Abstract
Background and objectives:
Pediatric patients frequently require deep sedation or general anesthesia for colonoscopy. This study was designed to compare the sedative efficacy of remifentanil-ketamine combination with propofol-ketamine combination in children undergoing colonoscopy.
Methods:
Seventy patients, between 2 and 16 years of age, scheduled for diagnostic colonoscopy were randomly allocated into two groups. Remifentanil-ketamine group received intravenous ketamine 2 mg.kg−1 and remifentanil 0.25 µg.kg−1 combination, followed by 0.1 µg.kg−1.min−1 remifentanil infusion. Propofol-ketamine group received intravenous propofol 1 and 2 mg.kg−1 ketamine combination, followed by 1 mg.kg−1.h−1 propofol infusion. In the case of children discomfort (cry, movement, and cough), remifentanil 0.1 µg.kg−1 in the remifentanil-ketamine group or propofol 0.5 mg.kg−1 in the propofol-ketamine group were administered to improve children discomfort. Despite the therapy given above, if children still experience discomfort, 1 mg.kg−1 of ketamine was administered as a rescue drug, regardless of the group. Ramsay sedation score, hemodynamic variables, drug requirements, gastroenterologists' satisfaction, colonoscopy duration, recovery time, and side effects were recorded throughout the procedure and the recovery period.
Results:
The percentage of patients with a Ramsay sedation score of 4 or higher during the procedure was 73.5 and 37.1% in remifentanil-ketamine and propofol-ketamine groups, respectively (p = 0.02). Systolic and diastolic blood pressure variables were significantly higher only after induction in the remifentanil-ketamine group than in the propofol-ketamine group (p = 0.015).
Conclusion:
Coadministration of ketamine with either remifentanil or propofol effectively and safely provides sedation and analgesia in children undergoing colonoscopy. Sedation scores were significantly better in remifentanil-ketamine group than in propofol-ketamine group.
KEYWORDS
Outpatient; Remifentanil; Ketamine; Propofol; Children; Colonoscopy
Resumo
Justificativa e objetivos:
Os pacientes pediátricos com frequência precisam de sedação profunda ou anestesia geral para colonoscopia. Este estudo foi desenhado para comparar a eficácia sedativa da combinação de remifentanil-cetamina e de propofol-cetamina em crianças submetidas à colonoscopia.
Métodos:
Setenta pacientes, entre 2-16 anos, programados para colonoscopia diagnóstica foram alocados randomicamente em dois grupos. O grupo remifentanil-cetamina recebeu a combinação de 2 mg.kg−1 de cetamina por via intravenosa e 0,25 µg.kg−1 de remifentanil; seguido de infusão de remifentanil (0,1 µg.kg−1.min−1). O grupo propofol-cetamina recebeu a combinação de 1 mg.kg−1 de propofol e 2 mg.kg−1 de cetamina; seguido de infusão de propofol (1 mg.kg−1.h−1). Em caso de desconforto das crianças (choro, movimento e tosse), remifentanil (0,1 µg.kg−1) seria administrado ao grupo remifentanil-cetamina ou propofol (0,5 mg.kg−1) ao grupo propofol-cetamina. A despeito da terapia acima citada, caso as crianças ainda sentissem desconforto, cetamina (1 mg.kg−1) seria administrada como fármaco de resgate, independentemente do grupo. Escore de sedação de Ramsay, variáveis hemodinâmicas, necessidade de medicamentos, satisfação dos gastroenterologistas, duração da colonoscopia, tempo de recuperação e efeitos colaterais foram registrados durante o procedimento e o período de recuperação.
Resultados:
O percentual de pacientes com escore 4 ou mais na escala de sedação de Ramsay durante o procedimento foi de 73,5% e 37,1% nos grupos remifentanil-cetamina e propofol-cetamina, respectivamente, (p = 0,02). As variáveis, pressão arterial sistólica e diastólica, foram significativamente maiores no grupo remifentanil-cetamina do que no grupo propofol-cetamina, mas somente após a indução (p = 0,015).
Conclusão:
A coadministração de cetamina com remifentanil ou propofol fornece sedação e analgesia de forma eficaz e segura em crianças submetidas à colonoscopia. Os escores de sedação foram significativamente melhores no grupo remifentanil-cetamina do que no grupo propofol-cetamina.
PALAVRAS-CHAVE
Paciente ambulatorial; Remifentanil; Cetamina; Propofol; Crianças; Colonoscopia
Introduction
Colonoscopy is a useful intervention in the diagnosis and treatment of intestinal diseases. The procedure itself can be painful for patients. The pain is caused by the inflation of the colon with gas, which allows for inspection. Although adult patients tolerate colonoscopy with conscious sedation, pediatric patients frequently require deep sedation or general anesthesia, because of their relatively high level of anxiety, lack of cooperation, and pain perception.11 Mahoney LB, Lightdale JR. Sedation of the pediatric and adolescent patient for GI procedures. Curr Treat Options Gastroenterol. 2007;10:412-21. In recent years, ketamine and propofol have been proposed as the sedation agents of choice during colonoscopy.22 Baykal TZ, Gulec H, Derelı N, et al. Propofol-ketamine combination: a choice with less complications and better hemodynamic stability compared to propofol? On a prospective study in a group of colonoscopy patients. Ir J Med Sci. 2016;185:699-704.
Propofol is a sedative-hypnotic agent with lack of analgesic properties leading to increased anesthetic or analgesic consumption during colonoscopy.33 Arora S. Combining ketamine and propofol ("Ketofol") for emergency department procedural sedation and analgesia: a review. West JEM. 2008;9:20-3.,44 Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983-8. Remifentanil is an ultra-short-acting opioid that offers rapid onset of action and rapid recovery time. However, it can cause dose-dependent respiratory depression.55 Battershill AJ, Keating GM. Remifentanil: a review of its analgesic and sedative use in the intensive care unit. Drugs. 2006;66:365-85.
Ketamine is an anesthetic that has been used as a sole or supplement for other sedative-hypnotic anesthetics for sedation and analgesia in children undergoing outpatient's procedures. In previous studies, ketamine combined with either propofol or remifentanil has been used safely in children.44 Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983-8.,66 Lee JA, Jeon YS, Noh HI, et al. The effect of ketamine with remifentanil for improving the quality of anaesthesia and recovery in paediatric patients undergoing middle-ear ventilation tube insertion. J Int Med Res. 2011;39:2239-46. However, when ketamine is used in combination with propofol or remifentanil, children may still become in pain or discomfort, disoriented, and movement during the procedure.44 Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983-8.,66 Lee JA, Jeon YS, Noh HI, et al. The effect of ketamine with remifentanil for improving the quality of anaesthesia and recovery in paediatric patients undergoing middle-ear ventilation tube insertion. J Int Med Res. 2011;39:2239-46.
As the ideal drug, dose, or combination is yet to be found to provide satisfactory sedation, analgesia, and comfort during the outpatient procedures, choice of drug, dose, or combination to achieve the desired clinical effect may be important.
This prospective, randomized, double-blind, controlled study was designed to compare the sedative efficacy and comfort of remifentanil-ketamine combination with propofol-ketamine combination in children undergoing colonoscopy. The hypothesis was that, in children undergoing colonoscopy, remifentanil-ketamine combination would provide better intraoperative sedation, children comfort, and less drug consumption compared with propofol-ketamine combination.
Material and methods
Patients
After obtaining ethical committee approval (2015-44/6) and informed parental and children consent, 70 ASA I-II children 2-16-year aged undergoing colonoscopy procedure with sedation were included in the study. This study was registered with the Clinical Research Information Service (NCT02602743).
Children with cardiovascular, cerebral, pulmonary, renal, or hepatic diseases were excluded from the study. The children had fasted for at least 6 h before the procedure. All patients were administered 0.5 mg.kg−1 midazolam orally with 10 mL of water as premedication, 30 min before the procedure. An intravenous line and standard anesthesia monitoring (including cardiovascular and respiratory monitoring) were in place during the sedation and recovery period. Children received 10 mL.kg−1.h−1 crystalloid infusion during the procedure and supplement oxygen (2-4 L.min−1) were applied via a nasal cannula.
Randomization
Patients were randomized by use of a randomized block design with block sizes of 10. Allocation was concealed with a pre-specified computer-generated randomization list.
Sedation protocol
The remifentanil-ketamine (RK) group received intravenous (iv) ketamine 2 mg.kg−1 (Ketalar flakon, Pfizer, New York, ABD) and iv remifentanil 0.25 µg.kg−1 (Ultiva flakon, GlaxoSmithKline, Brentford, England) combination for the induction of sedation within 1 min as bolus. Then, 0.1 µg.kg−1.min−1 remifentanil infusion was initiated and continued until the end of procedure for maintenance. The propofol-ketamine (PK) group received iv ketamine 2 mg.kg−1 and propofol 1 mg.kg−1 (Propofol ampul, Fresenius Kabi, Copenhagen, Denmark) combination for the induction of sedation. Then, iv propofol 1 mg.kg−1.h−1 infusion was initiated and continued until the end of procedure for maintenance. However, whenever children felt discomfort (movement, cough and cry), iv remifentanil 0.1 µg.kg−1 in RK group and iv propofol 0.5 mg.kg−1 in PK group was administered as a supplement dose. Despite the therapy given above, if children still experience discomfort, 1 mg.kg−1 of ketamine was administered intravenously as rescue dose, regardless of the group. An anesthetist in charge of the case administered study drugs infused fluids and supervised the children according to our study protocol, whilst a separate investigator blinded to the study groups recorded hemodynamic (systolic blood pressure [SBP], diastolic blood pressure [DBP], and heart rate [HR], Drager Infinity Kappa monitor) and respiratory parameters (respiratory rate [RR] and peripheral oxygen saturation [SpO2, Nellcor Oximax N-600 × monitor] and Ramsay sedation scores [RSSs])77 Ramsay MA, Savege TM, Simpson BR. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;22:656-9. at 5 min intervals from baseline (i.e., prior to induction) until the end of colonoscopy procedure.
Outcome variables
Hemodynamic and respiratory monitoring was followed during colonoscopy and recovery period. HR, SBP, DBP, SpO2, RR, and RSSs were recorded at 5 min intervals from baseline (i.e., prior to induction) until the end of colonoscopy procedure. Time to recovery (described as the time to achieve a recovery score = 7) was evaluated using Steward Recovery score at the end of procedure.88 Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J. 1975;22:111-3. Colonoscopy duration, supplemental and rescue drug consumption, and side effects (which occurred during colonoscopy and recovery period) such as respiratory depression, nausea, vomiting, hypotension, and bradycardia) were followed and recorded. Hypotension was defined as a 20% decrease in mean arterial pressure from baseline and treated with fluid infusion and ephedrine iv 1-5 mg). Bradycardia was defined as a 20% decrease in HR from baseline and treated with 0.02 mg.kg−1 atropine sulfate. SpO2 < 90% was considered as hypoxia and treated with oxygen supplementation via face mask. All colonoscopies were performed in the left lateral position by the same two pediatric gastroenterologists. At the end of the procedure, gastroenterologists were asked to give a score in terms of ease of attempt (1 = poor, 2 = moderate, 3 = good, 4 = excellent). Oral intake time and discharge time were also recorded.
Primary and secondary outcomes
The primary outcome of this study was to evaluate the efficacy of PK and RK combinations on RSSs during the procedure. The secondary end points were supplement and rescue drug consumptions, recovery time, gastroenterologist satisfaction scores, and side effects.
Power analysis
To ensure safe and effective sedation, it is desirable that an RSS be 4 or higher at all time during colonoscopy. In the preliminary study, in around 35% of the cases using propofol and ketamine, the RSS during the operation was 4 or higher; for those using remifentanil and ketamine, this percentage was 70%. Therefore, to obtain this difference being significant at the Type I error (alpha) level of 0.05 and the Type II error (1-power) level of 0.20, 30 children were required for each group.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 20.0 (IBM Inc., Armonk, NY, USA). Categorical variables were described as numbers and percentages, while continuous variables were shortened to mean and standard deviation values, and to median and minimum-maximum values. Categorical data were analyzed using a χ 2-test. A Kolmogorov-Smirnov test verified the normality of distribution for continuous variables. Continuous variables were compared using the Student's t-test or Mann-Whitney U-test, depending on whether or not the statistical hypotheses were supported. To investigate the change in the measures obtained in the time interval, the repeated measurements analysis was used. Statistical significance was accepted when p < 0.05.
Results
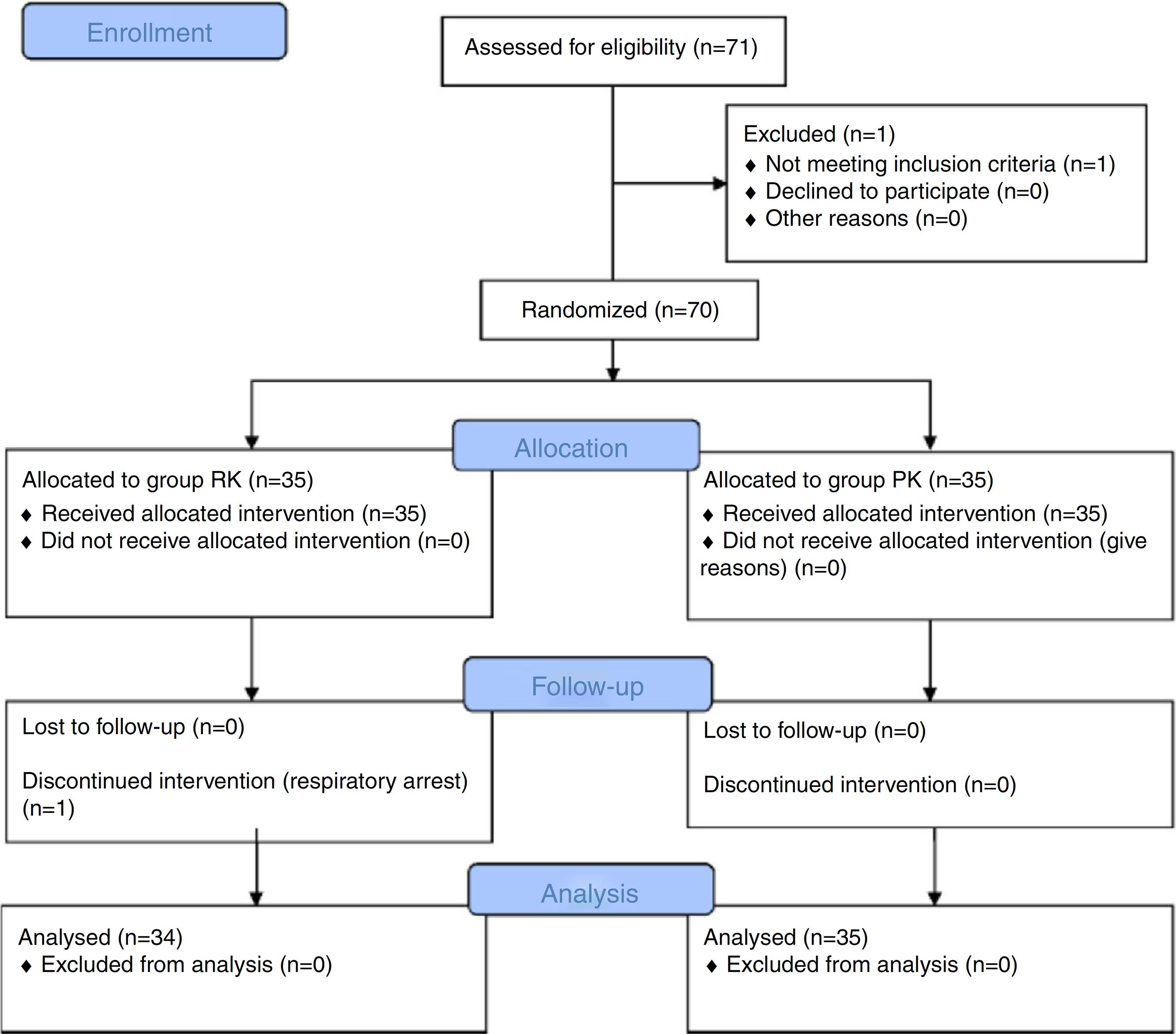
Fig. 1 shows the flow chart of children at various stages of the trial. Seventy children were recruited for this study and randomly assigned to one of the two groups. Only one child in the RK group experienced respiratory arrest (which supported via positive pressure ventilation) at induction and excluded from the study. Thus, 69 children completed the study. There were no statistically significant differences between the groups in terms of age, weight, gender, duration of colonoscopy, and time to recovery (Table 1).
There were no significant differences between the two study groups in terms of HR, SBP, and DBP during the procedure and recovery period. SBP and DBP variables were significantly higher only after induction in the RK group than in the PK group (p = 0.015, confidence interval (CI = 2.62-23.21 for SBP, p = 0.019; CI = 1.58-17.03 for DBP, t-test). HR was also higher after induction and at 5 and 10 min in the RK group than in the PK group (CI = 1.35-19.37 after induction, p = 0.025; CI = 4.92-24.44 at 5 min, p = 0.004; CI = 3.82-24.00 at 10 min, p = 0.008, t-test).
Repeated analysis showed that time affected both blood pressures and hearth rate variables. According to profile of analysis, the changes within time in SBP, DBP, and HR measurements were significantly different between groups: p = 0.01 for SBP (Fig. 2), p = 0.006 for DBP (Fig. 3), and p = 0.027 (Fig. 4) using repeated measurement analysis. Repeated analysis revealed an increasing trend in mean SBP, DBP, and HR variables in the RK group and a decreasing trend in the PK group. When we compared these variables between groups at a fixed time (i.e. at 5 or 10 min), there were no statistically significant differences between two groups with respect to hemodynamic variables (SBP, DBP, and HR).
SBP during the procedure. For both study groups, changes to SBP during the procedure were found to be statistically significant (p = 0.01 for SBP; repeated measurement analysis). RK, remifentanil-ketamine; PK, propofol-ketamine; min, minute.
DBP during the procedure. For both study groups, changes to DBP during the procedure were found to be statistically significant (p = 0.006 for DBP; repeated measurement analysis). RK, remifentanil-ketamine; PK, propofol-ketamine; min, minute.
HR during the procedure. For both study groups, changes to HR during the procedure were found to be statistically significant (p = 0.027, repeated measurement analysis). RK, remifentanil-ketamine; PK, propofol-ketamine; min, minute.
The percentage of patients with an RSS of 4 or higher during the procedure was 73.5 and 37.1% in RK and PK groups, respectively (p = 0.02, χ 2-test) (Table 2). Additionally, the sedation score at 10 min was significantly lower in the PK group than in the RK group (p = 0.02, Bonferroni-adjusted Mann-Whitney test).
Fifteen children (55.9%) in RK group needed supplement bolus dose of remifentanil and 22 children (62.9%) in the PK group needed supplement bolus dose of propofol. The number of children requiring supplement dose of study drugs were similar and there was no significant difference between the groups (p = 0.119, χ 2-test).
Eight children (23.5%) in the RK group and 15 children (42.9%) in the PK group needed rescue bolus dose of ketamine. Although ketamine requirement was greater in the PK group, there was no significant difference between the two groups (p = 0.089, χ 2-test).
Gastroenterologists' satisfaction scores were similar and there was no significant difference between the two groups (Table 1). Additionally, no significant difference was found in oral intake and discharge times.
Side effects during the procedure and recovery period are listed in Table 3. SpO2 and RR were within normal range throughout the study except one child experienced a transient decrease in SpO2 (<90%) in the RK group, but did not require intervention and resolved spontaneously. Bradycardia was the most frequent side effect during the procedure: four children in the RK group and five children in the PK group (p = 0.10, χ 2-test). Two patients in the RK group experienced nausea and vomited during the recovery period (p = 0.24, χ 2-test) and treated with iv 0.1-0.2 mg.kg−1 of ondansetron.
Discussion
The main finding of this study is that both RK and PK combinations provided effective sedation in children undergoing diagnostic colonoscopy. However, RK combination resulted in better intraoperative sedation and less rescue analgesic requirement than PK combination.
Colonoscopy is a painful procedure and frequently causes transient abdominal distress and pain.99 Stringer MD, Pinfield A, Revell L, et al. A prospective audit of paediatric colonoscopy under general anaesthesia. Acta Paediatr. 1999;88:199-202. Therefore, beside sedation, effective analgesic regimen is essential in pediatric colonoscopy.
Ketamine is a dissociative anesthetic and characterized by potent analgesia, sedation, and amnesia, while preserving spontaneous ventilation.1010 Drummond GB. Comparison of sedation with midazolam and ketamine: effect on airway muscle activity. Br J Anaesth. 1996;76:663-7. These properties make ketamine a suitable drug to provide anesthesia, sedation, amnesia, and analgesia in pediatric patients during brief painful or emotionally disturbing procedures.1010 Drummond GB. Comparison of sedation with midazolam and ketamine: effect on airway muscle activity. Br J Anaesth. 1996;76:663-7.,1111 Green SM, Johnson NE. Ketamine sedation for pediatric procedure: part 2, review and implications. Ann Emerg Med. 1990;19:1033-46. Proposed common loading doses of ketamine are 1.5-2.0 mg.kg−1 to provide dissociative state and generally, higher doses of ketamine is not recommended because of possible side effects such as prolonged recovery period, emergence delirium, dizziness, nausea, vomiting, and increased secretion.1111 Green SM, Johnson NE. Ketamine sedation for pediatric procedure: part 2, review and implications. Ann Emerg Med. 1990;19:1033-46.
12 von Ungern-Sternberg BS, Regil A, Frei FJ, et al. A deeper level of ketamine anesthesia does not affect functional residual capacity and ventilation distribution in healthy preschool children. Paediatr Anaesth. 2007;17:1150-5.-1313 Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46:10-20.
Combination of ketamine with other sedatives or analgesics is preferred to reduce these side effects and improve the quality of procedural sedation and analgesia.1414 Erden IA, Pamuk AG, Akinci SB, et al. Comparison of two ketamine-propofol dosing regimens for sedation during interventional radiology procedures. Minerva Anestesiol. 2010;76:260-5.,1515 Novak H, Karlsland Akeson P, Akeson J. Sedation with ketamine and low-dose midazolam for short-term procedures requiring pharyngeal manipulation in young children. Paediatr Anaesth. 2008;18:48-54. Lee et al. evaluated the effects of ketamine with remifentanil to improve the quality of anesthesia and postoperative recovery, following brief procedures in pediatric patients undergoing middle-ear ventilation tube insertion.66 Lee JA, Jeon YS, Noh HI, et al. The effect of ketamine with remifentanil for improving the quality of anaesthesia and recovery in paediatric patients undergoing middle-ear ventilation tube insertion. J Int Med Res. 2011;39:2239-46. They reported a lower patient movement scores, higher surgeon satisfaction scores, and shorter time to recovery in ketamine-remifentanil group compared with only ketamine group.
In the present study, we used remifentanil or propofol to augment sedative or analgesic efficacy of ketamine. Adding remifentanil to ketamine significantly improved RSS in RK than in PK group (RSS of 4 or higher; 73.5% vs. 37.1% in RK and PK groups, respectively). Although not significant, rescue ketamine requirement was also found smaller in remifentanil-ketamine group than in propofol-ketamine group.
Unlike remifentanil, with lack of analgesic properties, propofol has sedative, amnestic, and hypnotic effects. Previous studies evaluating the effects of ketamine-propofol combination during upper gastrointestinal endoscopy and colonoscopy have noted that combination of propofol with sedatives is a feasible and safer option.22 Baykal TZ, Gulec H, Derelı N, et al. Propofol-ketamine combination: a choice with less complications and better hemodynamic stability compared to propofol? On a prospective study in a group of colonoscopy patients. Ir J Med Sci. 2016;185:699-704.,44 Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983-8. When propofol is used alone for the management of brief outpatient procedures, relatively higher doses of propofol is needed to achieve a deep sedation. At high doses, it may cause hypotension and respiratory depression.22 Baykal TZ, Gulec H, Derelı N, et al. Propofol-ketamine combination: a choice with less complications and better hemodynamic stability compared to propofol? On a prospective study in a group of colonoscopy patients. Ir J Med Sci. 2016;185:699-704.,44 Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983-8. To avoid from side effects, combination of propofol with sedatives or analgesics has been advocated.1616 Disma N, Astuto M, Rizzo G, et al. Propofol sedation with fentanyl or midazolam during oesophagogastroduodenoscopy in children. Eur J Anaesthesiol. 2005;22:848-52.
In the present study, we add propofol to ketamine to improve sedation and reduce the amount of each drug. In propofol-ketamine group, the percentage of patients with an RSS of 4 or higher during the procedure was 37.1%. This ratio (RSSs) was significantly lower than in the remifentanil-ketamine group. Lower sedation scores in the PK group was attributed to the low dose of propofol used for the maintenance of drug.
In designing this study, one difficulty was determining dosages of each drug that would produce effective sedation while avoiding side effects. When the two drugs are combined at different dosages, many combinations are possible. In the literature, there is no reliable method of determining how much propofol a child will require as an adjunct to ketamine, far less how much will result in untoward side effects. The literatures on the dose of propofol used for sedation in children have been conflicting. In the present study, children in the PK group received bolus dose of ketamine (2 mg.kg−1) and propofol (1 mg.kg−1) combination for the induction of sedation, followed by 1 mg.kg−1.h−1 propofol infusion until the end of procedure, as maintenance. The dose of propofol was chosen small to avoid respiratory and hemodynamic side effects.
Coadministration of two drugs may potentiate the effect each agent and reduce the amount of drug consumption. In this study, coadministration of ketamine with remifentanil resulted in higher RSS and lower additional medication (supplement and rescue study drug requirement). Fifteen children (55.9%) in the RK group needed supplement bolus dose of remifentanil and 22 children (62.9%) in the PK group needed supplement bolus dose of propofol. Similarly, 8 children (23.5%) in the RK group and 15 children (42.9%) in the PK group needed rescue bolus dose of ketamine. Although ketamine requirement was greater in the PK group, there was no significant difference between the two groups.
Unlike remifentanil and propofol, ketamine has been reported to increase SBP, DBP, HR, and cardiac output, because of its sympathomimetic properties.1717 Lin C, Durieux ME. Ketamine and kids: an update. Paediatr Anaesth. 2005;15:91-7. In the present study, both RK and PK combinations provided stable hemodynamic parameters, and hemodynamic fluctuation (hypertension or tachycardia) was not reported. Although repeated analysis revealed an increasing trend in mean SBP, DBP, and HR variables in the RK group and a decreasing trend in the PK group, these differences were not considered as a clinically important.
In the PK group, no nausea or vomiting was reported, however in the RK group, two children complained of nausea and vomited during the recovery. Ketamine and remifentanil have been reported to lead emesis. Absence of nausea and vomiting in the PK group could be attributed to the antiemetic effect of propofol at low doses.1818 Erdem AF, Yoruk O, Alici HÁ, et al. Subhypnotic propofol infusion plus dexamethasone is more effective than dexamethasone alone for the prevention of vomiting in children after tonsillectomy. Paediatr Anaesth. 2008;18:878-83. Furthermore, Green et al. demonstrated that nausea and vomiting are less common after ketamine administration in children younger than 5 years.1919 Green SM, Kuppermann N, Rothrock SG, et al. Predictors of adverse events with intramuscular ketamine sedation in children. Ann Emerg Med. 2000;35:35-42. In our study, the children who experienced nausea and vomiting were 7 and 15 years old.
Agitation and hallucination during emergence may occur with ketamine use.2020 Petrack EM. Ketamine. Clin Ped Emerg Med. 2000;1:281-4. A combination of ketamine and short-acting benzodiazepine (midazolam) has frequently been used to preclude these side effects.2121 White PF, Way WL, Trevor AJ. Ketamine—its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119-36.,2222 Cartwright PD, Pingel SM. Midazolam and diazepam in ketamine anaesthesia. Anaesthesia. 1984;39:439-42. In the present study, we administered 0.5 mg.kg−1 midazolam orally to all patients in both study groups, as premedication; no such adverse effect was observed in children.
Opioids may provide dose-dependent respiratory depression and this effect can be aggravated with a second agent. In the present study, in the RK group, one child experienced respiratory arrest (which supported via positive pressure ventilation) at induction and excluded from the study. Additionally, transient respiratory depression and hypoxia (SpO2 < 90) occurred in one patient in the RK group and resolved spontaneously.
A limitation of this study is the wide age range of the patients. The age of a pediatric patient can affect sedative and analgesic agent consumption, as well as side effects and recovery time.2020 Petrack EM. Ketamine. Clin Ped Emerg Med. 2000;1:281-4.,2323 Law AK, Ng DK, Chan KK. Use of intramuscular ketamine for endoscopy sedation in children. Pediatr Int. 2003;45:180-5.,2424 Kaddu R, Bhattaccharya D, Metriyakool K, et al. Propofol compared with general anesthesia for pediatric GI endoscopy: is propofol better?. Gastrointest Endosc. 2002;55:27-32. In addition, we did not use target-controlled infusion (TCI) for propofol or remifentanil administration. TCI may provide better sedation and analgesia level, as it targets blood drug concentration.2525 Kim HS, Park HJ, Kim CS, et al. Combination of propofol and remifentanil target-controlled infusion for laryngeal mask airway insertion in children. Minerva Anestesiol. 2011;77:687-92.,2626 Nora FS. Total intravenous anesthesia as a target-controlled infusion: an evolutive analysis. Rev Bras Anestesiol. 2008;58:179-92. Furthermore, large number of children are needed to reveal this relationship between ketamine and other drugs.
In conclusion, coadministration of ketamine with either remifentanil or propofol effectively and safely provides sedation and analgesia in children undergoing colonoscopy. Sedation scores were significantly better in the RK group compared to the PK group.
References
-
1Mahoney LB, Lightdale JR. Sedation of the pediatric and adolescent patient for GI procedures. Curr Treat Options Gastroenterol. 2007;10:412-21.
-
2Baykal TZ, Gulec H, Derelı N, et al. Propofol-ketamine combination: a choice with less complications and better hemodynamic stability compared to propofol? On a prospective study in a group of colonoscopy patients. Ir J Med Sci. 2016;185:699-704.
-
3Arora S. Combining ketamine and propofol ("Ketofol") for emergency department procedural sedation and analgesia: a review. West JEM. 2008;9:20-3.
-
4Tosun Z, Aksu R, Guler G, et al. Propofol-ketamine vs propofol-fentanyl for sedation during pediatric upper gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:983-8.
-
5Battershill AJ, Keating GM. Remifentanil: a review of its analgesic and sedative use in the intensive care unit. Drugs. 2006;66:365-85.
-
6Lee JA, Jeon YS, Noh HI, et al. The effect of ketamine with remifentanil for improving the quality of anaesthesia and recovery in paediatric patients undergoing middle-ear ventilation tube insertion. J Int Med Res. 2011;39:2239-46.
-
7Ramsay MA, Savege TM, Simpson BR. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;22:656-9.
-
8Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J. 1975;22:111-3.
-
9Stringer MD, Pinfield A, Revell L, et al. A prospective audit of paediatric colonoscopy under general anaesthesia. Acta Paediatr. 1999;88:199-202.
-
10Drummond GB. Comparison of sedation with midazolam and ketamine: effect on airway muscle activity. Br J Anaesth. 1996;76:663-7.
-
11Green SM, Johnson NE. Ketamine sedation for pediatric procedure: part 2, review and implications. Ann Emerg Med. 1990;19:1033-46.
-
12von Ungern-Sternberg BS, Regil A, Frei FJ, et al. A deeper level of ketamine anesthesia does not affect functional residual capacity and ventilation distribution in healthy preschool children. Paediatr Anaesth. 2007;17:1150-5.
-
13Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46:10-20.
-
14Erden IA, Pamuk AG, Akinci SB, et al. Comparison of two ketamine-propofol dosing regimens for sedation during interventional radiology procedures. Minerva Anestesiol. 2010;76:260-5.
-
15Novak H, Karlsland Akeson P, Akeson J. Sedation with ketamine and low-dose midazolam for short-term procedures requiring pharyngeal manipulation in young children. Paediatr Anaesth. 2008;18:48-54.
-
16Disma N, Astuto M, Rizzo G, et al. Propofol sedation with fentanyl or midazolam during oesophagogastroduodenoscopy in children. Eur J Anaesthesiol. 2005;22:848-52.
-
17Lin C, Durieux ME. Ketamine and kids: an update. Paediatr Anaesth. 2005;15:91-7.
-
18Erdem AF, Yoruk O, Alici HÁ, et al. Subhypnotic propofol infusion plus dexamethasone is more effective than dexamethasone alone for the prevention of vomiting in children after tonsillectomy. Paediatr Anaesth. 2008;18:878-83.
-
19Green SM, Kuppermann N, Rothrock SG, et al. Predictors of adverse events with intramuscular ketamine sedation in children. Ann Emerg Med. 2000;35:35-42.
-
20Petrack EM. Ketamine. Clin Ped Emerg Med. 2000;1:281-4.
-
21White PF, Way WL, Trevor AJ. Ketamine—its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119-36.
-
22Cartwright PD, Pingel SM. Midazolam and diazepam in ketamine anaesthesia. Anaesthesia. 1984;39:439-42.
-
23Law AK, Ng DK, Chan KK. Use of intramuscular ketamine for endoscopy sedation in children. Pediatr Int. 2003;45:180-5.
-
24Kaddu R, Bhattaccharya D, Metriyakool K, et al. Propofol compared with general anesthesia for pediatric GI endoscopy: is propofol better?. Gastrointest Endosc. 2002;55:27-32.
-
25Kim HS, Park HJ, Kim CS, et al. Combination of propofol and remifentanil target-controlled infusion for laryngeal mask airway insertion in children. Minerva Anestesiol. 2011;77:687-92.
-
26Nora FS. Total intravenous anesthesia as a target-controlled infusion: an evolutive analysis. Rev Bras Anestesiol. 2008;58:179-92.
Publication Dates
-
Publication in this collection
Nov-Dec 2018
History
-
Received
3 July 2017 -
Accepted
22 June 2018 -
Published
11 Aug 2018