas. A espessura do pâncreas foi de 0,51±0,1cm e o ducto pancreático mediu 0,12±0,02cm. O estômago relaciona-se à esquerda com o baço e a direita com o duodeno proximal e colón transverso. Sua espessura de parede mensurada foi de 0,16±0,05cm. A aorta abdominal possui diâmetro de 0,43±0,04cm e fluxo vascular de 95,2±2,16cm/s. Este estudo caracterizou os órgãos e vasos sanguíneos abdominais de cutias, por meio de ultrassonografia modo B e Doppler, o que permitiu definir o tamanho, formato, posição, ecogenicidade, ecotextura dos constituintes anatômicos, além de estabelecer valores de referência para a rede vascular e fluxo sanguíneo na espécie.
TERMOS DE INDEXAÇÃO:
Ultrassonografia abdominal; Modo B; Doppler; cutias; Dasyprocta prymnolopha; ultrassom; hemodinâmica; abdome; morfologia; Hystricomorpha
Introduction
Agoutis are small wild animals, whose body weight can reach 4 kg and are found throughout Brazil. They belong to the classe Mammalia, order Rodentia, suborder Hystricomorpha, family Dasyproctidae and genus Dasyprocta (Moojen 1952Moojen J. 1952. Os Roedores do Brasil. Instituto Nacional do Livro, Rio de Janeiro. 215p., Lange & Jablonski 1981Lange R.B. & Jablonski E.F. 1981. Lista prévia dos Mammalia do Estado do Paraná. Revta Estud. Biol., PUCPR, 4:1-35.).
These rodents are considered important seed dispersers, especially for big trees, and there are tree species that depend exclusively on them for their territorial distribution and they therefore determine the spatial recruitment and germination of these plant species (Forget & Milleron 1991Forget P.M. & Milleron T. 1991. Evidence for secondary seed dispersal by rodents in Panama. Ecologia 87(4):596-599. PMid:28313705., Emmons & Feer 1997Emmons L.H. & Feer F. 1997. Neotropical Rainforest Mammals: a field guide. University of Chicago Press, Chicago. 2nd ed. 134p.). They play a fundamental role in nature, because they are earth-digging animals and promote soil oxygenation. Their urine and feces help to return nutrients and minerals salts to the soil (Peres & Baider 1997Peres C.A. & Baider C. 1997. Seed dispersal, spatial distribution and population structure of Brazil nut trees (Bertholletia excelsa) in Southeastern Amazonia. J. Trop. Ecol. 13(04):595-616. http://dx.doi.org/10.1017/S0266467400010749.
https://doi.org/10.1017/S026646740001074...
, Asquith et al. 1999Asquith N.M., Terborgh J., Arnold E. & Riveros M. 1999. The fruits the agouti ate: Hymenaea courbaril seed fate when its disperser is absent. J. Trop. Ecol. 15(2):229-235. http://dx.doi.org/10.1017/S0266467499000772.
https://doi.org/10.1017/S026646749900077...
). Especially, the agoutis act in the aggregated distribution of seeds from trees in scales of a few hundred meters in relation to the mother tree, making their contribution clear from the ecological point of view (Fragoso 1997Fragoso J.M.V. 1997. Tapir-generated seed shadows: scale-dependent patchiness in the Amazon rain forest. J. Ecol. 85(4):519-529. http://dx.doi.org/10.2307/2960574.
https://doi.org/10.2307/2960574...
). These animals are important links in the food chain because they contribute to maintaining the environmental balance (Hosken & Silveira 2001Hosken F.M. & Silveira A.C. 2001. Criação de Cutias. Aprenda Fácil, Viçosa. 234p.).
Many studies have already been developed on wild species to describe in the best way possible the morphology of abdominal organs by B scan ultrasound. However, even with the ultrasonographic knowledge of these tissues, it is still necessary to carry out future research that shows the vascular indices of abdominal vessels, and their hemodynamic, organic deformation and interaction with adjacent organs.
Previous ultrasonographic studies on wild species that discuss morphological aspects of abdominal structures include those on ant eaters Myrmecophaga tridactyla (Lopes et al. 2015Lopes E.R., Morgado T.O., Meireles Y.S., Jorge A.A., Zago A.A.Q., Corrêa S.H.R., Paz R.C.R. & Nespóli P.B. 2015. Ultrassonografia abdominal de tamanduás-bandeira (Myrmecophaga tridactyla Linnaeus, 1758) mantidos em cativeiro. Pesq. Vet. Bras. 35(11):919-924. http://dx.doi.org/10.1590/S0100-736X2015001100008.
https://doi.org/10.1590/S0100-736X201500...
), crab-eating foxes Cerdocyon thous (Silva et al. 2014Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
https://doi.org/10.1590/S0100-736X201400...
), pacas Cuniculus paca (Oliveira et al. 2003Oliveira F.S., Machado M.R.F. & Canola J.C. 2003. Real time B-mode ultrasound in pacas pregnancy (Agouti paca Linnaeus, 1766). Braz. J. Vet. Res. Anim. Sci. 40(1):73-78. http://dx.doi.org/10.1590/S1413-95962003000100009.
https://doi.org/10.1590/S1413-9596200300...
, 2007Oliveira F.S., Machado M.R.F., Canola J.C. & Camargo M.H.B. 2007. Uniparidade em pacas criadas em cativeiro (Agouti paca Linnaeus, 1766). Arq. Bras. Med. Vet. Zootec. 59(2):387-389. http://dx.doi.org/10.1590/S0102-09352007000200019.
https://doi.org/10.1590/S0102-0935200700...
, Feliciano et al. 2014Feliciano M.A.R., Barros F.F.P.C., Coutinho L.N., Brito M.B.S., Uscategui R.R., Santos V.J.C., Almeida V.T., Kawanami A.E., Nociti R.P., Machado M.R.F. & Vicente W.R.R. 2014. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Scient. Vet. 42:1-6.), agoutis Dasyprocta prymnolopha (Sousa et al. 2012Sousa F.C., Alves F.R., Fortes E.A., Ferraz M.S., Machado Júnior A.A., Menezes D.J. & Carvalho M.A. 2012. Pregnancy in Histrycomorpha: gestacional age and embryonic-fetal development of agouti (Dasyprocta prymnolopha Wagler, 1831) estimated by ultrasonography. Theriogenology 78(6):1278-1285. http://dx.doi.org/10.1016/j.theriogenology.2012.05.023. PMid:22898012.
https://doi.org/10.1016/j.theriogenology...
, 2016Sousa F.C.A., Pessoa G.T., Moura L.S., Rodrigues R.P.S., Diniz A.N., Souza A.B., Silva E.G., Sanches M.P., Silva-Filho O.F., Guerra P.C., Sousa J.M., Neves W.C. & Alves F.R. 2016. Doppler ultrasound of the placenta and maternal and fetal vessels during normal gestacion in captive agoutis (Dasyprocta prymnolopha Wagler, 1831). Theriogenology 86(8):1921-1930. http://dx.doi.org/10.1016/j.theriogenology.2016.06.006. PMid:27458115.
https://doi.org/10.1016/j.theriogenology...
, 2017Sousa F., Pessoa G.T., Moura L.S., Araújo J.R., Rodrigues R., Barbosa M., Diniz A.N., Souza A.B., Silva E.G., Lucena L.U., Sanches M.P., Silva-Filho O.F., Guerra P.C., Sousa J.M., Neves W.C. & Alves F.R. 2017. Organogenesis and foetal haemodynamics during the normal gestacion of healthy black-rumped agoutis (Dasyprocta prymnolopha Wagler, 1831) bred in captivity. Reprod. Domest. Anim. 52(1):60-66. http://dx.doi.org/10.1111/rda.12803. PMid:27687997.
https://doi.org/10.1111/rda.12803...
), coatis Nasua nasua (Ribeiro et al. 2013Ribeiro R.G., Costa A.P., Bragato N., Fonseca A.M., Duque J.C., Prado T.D., Silva A.C. & Borges N.C. 2013. Normal sonographic anatomy of the abdomen coatis (Nasua nasua Linnaeus, 1766). BMC Vet. Res. 9(1):124. http://dx.doi.org/10.1186/1746-6148-9-124. PMid:23800301.
https://doi.org/10.1186/1746-6148-9-124...
), collared peccary Tayassu tajacu (Peixoto et al. 2012Peixoto G.C.X., Oliveira I.R.S., Alves N.D., Oliveira M.F. & Silva A.R. 2012. Abdominal exploration in captive collared peccaries (Tayassu tajacu) by ultrasonography. Anat. Histol. Embryol. 41(4):256-261. http://dx.doi.org/10.1111/j.1439-0264.2011.01129.x. PMid:22220558.
https://doi.org/10.1111/j.1439-0264.2011...
), robust capuchin monkey Cebus apella (Alves et al. 2007Alves F.R., Costa F.B., Arouche M.M.S., Barros A.C.E., Miglino M.A., Vulcano L.C. & Guerra P.C. 2007. Avaliação ultra-sonográfica do sistema urinário, fígado e útero do macaco-prego, Cebus apella. Pesq. Vet. Bras. 27(9):377-382. http://dx.doi.org/10.1590/S0100-736X2007000900004.
https://doi.org/10.1590/S0100-736X200700...
), cheetahs Acinonyx jubatus (Carstens et al. 2006Carstens A., Kirberger R.M., Spotswood T., Wagner W.M. & Grimbeek R.J. 2006. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet. Radiol. Ultrasound 47(4):376-383. http://dx.doi.org/10.1111/j.1740-8261.2006.00157.x. PMid:16863057.
https://doi.org/10.1111/j.1740-8261.2006...
) and common marmosets Callithrix jacchus (Wagner & Kirberger 2005Wagner W.M. & Kirberger R.M. 2005. Transcutaneous ultrasonography of the abdômen in the normal common marmoset (Callithrix jacchus). Vet. Radiol. Ultrasound 46(3):251-258. http://dx.doi.org/10.1111/j.1740-8261.2005.00045.x. PMid:16050285.
https://doi.org/10.1111/j.1740-8261.2005...
).
Based on these studies, the need was perceived for new research that generates information to facilitate diagnosis for the wild animal clinical veterinarian, because these species have been little studied to date and are a great perspective for the biodiversity of the planet. Although there is knowledge of abdominal organ in B scan, it is essential to obtain ultrasound parameters in Doppler ultrasonography because these would serve as reference for the diagnosis of various abnormalities.
The objective of the present study was the B scan and Doppler ultrasound characterization of the abdominal organs of healthy agouti reared in captivity. The results of this study could contribute to providing new information regarding ultrasound morphology and the hemodynamic parameters of abdominal blood vessels of this wild species in the scientific environment. Furthermore, this research would serve as a strong base for initial illness diagnosis and will favor a more effective clinical surgical approach with ecological conservation of these wild rodents.
Materials and Methods
Fifteen adult, clinically healthy agoutis (Dasyprocta prymnolopha Wagler, 1831) were used, eight males and seven females, 2-3 years old and weighing 2.5 to 3kg, from the Nucleus for Wild Animal Study and Conservation (Núcleo de Estudos e Preservação de Animais Silvestres - NEPAS) (Registration IBAMA no. 02/08-618) of the Agrarian Science Centre (CCA) at the Federal University of Piauí (UFPI), Teresina, Piauí, Brazil.
The protocols used in this study were approved by the Ethics Committee for Animal Experimentation (Comitê de Ética em Experimentação Animal) - CEEA/UFPI (no. 013/15) and authorized by the Ministry of the Environment, through the Authorization and Information on Biodiversity System (SISBIO) of the Brazilian Institute of Environment and Renewable Natural Resources - IBAMA (no. 47199-1).
The animals were subjected to 12 hours fast from solid foods and 6 hours fast from water, and captured in their enclosures with a landing net. An association of 40mg/kg ketamine hydrochloride (Vetanarcol?) with 1mg/kg hydrochloride xylazine (Xylazine?) was used intramuscular for chemical restraint. After the anesthetic protocol, the vital parameters were evaluated throughout the experiment. The heart rate measured using a Littmann cardiology IV stethoscope (Littmann?, NYC, USA). Blood pressure was evaluated with the animals placed in right lateral recumbency, and noninvasive oscillometric equipment was used for measuring blood pressure (PetMap?, San Diego, USA). Five measurements of systolic blood pressure were taken, and the standard cuff equipment was 40% of the circumference of the animal (a third of the average radius and ulna).
To carry out the ultrasound exams, the specimens were positioned in dorsal decubitus, the abdominal surface was shaved and the abdomen was scanned, after applying ultrasound conductor gel (Carbogel?), using the M-Turbo apparatus (Sonosite FUJIFILM?) associated to a 4-8 MHz frequency sectorial transductor (P10x). A 6-13MHz frequency linear transductor (HFL38x) was used to better visualize the parenchyma and cavity organ architecture.
The morphology was assessed of abdominal organs such as the urinary bladder, kidneys, adrenal glands, spleen, liver, gallbladder, pancreas, stomach and intestinal loops. The flow pattern of the abdominal aorta, renal arteries, inferior vena cava and the intra-hepatic veins (portal veins and hepatic veins) was established by spectral and colored Doppler giving values of the systolic peak speed and the resistivity index.
The data were submitted to the normality test (Shapiro-Wilk and Kolmogorov-Smirnov test) and the means were analyzed later by the Student t-test to interpret the parameters, considering a 5% (p<0.05) confidence interval.
Results
Vital parameters (heart rate and blood pressure)
All agoutis presented a satisfactory response to sedation, and there were no deaths or hypotension, due to the anesthetic protocol used. The heart rate measured during the study time ranged from 120 to 135 bpm (±128 bpm). Measured arterial pressure was measured ranging from 110 to 125mm Hg (±117mmHg).
Urinary bladder
The urinary bladder was oval and located between the abdominal and pelvic transition region. It had normal anechogenic intraluminal content (urine). The wall was hyperechogenic, thin, smooth and regular throughout its anatomic path, with 0.09±0.03cm mean thickness (Fig.1a).
Ultrasound imaging in B-mode, color and spectral Doppler of the abdominal organs of the agouti. (a) Ultrasonographic aspects of the urinary vesicle. Note the smooth and echogenic walls with a slight amount of sediment on the interior. (b,d) Color flow and B-mode renal morphology of the right and left kidneys, respectively, showing the usual echotexture and parenchymal echogenicity and preserved corticomedullary limit. (c,e) Pattern of flow of the renal artery, arcuate (arrowhead) and interlobar (arrow) arteries observed with color Doppler. The pulsed Doppler demonstrates well-defined systolic and diastolic peaks.
Right and left kidney
Both kidneys presented retroperitoneal topography. The caudal spleen edge showed synthopia with the left kidney cranial pole, while the right lateral hepatic lobe was intimately related to the right kidney cranial pole and the latter was positioned cranial to the left kidney. The kidneys showed homogeneous and thin echotexture and preserved global echogenicity, hyperechogenic in relation to the spleen (left kidney) and isoechogenic or discretly hyperechogenic in relation to the liver (right kidney). The cortex-medullar ratio was preserved, individual and the pelvic recess was free from stenosis, dilations and obstructive processes (Fig.1b and 1d).
The right and left kidneys were similar in size for both length and height, showing that the morphometric values did not differ statistically (p>0.05) compared to the means of the right and left kidneys. The aorta/kidney ratio was measured in all the agoutis studied, and showed a mean value of 7.20±0.27 for the right kidney and 6.864±0.10 for the left kidney, with no statistical difference (p>0.05) between their means (Table 1). High positive correlation was observed when the kidney length and aorta diameter were compared r=0.97 (aorta diameter versus right kidney length) and r=0.94 (aorta diameter versus left kidney links) (Table 1, Fig.2).
Correlation between the renal length and aortic diameter of agouti, Teresina/PI, Brazil, 2016. (a) Correlation between the right kidney and diameter of the aorta. (b) Correlation existing between the left kidney and diameter of the aorta.
Flow assessment in the Doppler exam allowed study of the perfusion through the renal artery. Colored Doppler permitted delimitation of the flow path and direction of this artery through the renal hilum and there was no presence of stenosis, dilations or hypoflows. It divided and formed the arched arteries that were very visible on the cortex-medullar edge (Fig.1b and 1d).
The spectral Doppler trace showed systolic and diastolic peaks, wide and thread-like, showing low flow resistance, with a continuous and full diastolic portion, that gradually decreased during the diastole (75.83±1.42cm/s for the right kidney and 80.43±1.22 cm/s for the left kidney (Fig.1c and 1e). The resistivity indices for these animals showed mean values of 0.54±0.16 for the right kidney and 0.50±0.11 for the left kidney, and there were no statistical differences for these variables (p>0.05) (Table 1).
Abdominal aorta
It followed a traditional path, passing dorsal to the caudal vena cava at the level of the space between the fifth and the sixth lumbar vertebra, giving rise to the deep circumflex arteries, returning ventral and placed to the left of the caudal vena cava. Its diameter was 0.43±0.04cm and vascular flow was measured at 95.2±2.16cm/s (Fig.3a and 3b).
Ultrasound imaging in B-mode, color and spectral Doppler of the abdominal organs of the agouti. (a,b) The color and pulsed characteristics of the abdominal aorta (Ao). Laminar flow was observed with a flat and high systolic peak, a wide spectral window, and flow pattern of high resistivity. (c) The right adrenal gland (R Adr) is located dorsal to the caudal vena cava. (d) The left adrenal gland (L adr) was seen cranially to the left renal artery, ventrally to the aorta and in varied coexistence with the cranial pole of the left kidney (Lk).
Right and left adrenal gland
The adrenal glands were characterized as paired bilobate kidney shaped glands, with greater anatomical variation in the right adrenal that in most of the cases was triangular in shape. They were placed vertically between the second and third lumbar vertebra, in the retroperitoneal space. The right adrenal gland was located cranial to the right renal artery, medial to the right kidney cranial pole, in intimate relationship with the origin of the cranial celiac and mesenteric arteries, dorsal to the caudal vena cava (Fig.3c). The left adrenal was shown to be cranial to the left renal artery, ventral to the aorta and in varied synthopia with the left kidney cranial pole, depending on the localization of this organ (Fig.3d). The right adrenal gland measured 0.61-1.18cm (0.97±0.14cm) in length and its diameter ranged from 0.17 to 0.32cm (0.22±0.06cm). The left adrenal was 0.62-1.16 (0.92±0.14cm) long and its diameter was 0.14 to 0.25cm (0.20±0.03cm). There was no difference between the means for the variables studied, when the adrenal glands were compared (p>0.05).
Spleen
The spleen was filiform in shape, with pointed poles and 1.02±0.18cm diameter and a synthopic relationship with the greater curvature of the stomach and the left kidney cranial pole that varied during respiratory movements. Its echotexture was thin and homogeneous throughout its extension, but echogenic compared to the renal cortex (Fig.4a and 4d).
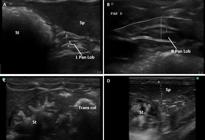
Ultrasound imaging in B-mode of the abdominal organs of the agouti. (A,B) Left and Right lobes of pancreas, respectively. Its echotexture was homogeneous and hyperechogenic. The pancreatic duct was distinguished within this organ by the echogenic characteristic of its walls. (C) The stomach, echogenic and with homogeneous echotexture, and its closed relationship with the transverse colon. (D) The spleen with filiform shape, with pointed poles, maintaining a syntopic relation with the greater curvature of the stomach.
Pancreas
The pancreas was clearly characterized among the other abdominal structures. The right lobe was located dorsal medial to the descending duodenum, ventral to the right kidney and ventral lateral to the portal vein. The left pancreatic lobe showed synthopia with the spleen, greater curvature of the stomach and the left kidney cranial pole. The pancreas showed intimate relation with the pyloric portion of the stomach, mid-cranial to the right kidney and ventral to the portal vein. Its echotexture was homogeneous. The pancreatic duct was distinguished inside this organ by the echogenic characteristic of its walls. It was not possible to discern precisely the extension of each pancreatic lobe. The pancreas was 0.51±0.1cm thick and the pancreatic duct measured 0.12±0.02cm (right), while the left pancreatic lobe was 0.36±0.25 cm thick and the pancreatic duct measured 0.10±0.04 cm (Fig.4a and 4b).
Stomach and intestinal loops
The stomach was placed caudal to the liver, in the longitudinal access. It had homogeneous echogenicity and echotexture. It was positioned to the left of the spleen and to the right of the proximal intestine and transverse colon and it was 0.16±0.05cm thick. The proximal intestine was 0.3±0.15cm thick and was placed laterally to the right wall. The other segments of the duodenum and intestinal loops could not be properly identified because the quantity of gas present in intestinal loops during the exam (Fig.3c, 4a and 4d).
Liver, gallbladder and caudal vena cava
The liver of the agouti occupies all the cranial space of the abdominal cavity, in direct contact with the diaphragm (echogenic and reflexive surface visualized on its interface with the thoracic cavity) (Fig.5a). This organ presented impressions of adjacent structures that can be properly described and referenced, relating to the stomach centrally, right kidney (hepatorenal fossa) and duodenum in its more cranial portion. Its echotexture is fine and homogeneous, hyperechogenic in relation to the renal cortical parenchyma and hyperechogenic in relation to the spleen. The gallbladder was observed in longitudinal and subcostal access (xifoid process level), and was visualized as a thin-walled oval structure, echogenic with anechogenic content in its interior.
Ultrasound imaging in B-mode, color and spectral Doppler of the liver of agouti. (a) The liver of agouti occupies all the cranial space of the abdominal cavity, in direct contact with the diaphragm. (b) Using color Doppler, the portal flow showed a color map with a red (hepatopetal) signal, while the hepatic veins showed a blue (hepatofugal) signal. Note the laminar flow in both vessels. (c) The portal vein showed a relatively broad and linear pulse discretely influenced by the respiratory phase. (d) The Pulsed Doppler in the hepatic vein showed a polyphasic flow, with two retrograde peaks and one anterograde peak. (e,f) The profile wave of the caudal vena cava, laminar and with two evident anterograde peaks.
The two-dimensional ultrasound examination of the intrahepatic vascular flow in the agoutis allowed individualization of the portal vein (PV) and hepatic veins (HV). The portal veins were different, especially due to the echogenic pattern of their walls, compared to the hepatic veins. In the colored Doppler the portal flow showed a red sign (hepatopetal), while the HV presented a blue sign (hepatofugal) (Fig.5b). These vessels presented laminal flow, when tested with correctly adjusted pulse repetition frequency (PRF). The pulsed Doppler showed a wavy and wide flow pattern due to the discreet influence of the respiratory phase on the PV, whose calculated speed was 20.34±1.25cm/s, with 0.47±0.03 resistivity index (IR) (Fig.5c). The HV showed polyphasic flow, with a peak above the baseline and two peaks below this line, reflecting the influence of the heart cycle on this vessel, showing a 21.23±0.41cm/s flow velocity (Fig.5d). The cava caudal vein (VCC) receives the hepatic veins and was shown in distal plane to the liver, in a left subcostal access. The flow velocity for this vessel was 49.91±2.75 cm/s and the IR was 0.45±0.02 (Fig.5e and 5f).
Discussion
Abdominal access by ultrasonography to the longitudinal, transversal and dorsal axes in agouti was made sequentially and systematically. The organs of the abdominal cavity were assessed and characterized regarding size, position, shape, echogenic, echotexture and angio architecture. Sectoral (4 to 8MHz) and linear (6 to 13MHz) transductors were essentially compatible to lateral and axial resolution to obtain diagnostic images of the abdominal organs of Dasyprocta prymnolopha.
For a better sonographic approach, the animals were submitted to water and food fasts of six and 12 hours, respectively. However, Garcia & Froes (2014)Garcia D.A.A. & Froes T.R. 2014. Importance of fasting in preparing dogs for abdominal ultrasound examination of specific organs. J. Small. Anim. Pract. 55(12):630-634. http://dx.doi.org/10.1111/jsap.12281. PMid:25377227.
https://doi.org/10.1111/jsap.12281...
in a study on preparing dogs for B scan and Doppler ultrasonography examination reported that there was no significant difference in image interpretation between animals that had fasted and those that had not. This information may be beneficial especially for critical patients that are under risk of complications in consequence of prolonged fasting, such as diabetic dogs and/or puppies.
In general, chemical means are not used to restrain the patient during ultrasound examination. However, as agoutis are wild specimens and aiming for better immobilization of the animal, a dissociative anesthetic association was used consisting of ketamine and xylazine. This protocol was previously used by Oliveira et al. (2006)Oliveira F.S., Martins L.L., Duque J.C., Pauloni A.P. & Valadão C.A.A. 2006. Anestesia epidural em cutias (Dasyprocta azarae). Acta Scient. Vet. 34:89-91. and promoted sufficient tranquillization to perform the ultrasonography procedures. The animals showed a heart rate ranging from 120 to 135 bpm (±128 bpm) and blood pressure measured between 110 to 125 mmHg (±117 mmHg), and no hypotensive effect was observed, promoted by the anesthetic protocol used, which could influence the Doppler measurement values.
The ultrasound assessment of the urinary system of this wild species showed many anatomic and topographic similarities with other domestic and wild animals. The urinary bladder presented location, shape and synthopia similar to the crab-eating fox Cerdocyon thous (Silva et al. 2014Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
https://doi.org/10.1590/S0100-736X201400...
), paca Cuniculus paca (Oliveira et al. 2003Oliveira F.S., Machado M.R.F. & Canola J.C. 2003. Real time B-mode ultrasound in pacas pregnancy (Agouti paca Linnaeus, 1766). Braz. J. Vet. Res. Anim. Sci. 40(1):73-78. http://dx.doi.org/10.1590/S1413-95962003000100009.
https://doi.org/10.1590/S1413-9596200300...
, 2007Oliveira F.S., Machado M.R.F., Canola J.C. & Camargo M.H.B. 2007. Uniparidade em pacas criadas em cativeiro (Agouti paca Linnaeus, 1766). Arq. Bras. Med. Vet. Zootec. 59(2):387-389. http://dx.doi.org/10.1590/S0102-09352007000200019.
https://doi.org/10.1590/S0102-0935200700...
, Feliciano et al. 2014Feliciano M.A.R., Barros F.F.P.C., Coutinho L.N., Brito M.B.S., Uscategui R.R., Santos V.J.C., Almeida V.T., Kawanami A.E., Nociti R.P., Machado M.R.F. & Vicente W.R.R. 2014. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Scient. Vet. 42:1-6.), coati Nasua nasua (Ribeiro et al. 2013Ribeiro R.G., Costa A.P., Bragato N., Fonseca A.M., Duque J.C., Prado T.D., Silva A.C. & Borges N.C. 2013. Normal sonographic anatomy of the abdomen coatis (Nasua nasua Linnaeus, 1766). BMC Vet. Res. 9(1):124. http://dx.doi.org/10.1186/1746-6148-9-124. PMid:23800301.
https://doi.org/10.1186/1746-6148-9-124...
) and cheetah Acinonyx jubatus (Carstens et al. 2006Carstens A., Kirberger R.M., Spotswood T., Wagner W.M. & Grimbeek R.J. 2006. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet. Radiol. Ultrasound 47(4):376-383. http://dx.doi.org/10.1111/j.1740-8261.2006.00157.x. PMid:16863057.
https://doi.org/10.1111/j.1740-8261.2006...
). However, compared to a New World monkey species, the common marmoset Callithrix jacchus, the shape diverged, because in this non-human primate species the shape is multilobulate (Wagner & Kirberger 2005Wagner W.M. & Kirberger R.M. 2005. Transcutaneous ultrasonography of the abdômen in the normal common marmoset (Callithrix jacchus). Vet. Radiol. Ultrasound 46(3):251-258. http://dx.doi.org/10.1111/j.1740-8261.2005.00045.x. PMid:16050285.
https://doi.org/10.1111/j.1740-8261.2005...
) that differs from the oval shape observed in the agoutis. Another key point is the mean thickness of the urinary bladder wall, because it is thinner when contrasted with data on other species. In the agoutis, the reference value established in the present study was 0.09±0.03cm, different from that determined by Alves et al. (2007)Alves F.R., Costa F.B., Arouche M.M.S., Barros A.C.E., Miglino M.A., Vulcano L.C. & Guerra P.C. 2007. Avaliação ultra-sonográfica do sistema urinário, fígado e útero do macaco-prego, Cebus apella. Pesq. Vet. Bras. 27(9):377-382. http://dx.doi.org/10.1590/S0100-736X2007000900004.
https://doi.org/10.1590/S0100-736X200700...
in the robust capuchin monkey Cebus apella of 0.2cm and Peixoto et al. (2012)Peixoto G.C.X., Oliveira I.R.S., Alves N.D., Oliveira M.F. & Silva A.R. 2012. Abdominal exploration in captive collared peccaries (Tayassu tajacu) by ultrasonography. Anat. Histol. Embryol. 41(4):256-261. http://dx.doi.org/10.1111/j.1439-0264.2011.01129.x. PMid:22220558.
https://doi.org/10.1111/j.1439-0264.2011...
in the collared peccary Tayassu tajacu of 0.20±0.08cm.
The kidneys of all the agoutis studied presented similar ultrasound appearance to dog kidneys with no evidence of acute or chronic kidney disease (Hart et al. 2013Hart D.V., Winter M.D., Conway J. & Berry C.R. 2013. Ultrasound appearance of the outer medula in dogs without renal dysfunction. Vet. Radiol. Ultrasound 54(6):652-658. PMid:23738847.) and no nematode parasites (Veiga et al. 2011Veiga C.C.P., Azevedo F.D., Fernandes J.I. & Scott F.B. 2011. Ultrassonografia e dopplervelocimetria na avaliação renal de cães parasitados por Dioctophyma renale: relato de caso. Revta Bras. Med. Vet. 33:151-154.). Regarding the renal morphometry, our measurements were within the maximum and minimum limits established by Banzato et al. (2015)Banzato T., Bellini L., Contiero B., Selleri P. & Zotti A. 2015. Abdominal ultrasound features and reference values in 21 healthy rabbits. Vet. Rec. 176(4):101. http://dx.doi.org/10.1136/vr.102657. PMid:25362002.
https://doi.org/10.1136/vr.102657...
in rabbits of 2.8±0.34cm and 1.62±0.17cm for length and width, respectively, for the right kidney and 2.86±0.33cm and 1.72±0.19cm for the left kidney, length and width, respectively. The 1:1 cortex-medullar ratio observed in these wild rodents was individualized and preserved, as observed by Silva et al. (2014)Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
https://doi.org/10.1590/S0100-736X201400...
in crab-eating foxes (Cerdocyon thous) and by Feliciano et al. (2014)Feliciano M.A.R., Barros F.F.P.C., Coutinho L.N., Brito M.B.S., Uscategui R.R., Santos V.J.C., Almeida V.T., Kawanami A.E., Nociti R.P., Machado M.R.F. & Vicente W.R.R. 2014. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Scient. Vet. 42:1-6. in pacas (Cuniculus paca). However, a difference was observed in the cortex-medullar ratio compared with non-human primates (Cebus apella), that showed a 2:1 ratio (Alves et al. 2007Alves F.R., Costa F.B., Arouche M.M.S., Barros A.C.E., Miglino M.A., Vulcano L.C. & Guerra P.C. 2007. Avaliação ultra-sonográfica do sistema urinário, fígado e útero do macaco-prego, Cebus apella. Pesq. Vet. Bras. 27(9):377-382. http://dx.doi.org/10.1590/S0100-736X2007000900004.
https://doi.org/10.1590/S0100-736X200700...
).
Kidney size is a very important parameter to assess kidney disease in dogs. However, due to the wide variability in the dog body conformation, absolute measurements cannot be used as a reliable source for kidney assessment by ultrasonography. Mareschal et al. (2007)Mareschal A., d’Anjou M.A., Moreau M., Alexander K. & Beauregard G. 2007. Ultrasonographic measurement of kidney-to-aorta ratio as a method of estimating renal size in dogs. Vet. Radiol. Ultrasound 48(5):434-438. http://dx.doi.org/10.1111/j.1740-8261.2007.00274.x. PMid:17899978.
https://doi.org/10.1111/j.1740-8261.2007...
standardized a portion between the kidney lengths and the aorta luminal diameter, establishing that the kidney size should be considered reduced if the kidneys:aorta ratio was greater than 5.5 and increased if it was greater than 9:1. Starting from this premise, we standardized the kidney:aorta ratio for agoutis, but differently to that found in dogs, the species under study did not present great variability in body conformation. Thus, in these rodents the kidney size is considered reduced or increased if the kidney:aorta ratio is smaller or bigger than the mean values of 7.20±0.27 and 6.86±0.10, for the right and left kidneys respectively.
The renal artery blood perfusion in the colored Doppler showed no stenosis, dilations or hypoflows. In spectral Doppler arterial flow velocity of 75.83±1.42cm/s and 80.43±1.22cm/s was observed for the right and left kidneys, respectively. These values are very close to those reported by Melo et al. (2006)Melo M.B., Veado J.C.C., Silva E.F., Moreira S.M. & Passos L.M.F. 2006. Dopplerfluxometria das artérias renais: valores normais das velocidades sistólica e diastólica e do índice resistivo nas artérias renais principais. Arq. Bras. Med. Vet. Zootec. 58(4):691-693. http://dx.doi.org/10.1590/S0102-09352006000400040.
https://doi.org/10.1590/S0102-0935200600...
for dogs. These authors observed that the renal arterial flow velocity was 79.96±8.82cm/s for the right kidney and 80.22±6.99cm/s for the left kidney. In spite of the similarity found in the arterial flow velocity values, this did not occur with the resistivity index, because in dogs this parameter shows higher values (0.64±0.04 - principal right artery and 0.63±0.028 - principal left artery) than those observed in agouti (0.54±0.16 - principal right artery and 0.50±0.11 - principal left artery). Carstens et al. (2006)Carstens A., Kirberger R.M., Spotswood T., Wagner W.M. & Grimbeek R.J. 2006. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet. Radiol. Ultrasound 47(4):376-383. http://dx.doi.org/10.1111/j.1740-8261.2006.00157.x. PMid:16863057.
https://doi.org/10.1111/j.1740-8261.2006...
studied cheetahs (Acinonyx jubatus) and observed a 0.58 mean resistivity index, and found a smaller value than that determined in dogs (Melo et al. 2006Melo M.B., Veado J.C.C., Silva E.F., Moreira S.M. & Passos L.M.F. 2006. Dopplerfluxometria das artérias renais: valores normais das velocidades sistólica e diastólica e do índice resistivo nas artérias renais principais. Arq. Bras. Med. Vet. Zootec. 58(4):691-693. http://dx.doi.org/10.1590/S0102-09352006000400040.
https://doi.org/10.1590/S0102-0935200600...
) and bigger than that established in agoutis by our study. However, it is emphasized that Carstens et al. (2006)Carstens A., Kirberger R.M., Spotswood T., Wagner W.M. & Grimbeek R.J. 2006. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet. Radiol. Ultrasound 47(4):376-383. http://dx.doi.org/10.1111/j.1740-8261.2006.00157.x. PMid:16863057.
https://doi.org/10.1111/j.1740-8261.2006...
only managed to assess his parameter in three animals for the left kidney and two animals for the right kidney, while in our research this index was assessed in all the specimens studied.
The assessment of the adrenal glands showed no presence of intervenient variables, because the animals were not restless and did not have tachypnea resulting from the stress of physical restraint. Although this organ is difficult to be seen by ultrasonography, there was no problem regarding obtaining ultrasound images in the agoutis studied. The acoustic window used to capture the images did not interfere with the morphological characteristics.
In dogs, the limit value reported to suggest an increase in the adrenal gland is 0.74cm (Barthez et al. 1995Barthez P.Y., Nyland T.G. & Feldman E.C. 1995. Ultrasonographic evaluation of the adrenal glands in dogs. J. Am. Vet. Med. Assoc. 207(9):1180-1183. PMid:7559065., 1998Barthez P.Y., Nyland T.G. & Feldman E.C. 1998. Ultrasonography of the adrenal glands in the dog, cat, and ferret. Vet. Clin. N. Am., Small. Anim. Pract. 28(4):869-885. PMid:9698619.). From this information, Soulsby et al. (2015)Soulsby S.N., Holland M., Hudson J.A. & Behrend E.N. 2015. Ultrasonographic evaluation of adrenal gland size compared to body weight in normal dogs. Vet. Radiol. Ultrasound 56(3):317-326. http://dx.doi.org/10.1111/vru.12236. PMid:25496665.
https://doi.org/10.1111/vru.12236...
stratified maximum values according to animal size, using the thickness of the adrenal caudal pole in the sagittal plane, thus for dogs weighing <10kg, 10-30kg and >30kg, the limits were 0.54cm; 0.68cm and 0.80cm, respectively. The agoutis in the present research had mean of 0.22±0.06cm and 0.20±0.03cm for the right and left adrenal glands, respectively. These animals fit in the <10kg weight range, corresponding to small sized dogs. In another panorama, lagomorphs, such as the rabbit, have thicker adrenal glands (0.34±0.08cm and 0.38±0.11cm for right and left adrenal glands respectively) than those observed in agoutis.
The agouti spleen presented thin and homogeneous echotexture, as observed by Silva et al. (2014)Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
https://doi.org/10.1590/S0100-736X201400...
in crab-eating foxes (Cerdocyon thous), Feliciano et al. (2014)Feliciano M.A.R., Barros F.F.P.C., Coutinho L.N., Brito M.B.S., Uscategui R.R., Santos V.J.C., Almeida V.T., Kawanami A.E., Nociti R.P., Machado M.R.F. & Vicente W.R.R. 2014. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Scient. Vet. 42:1-6. in pacas (Cuniculus paca) and Peixoto et al. (2012)Peixoto G.C.X., Oliveira I.R.S., Alves N.D., Oliveira M.F. & Silva A.R. 2012. Abdominal exploration in captive collared peccaries (Tayassu tajacu) by ultrasonography. Anat. Histol. Embryol. 41(4):256-261. http://dx.doi.org/10.1111/j.1439-0264.2011.01129.x. PMid:22220558.
https://doi.org/10.1111/j.1439-0264.2011...
and wild pigs. In addition, greater echogenicity was observed compared with the left kidney, a fact also observed in crab-eating foxes (Cerdocyon thous) by Silva et al. (2014)Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
https://doi.org/10.1590/S0100-736X201400...
and in coatis (Nasua nasua) by (Ribeiro et al. 2013Ribeiro R.G., Costa A.P., Bragato N., Fonseca A.M., Duque J.C., Prado T.D., Silva A.C. & Borges N.C. 2013. Normal sonographic anatomy of the abdomen coatis (Nasua nasua Linnaeus, 1766). BMC Vet. Res. 9(1):124. http://dx.doi.org/10.1186/1746-6148-9-124. PMid:23800301.
https://doi.org/10.1186/1746-6148-9-124...
).
The liver, largest gland and second-largest organ of these rodents occupies all the cranial space of the abdominal cavity showing homogeneous texture and visible gallbladder in all the animals with rounded shape filled with anechogenic content. These ultrasound morphological characteristics were also found in procyonidae (Ribeiro et al. 2013Ribeiro R.G., Costa A.P., Bragato N., Fonseca A.M., Duque J.C., Prado T.D., Silva A.C. & Borges N.C. 2013. Normal sonographic anatomy of the abdomen coatis (Nasua nasua Linnaeus, 1766). BMC Vet. Res. 9(1):124. http://dx.doi.org/10.1186/1746-6148-9-124. PMid:23800301.
https://doi.org/10.1186/1746-6148-9-124...
). In New World monkeys the gallbladder morphology differs from that observed in agoutis, and is characterized by as multilobulate with wide and thread-like cystic duct (Wagner & Kirberger 2005Wagner W.M. & Kirberger R.M. 2005. Transcutaneous ultrasonography of the abdômen in the normal common marmoset (Callithrix jacchus). Vet. Radiol. Ultrasound 46(3):251-258. http://dx.doi.org/10.1111/j.1740-8261.2005.00045.x. PMid:16050285.
https://doi.org/10.1111/j.1740-8261.2005...
).
It was observed in the agouti that the HV (21.23±0.41cm/s) and CCV vein (49.91±2.75cm/s) flow velocities were close to the velocities established in the same vessels by Carstens et al. (2006)Carstens A., Kirberger R.M., Spotswood T., Wagner W.M. & Grimbeek R.J. 2006. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet. Radiol. Ultrasound 47(4):376-383. http://dx.doi.org/10.1111/j.1740-8261.2006.00157.x. PMid:16863057.
https://doi.org/10.1111/j.1740-8261.2006...
in cheetahs (21.23±0.41cm/s and 33.8±19.8cm/s respectively). In spite of this, the flow velocity of the agouti portal vein was 75% C greater than that shown in these wild cats. The HV flow pattern shown by pulsed Doppler was characterized by a peak above the baseline and two peaks below it, showing the influence of the heart cycle on this blood vessel. Corroborating our results, Huang et al. (2004)Huang T.L., Chen T.Y., Tsang L.L., Sun P.L., Chen Y.S., Wang C.C., Wang S.H., Lin T.S., Chiang Y.C., Chiu K.W., Eng H.L., Jawan B., Cheng Y.F. & Chen C.L. 2004. Hepatic venous stenosis in partial liver graft transplantation detected by color Doppler ultrasound before and after radiological interventional management. Transplant. Proc. 36(8):2342-2343. http://dx.doi.org/10.1016/j.transproceed.2004.07.067. PMid:15561243.
https://doi.org/10.1016/j.transproceed.2...
stated that the hepatic vein flow is biphasic and can be correlated with respiration or pressure alterations in the right atrium. Bogin et al. (2005)Bogin V., Marcos A. & Shaw-Stiffel T. 2005. Budd-Chiari syndrome: in evolution. Eur. J. Gastroenterol. Hepatol. 17(1):33-35. http://dx.doi.org/10.1097/00042737-200501000-00007. PMid:15647637.
https://doi.org/10.1097/00042737-2005010...
and Yoshimoto et al. (2001)Yoshimoto K., Yakushiji K., Ijuin H., Ono N., Hashiguchi M., Imamura R., Ogata H., Okamura T., Sata M. & Hashimoto H. 2001. Colour Doppler ultrasonography of a segmental branch of the portal vein is useful for early diagnosis and monitoring of the therapeutic course of veno-occlusive disease after allogenic haematopoietic stem cell transplantation. Brit. J. Haematol. 115(4):945-948. http://dx.doi.org/10.1046/j.1365-2141.2001.03213.x. PMid:11843831.
https://doi.org/10.1046/j.1365-2141.2001...
reported that the flow velocity of the hepatic and portal veins are important in assessing veno-oclusive diseases in human beings, especially with inverted flow from the portal vein and low velocity monophasic hepatic flow.
Barberet et al. (2008)Barberet V., Schreurs E., Rademacher N., Nitzl D., Taeymans O., Duchateau L. & Saunders J.H. 2008. Quantification of the effect of various patient and image factors on ultrasonographic detection of select canine abdominal organs. Vet. Radiol. Ultrasound 49(3):273-276. http://dx.doi.org/10.1111/j.1740-8261.2008.00365.x. PMid:18546785.
https://doi.org/10.1111/j.1740-8261.2008...
quantified in one hundred dog patients the detection of ultrasound images of the pancreas and verified that the left pancreatic lobe, pancreas body and left pancreatic lobe had frequencies of 56%, 60% and 87%, respectively. In agoutis, they were both visualized in all the animals, in synthopia with organs considered key for identifying this gland, such as the descending duodenum, kidneys, portal vein and stomach. The total extension of the pancreatic lobes was difficult to distinguish from the other mesenteric structures.
The ultrasound characteristics of the agouti stomach were similar to those described for pacas Cuniculus paca (Feliciano et al. 2014Feliciano M.A.R., Barros F.F.P.C., Coutinho L.N., Brito M.B.S., Uscategui R.R., Santos V.J.C., Almeida V.T., Kawanami A.E., Nociti R.P., Machado M.R.F. & Vicente W.R.R. 2014. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Scient. Vet. 42:1-6.) and wild dogs Cerdocyon thous (Heleno et al. 2011Heleno A.R., Santos L.M., Miglino M.A., Peres J.A. & Guerra R.R. 2011. Biometria, histologia e morfometria do sistema digestório do cachorro-do-mato (Cerdocyon thous) de vida livre. Biotemas 24:111-199. http://dx.doi.org/10.5007/2175-7925.2011v24n4p111
https://doi.org/10.5007/2175-7925.2011v2...
). The stomach walls (0.16±0.05cm) and duodenum (0.3±0.15cm) of agoutis are thicker than those of the rabbit stomach (0.10±0.01cm) and duodenum (0.19±0.04cm) (Banzato et al. 2015Banzato T., Bellini L., Contiero B., Selleri P. & Zotti A. 2015. Abdominal ultrasound features and reference values in 21 healthy rabbits. Vet. Rec. 176(4):101. http://dx.doi.org/10.1136/vr.102657. PMid:25362002.
https://doi.org/10.1136/vr.102657...
), but crab-eating foxes (0.39±0.05cm) (Silva et al. 2014Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
https://doi.org/10.1590/S0100-736X201400...
) and collared peccaries (0.42±0.28cm) (Peixoto et al. 2012Peixoto G.C.X., Oliveira I.R.S., Alves N.D., Oliveira M.F. & Silva A.R. 2012. Abdominal exploration in captive collared peccaries (Tayassu tajacu) by ultrasonography. Anat. Histol. Embryol. 41(4):256-261. http://dx.doi.org/10.1111/j.1439-0264.2011.01129.x. PMid:22220558.
https://doi.org/10.1111/j.1439-0264.2011...
) have much thicker stomach walls than agoutis.
The diagnostic assessment of the abdominal vessels, whether by arteriography or ultrasonography, is still little used in small animal clinical routine. The mean values of the vascular indices of the agouti abdominal aorta (95.2±2.16cm/s) were similar to those described in the literature by Carvalho et al. (2008)Carvalho C.F., Chammas M.C., Stermann F.D.A., Barros N. & Cerri G.G. 2008. Ultra-sonografia dúplex-Doppler na avaliação morfológica e hemodinâmica das artérias aorta e mesentérica cranial em cães. Braz. J. Vet. Res. Anim. Sci. 45(1):24-31. http://dx.doi.org/10.11606/issn.1678-4456.bjvras.2008.26716.
https://doi.org/10.11606/issn.1678-4456....
for the domestic dog (95.49±35.43cm/s).
Conclusion
This study characterized the agouti abdominal organs and blood vessels by B scan and Doppler ultrasonography, what allowed definition of the size, shape composition, echogenicity and echotexture of the anatomic constituents, and also established reference values for the vessel network and blood flow.
Acknowledgements
The authors thank the University Veterinary Hospital, Federal University of Piauí (HVU-UFPI), in name of Professor Dr. João Macedo de Sousa (Diagnosis by Image Setor, HUV-UFPI) and the Nucleus for Wild Animal Study and Conservation (Núcleo de Estudos e Preservação de Animais Silvestres (NEPAS) of teh Federal University of Pauí in name of Dr. Paulo Marques Costa. They also thank the Coordination for Improvement of Higher Education Personnel (CAPES) for conceding a doctorate grant.
References
- Alves F.R., Costa F.B., Arouche M.M.S., Barros A.C.E., Miglino M.A., Vulcano L.C. & Guerra P.C. 2007. Avaliação ultra-sonográfica do sistema urinário, fígado e útero do macaco-prego, Cebus apella Pesq. Vet. Bras. 27(9):377-382. http://dx.doi.org/10.1590/S0100-736X2007000900004.
» https://doi.org/10.1590/S0100-736X2007000900004 - Asquith N.M., Terborgh J., Arnold E. & Riveros M. 1999. The fruits the agouti ate: Hymenaea courbaril seed fate when its disperser is absent. J. Trop. Ecol. 15(2):229-235. http://dx.doi.org/10.1017/S0266467499000772.
» https://doi.org/10.1017/S0266467499000772 - Banzato T., Bellini L., Contiero B., Selleri P. & Zotti A. 2015. Abdominal ultrasound features and reference values in 21 healthy rabbits. Vet. Rec. 176(4):101. http://dx.doi.org/10.1136/vr.102657. PMid:25362002.
» https://doi.org/10.1136/vr.102657 - Barberet V., Schreurs E., Rademacher N., Nitzl D., Taeymans O., Duchateau L. & Saunders J.H. 2008. Quantification of the effect of various patient and image factors on ultrasonographic detection of select canine abdominal organs. Vet. Radiol. Ultrasound 49(3):273-276. http://dx.doi.org/10.1111/j.1740-8261.2008.00365.x. PMid:18546785.
» https://doi.org/10.1111/j.1740-8261.2008.00365.x - Barthez P.Y., Nyland T.G. & Feldman E.C. 1995. Ultrasonographic evaluation of the adrenal glands in dogs. J. Am. Vet. Med. Assoc. 207(9):1180-1183. PMid:7559065.
- Barthez P.Y., Nyland T.G. & Feldman E.C. 1998. Ultrasonography of the adrenal glands in the dog, cat, and ferret. Vet. Clin. N. Am., Small. Anim. Pract. 28(4):869-885. PMid:9698619.
- Bogin V., Marcos A. & Shaw-Stiffel T. 2005. Budd-Chiari syndrome: in evolution. Eur. J. Gastroenterol. Hepatol. 17(1):33-35. http://dx.doi.org/10.1097/00042737-200501000-00007. PMid:15647637.
» https://doi.org/10.1097/00042737-200501000-00007 - Carstens A., Kirberger R.M., Spotswood T., Wagner W.M. & Grimbeek R.J. 2006. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet. Radiol. Ultrasound 47(4):376-383. http://dx.doi.org/10.1111/j.1740-8261.2006.00157.x. PMid:16863057.
» https://doi.org/10.1111/j.1740-8261.2006.00157.x - Carvalho C.F., Chammas M.C., Stermann F.D.A., Barros N. & Cerri G.G. 2008. Ultra-sonografia dúplex-Doppler na avaliação morfológica e hemodinâmica das artérias aorta e mesentérica cranial em cães. Braz. J. Vet. Res. Anim. Sci. 45(1):24-31. http://dx.doi.org/10.11606/issn.1678-4456.bjvras.2008.26716.
» https://doi.org/10.11606/issn.1678-4456.bjvras.2008.26716 - Emmons L.H. & Feer F. 1997. Neotropical Rainforest Mammals: a field guide. University of Chicago Press, Chicago. 2nd ed. 134p.
- Feliciano M.A.R., Barros F.F.P.C., Coutinho L.N., Brito M.B.S., Uscategui R.R., Santos V.J.C., Almeida V.T., Kawanami A.E., Nociti R.P., Machado M.R.F. & Vicente W.R.R. 2014. Conventional and Doppler abdominal ultrasonography in pacas (Cuniculus paca). Acta Scient. Vet. 42:1-6.
- Forget P.M. & Milleron T. 1991. Evidence for secondary seed dispersal by rodents in Panama. Ecologia 87(4):596-599. PMid:28313705.
- Fragoso J.M.V. 1997. Tapir-generated seed shadows: scale-dependent patchiness in the Amazon rain forest. J. Ecol. 85(4):519-529. http://dx.doi.org/10.2307/2960574.
» https://doi.org/10.2307/2960574 - Garcia D.A.A. & Froes T.R. 2014. Importance of fasting in preparing dogs for abdominal ultrasound examination of specific organs. J. Small. Anim. Pract. 55(12):630-634. http://dx.doi.org/10.1111/jsap.12281. PMid:25377227.
» https://doi.org/10.1111/jsap.12281 - Hart D.V., Winter M.D., Conway J. & Berry C.R. 2013. Ultrasound appearance of the outer medula in dogs without renal dysfunction. Vet. Radiol. Ultrasound 54(6):652-658. PMid:23738847.
- Heleno A.R., Santos L.M., Miglino M.A., Peres J.A. & Guerra R.R. 2011. Biometria, histologia e morfometria do sistema digestório do cachorro-do-mato (Cerdocyon thous) de vida livre. Biotemas 24:111-199. http://dx.doi.org/10.5007/2175-7925.2011v24n4p111
» https://doi.org/10.5007/2175-7925.2011v24n4p111 - Hosken F.M. & Silveira A.C. 2001. Criação de Cutias. Aprenda Fácil, Viçosa. 234p.
- Huang T.L., Chen T.Y., Tsang L.L., Sun P.L., Chen Y.S., Wang C.C., Wang S.H., Lin T.S., Chiang Y.C., Chiu K.W., Eng H.L., Jawan B., Cheng Y.F. & Chen C.L. 2004. Hepatic venous stenosis in partial liver graft transplantation detected by color Doppler ultrasound before and after radiological interventional management. Transplant. Proc. 36(8):2342-2343. http://dx.doi.org/10.1016/j.transproceed.2004.07.067. PMid:15561243.
» https://doi.org/10.1016/j.transproceed.2004.07.067 - Lange R.B. & Jablonski E.F. 1981. Lista prévia dos Mammalia do Estado do Paraná. Revta Estud. Biol., PUCPR, 4:1-35.
- Lopes E.R., Morgado T.O., Meireles Y.S., Jorge A.A., Zago A.A.Q., Corrêa S.H.R., Paz R.C.R. & Nespóli P.B. 2015. Ultrassonografia abdominal de tamanduás-bandeira (Myrmecophaga tridactyla Linnaeus, 1758) mantidos em cativeiro. Pesq. Vet. Bras. 35(11):919-924. http://dx.doi.org/10.1590/S0100-736X2015001100008.
» https://doi.org/10.1590/S0100-736X2015001100008 - Mareschal A., d’Anjou M.A., Moreau M., Alexander K. & Beauregard G. 2007. Ultrasonographic measurement of kidney-to-aorta ratio as a method of estimating renal size in dogs. Vet. Radiol. Ultrasound 48(5):434-438. http://dx.doi.org/10.1111/j.1740-8261.2007.00274.x. PMid:17899978.
» https://doi.org/10.1111/j.1740-8261.2007.00274.x - Melo M.B., Veado J.C.C., Silva E.F., Moreira S.M. & Passos L.M.F. 2006. Dopplerfluxometria das artérias renais: valores normais das velocidades sistólica e diastólica e do índice resistivo nas artérias renais principais. Arq. Bras. Med. Vet. Zootec. 58(4):691-693. http://dx.doi.org/10.1590/S0102-09352006000400040.
» https://doi.org/10.1590/S0102-09352006000400040 - Moojen J. 1952. Os Roedores do Brasil. Instituto Nacional do Livro, Rio de Janeiro. 215p.
- Oliveira F.S., Machado M.R.F. & Canola J.C. 2003. Real time B-mode ultrasound in pacas pregnancy (Agouti paca Linnaeus, 1766). Braz. J. Vet. Res. Anim. Sci. 40(1):73-78. http://dx.doi.org/10.1590/S1413-95962003000100009.
» https://doi.org/10.1590/S1413-95962003000100009 - Oliveira F.S., Machado M.R.F., Canola J.C. & Camargo M.H.B. 2007. Uniparidade em pacas criadas em cativeiro (Agouti paca Linnaeus, 1766). Arq. Bras. Med. Vet. Zootec. 59(2):387-389. http://dx.doi.org/10.1590/S0102-09352007000200019.
» https://doi.org/10.1590/S0102-09352007000200019 - Oliveira F.S., Martins L.L., Duque J.C., Pauloni A.P. & Valadão C.A.A. 2006. Anestesia epidural em cutias (Dasyprocta azarae). Acta Scient. Vet. 34:89-91.
- Peixoto G.C.X., Oliveira I.R.S., Alves N.D., Oliveira M.F. & Silva A.R. 2012. Abdominal exploration in captive collared peccaries (Tayassu tajacu) by ultrasonography. Anat. Histol. Embryol. 41(4):256-261. http://dx.doi.org/10.1111/j.1439-0264.2011.01129.x. PMid:22220558.
» https://doi.org/10.1111/j.1439-0264.2011.01129.x - Peres C.A. & Baider C. 1997. Seed dispersal, spatial distribution and population structure of Brazil nut trees (Bertholletia excelsa) in Southeastern Amazonia. J. Trop. Ecol. 13(04):595-616. http://dx.doi.org/10.1017/S0266467400010749.
» https://doi.org/10.1017/S0266467400010749 - Ribeiro R.G., Costa A.P., Bragato N., Fonseca A.M., Duque J.C., Prado T.D., Silva A.C. & Borges N.C. 2013. Normal sonographic anatomy of the abdomen coatis (Nasua nasua Linnaeus, 1766). BMC Vet. Res. 9(1):124. http://dx.doi.org/10.1186/1746-6148-9-124. PMid:23800301.
» https://doi.org/10.1186/1746-6148-9-124 - Silva A.S.L., Feliciano M.A.R., Motheo T.F., Oliveira J.P., Kawanami A.E., Werther K., Palha M.D.C. & Vicente W.R.R. 2014. Mode B ultrasonography and abdominal Doppler in crab-eating-foxes (Cerdocyon thous). Pesq. Vet. Bras. 34(Suppl.1):23-28. http://dx.doi.org/10.1590/S0100-736X2014001300005.
» https://doi.org/10.1590/S0100-736X2014001300005 - Soulsby S.N., Holland M., Hudson J.A. & Behrend E.N. 2015. Ultrasonographic evaluation of adrenal gland size compared to body weight in normal dogs. Vet. Radiol. Ultrasound 56(3):317-326. http://dx.doi.org/10.1111/vru.12236. PMid:25496665.
» https://doi.org/10.1111/vru.12236 - Sousa F., Pessoa G.T., Moura L.S., Araújo J.R., Rodrigues R., Barbosa M., Diniz A.N., Souza A.B., Silva E.G., Lucena L.U., Sanches M.P., Silva-Filho O.F., Guerra P.C., Sousa J.M., Neves W.C. & Alves F.R. 2017. Organogenesis and foetal haemodynamics during the normal gestacion of healthy black-rumped agoutis (Dasyprocta prymnolopha Wagler, 1831) bred in captivity. Reprod. Domest. Anim. 52(1):60-66. http://dx.doi.org/10.1111/rda.12803. PMid:27687997.
» https://doi.org/10.1111/rda.12803 - Sousa F.C., Alves F.R., Fortes E.A., Ferraz M.S., Machado Júnior A.A., Menezes D.J. & Carvalho M.A. 2012. Pregnancy in Histrycomorpha: gestacional age and embryonic-fetal development of agouti (Dasyprocta prymnolopha Wagler, 1831) estimated by ultrasonography. Theriogenology 78(6):1278-1285. http://dx.doi.org/10.1016/j.theriogenology.2012.05.023. PMid:22898012.
» https://doi.org/10.1016/j.theriogenology.2012.05.023 - Sousa F.C.A., Pessoa G.T., Moura L.S., Rodrigues R.P.S., Diniz A.N., Souza A.B., Silva E.G., Sanches M.P., Silva-Filho O.F., Guerra P.C., Sousa J.M., Neves W.C. & Alves F.R. 2016. Doppler ultrasound of the placenta and maternal and fetal vessels during normal gestacion in captive agoutis (Dasyprocta prymnolopha Wagler, 1831). Theriogenology 86(8):1921-1930. http://dx.doi.org/10.1016/j.theriogenology.2016.06.006. PMid:27458115.
» https://doi.org/10.1016/j.theriogenology.2016.06.006 - Veiga C.C.P., Azevedo F.D., Fernandes J.I. & Scott F.B. 2011. Ultrassonografia e dopplervelocimetria na avaliação renal de cães parasitados por Dioctophyma renale: relato de caso. Revta Bras. Med. Vet. 33:151-154.
- Wagner W.M. & Kirberger R.M. 2005. Transcutaneous ultrasonography of the abdômen in the normal common marmoset (Callithrix jacchus). Vet. Radiol. Ultrasound 46(3):251-258. http://dx.doi.org/10.1111/j.1740-8261.2005.00045.x. PMid:16050285.
» https://doi.org/10.1111/j.1740-8261.2005.00045.x - Yoshimoto K., Yakushiji K., Ijuin H., Ono N., Hashiguchi M., Imamura R., Ogata H., Okamura T., Sata M. & Hashimoto H. 2001. Colour Doppler ultrasonography of a segmental branch of the portal vein is useful for early diagnosis and monitoring of the therapeutic course of veno-occlusive disease after allogenic haematopoietic stem cell transplantation. Brit. J. Haematol. 115(4):945-948. http://dx.doi.org/10.1046/j.1365-2141.2001.03213.x. PMid:11843831.
» https://doi.org/10.1046/j.1365-2141.2001.03213.x
Publication Dates
-
Publication in this collection
Apr 2018
History
-
Received
29 June 2017 -
Accepted
07 Sept 2017