Abstract
The aim of the present work was to study the in-vitro cytotoxic effects of different concentrations of aflatoxin B1 (AFB1) on broiler lymphocytes. Lymphocyte-rich mononuclear cells were separated by Ficoll-Histopaque density and cultured in 96-wellplates containing the evaluated AFB1 concentrations in 5% CO2 atmosphere at 39°C. Thereafter, MTT, PicoGreen, and reactive oxygen species assays were performed. Cell viability decreased in the presence of 10 µg/mL AFB1 at 48 h (p < 0.05) and of 10 and 20 µg/mL AFB1 at 72 h (p < 0.01 and p < 0.001, respectively) when compared to the control (0 µg/mL). However, a dose-dependent increase in the cell-free DNA at 24 h was observed at 1, 10 and 20 µg/mL (p < 0.001). ROS formation significantly increased at 24 h at all concentrations (p < 0.001). The in-vitro results demonstrate that AFB1 is cytotoxic and causes biomolecular oxidative damage in broiler lymphocytes.
Aflatoxin B1; Broilers; Lymphocytes; PicoGreen; Reactive oxygen species
In-vitro cytotoxicity of aflatoxin B1 to broiler lymphocytes of broiler chickens
Zimmermann CEPI; Machado AKII; Cadoná FCII; Jaques JASII; Schlemmer KBI; Lautert CIII; Cruz IBMI,II; Zanette RAI; Leal DBRII; Santurio JMI
IPrograma de Pós-graduação em Farmacologia, Centro de Ciências da Saúde, Universidade Federal de Santa Maria, Santa Maria, Brazil
IIPrograma de Pós-graduação em Bioquímica Toxicológica, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria, Santa Maria, Brazil
IIIFaculdade de Veterinária/Setor de Micologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Correspondence Corresponding author e-mail address: Prof. Dr. Janio Morais Santurio Laboratório de Pesquisas Micológicas, Departamento de Microbiologia e Parasitologia, Campus UFSM, Prédio 20, Sala 4139, Universidade Federal de Santa Maria, 97105-900 Santa Maria, RS, Brazil. Phone/Fax: + 55 55 3220 8906 E-mail: janio.santurio@gmail.com
ABSTRACT
The aim of the present work was to study the in-vitro cytotoxic effects of different concentrations of aflatoxin B1 (AFB1) on broiler lymphocytes. Lymphocyte-rich mononuclear cells were separated by Ficoll-Histopaque density and cultured in 96-wellplates containing the evaluated AFB1 concentrations in 5% CO2 atmosphere at 39°C. Thereafter, MTT, PicoGreen, and reactive oxygen species assays were performed. Cell viability decreased in the presence of 10 µg/mL AFB1 at 48 h (p < 0.05) and of 10 and 20 µg/mL AFB1 at 72 h (p < 0.01 and p < 0.001, respectively) when compared to the control (0 µg/mL). However, a dose-dependent increase in the cell-free DNA at 24 h was observed at 1, 10 and 20 µg/mL (p < 0.001). ROS formation significantly increased at 24 h at all concentrations (p < 0.001). The in-vitro results demonstrate that AFB1 is cytotoxic and causes biomolecular oxidative damage in broiler lymphocytes.
Keywords: Aflatoxin B1; Broilers; Lymphocytes; PicoGreen; Reactive oxygen species.
Introduction
Aflatoxins are secondary metabolites of Aspergillus fungi, including A. flavus, A. parasiticus, and A. nomius (Hamid et al., 2013; Kalpana et al., 2012). The most common aflatoxins are B1, B2, G1 and G2 that are naturally present in many food products, and M1 and M2 are found in milk, dairy products, eggs, meat, and urine (Oliveira et al., 2000).
The toxigenic potential of aflatoxins dependends on their dose and duration of intake, as well as on animal species, age, and nutritional status (Marai & Asker, 2008). It is directly associated with its rapid absorption in the gastrointestinal tract and immediate binding to serum proteins, such as albumin (Santurio, 2000). AFB1 is recognized as one of the most potent known liver carcinogens, and has genotoxic, immunotoxic, and other adverse effects on several animal species, including poultry (Kalpana et al., 2012).
In addition of its high mutagenic and teratogenic potential, the constant daily intake of small amounts of AFB1 is responsible for the induction and modulation of diseases in humans and animals (Lewis et al., 1999). AFB1 mainly affects cell-mediated immunity (Williams et al., 2004), reducing lymphocyte proliferation and cytokine production in experimental animals (Abbès et al., 2010). Moreover, AFB1 is able to generate reactive oxygen species (ROS), which may have a dual role, acting as toxic bioproducts that alter the cellular function and viability and as key participants in cell regulation and signaling (Shen et al., 1996). The carcinogenic action of AFB1 requires its metabolic activation by cytochrome (CYP) P-450, which is primarily responsible for the activation of AFB1 to produce the ultimate carcinogen AFB1-8,9-epoxide (Marai & Asker, 2008), and binds to DNA and RNA (Bbosa et al., 2013). DNA damage also occurs when ROS synthesis exceeds the capacity of body antioxidant defenses to eliminate them (Bischoff & Ramaiah, 2007).
Chronic AFB1 exposure causes significant losses in the poultry industry (Sur & Celik, 2003). Contamination with AFB1 can negatively affect broiler chickens in a variety of ways, including reduced Þtness, altered immune function (Yunus et al., 2011), decreased survival (Azzam & Gabal, 1998), and reduced humoral immune response to vaccines (Gabal & Azzam, 1998).
Based on this context, the aim of the present study was to use MTT, PicoGreen fluorescence and the 2',7' dichlorofluorescein diacetate (DCFH-DA) assays to investigate the effects of AFB1 on lymphoid cells of broiler chickens cultivated in vitro.
Materials and methods
Chemicals
AFB1 (C17H12O6 - 5mg), RPMI-1640 medium, Ficoll-Histopaque density®, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), sodium bicarbonate, penicillin/streptomycin solution and DCFH-DA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fetal calf serum was obtained from Cultilab (Campinas, SP, BR), Vybrant® MTT cell Proliferation Assay Kit and Quant-iT™ PicoGreen® Reagent from Invitrogen (Eugene, UK).
Birds
Broiler chickens (Gallus gallus domesticus) between 38 and 42 days of age and weighing 2-2.5 kg were used in the experiments. Birds were obtained from a local processing plant, after being stunned by electronarcosis, and bled by sectioning the large vessels of the neck. All experimental procedures were conducted according to Normative Instruction N° 3, January 17th, 2000 (Regulation of Technical Methods for the Humane Slaughter of Animals; Brasil, 2000).
AFB1
AFB1 (5 mg - Sigma-Aldrich Co.) was first dissolved in 99% ethanol (Mehrzad et al., 2011). Further dilutions were made with RPMI 1640 complete medium containing 9.7 mM HEPES and 24 mM sodium bicarbonate, supplemented with 10% heat-inactivated fetal bovine serum and 2.5 IU penicillin/streptomycin. AFB1 was added to the medium containing the isolated lymphocytes at the final concentrations of 0.1, 1, 10, and 20 µg/mL with 0.5 % v/v of ethanol in the culture cell. Vehicle control cells were prepared in the same manner as AFB1 treated samples, including the addition of the vehicle (0.5% ethanol) instead of AFB1.
Peripheral blood mononuclear cell preparation
Lymphocyte-rich mononuclear cells of broiler chickens were isolated from blood collected with 7.2 mg dipotassium EDTA and separated on Ficoll-Histopaque density system (Sigma-Aldrich Co.) as described by Böyum (1968) and Nathanson (1982), except that lymphocytes were centrifuged at 1000 rpm for 5 min with culture medium to completely remove platelets. Next, the pellet was suspended in culture medium and stored in a culture flask in a 5% CO2 atmosphere at 39° C for 2 h to allow adhesion of monocytes to the surface of the bottle, which times occur among lymphocytes (non adherent). After incubation, cell suspensions were transferred to centrifuge tubes and centrifuged at 1500 rpm for 10 min. The supernatants were discarded, the dry pellets containing lymphocytes were suspended in 3 mL of culture medium, and cell viability was measured using trypan blue dye (1:2). This protocol was performed within 2 h following blood collection.
Lymphocytes were suspended at a density of 0.7 x 105 cells/mL in RPMI 1640-enriched culture complete medium. Cells were seeded in triplicate in 96-well tissue culture plates under an atmosphere of 5% CO2 at 39°C, and treated with increasing concentrations of AFB1 (0, 0.1, 1, 10 and 20 µg/mL). The density of the cells that were seeded was equivalent to 75% confluence.
MTT assay
Cytotoxicity was evaluated by MTT reduction assay (Vybrant MTT cell Proliferation Assay Kit), which is based on the cleavage of tetrazolium salts via the activity of mitochondrial succinate dehydrogenase in metabolically active cells that yield a colored formazan product (Mosmann, 1983). Since the conversion takes place in living cells, the amount of formazan produced directly corresponds to the number of viable cells. Absorbance was measured in microplate spectrophotometer at 540 nm. This assay was performed 24, 48 and 72 h after AFB1 exposure. All tests were performed in 96-well microplates in triplicate. The results were expressed as optical density (OD540).
PicoGreen fluorescence assay
The PicoGreen fluorescence assay measures the presence of double-stranded (ds) DNA, which is an indicative of cytotoxicity (Swarup et al., 2011). This assay was performed 24, 48 and 72 h after exposure, according to the protocol supplied by the manufacturer (Quant ItTM, Invitrogen). PicoGreen dye was diluted to 1:200 with TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.5) and incubated with each supernatant sample in the dark at room temperature in black 96-well microplates for 5 min. All fluorescence measurements were recorded with a fluorimeter. Fluorescence emissions of PicoGreen alone (blank) and PicoGreen with supernatant were recorded at 520 nm using an excitation wavelength of 480 nm at 25°C. A standard curve was generated using the lambda DNA standard provided by the manufacturer. All calibration samples were assayed in quintuplicate. Baseline fluorescence was determined with a TE blank, which average was subtracted from the average fluorescence of the other samples. The results were expressed as fluorescence (pg/mL of dsDNA).
Determination of intracellular ROS formation
Intracellular ROS concentrations were determined using the fluorescence probe DCFH-DA, which is a well-established compound used to detect and quantify free radicals, particularly intracellular hydrogen peroxide (H2O2). DCFH-DA is transported across the cell membrane and it is deacetylated by cytosolic esterases to form non-ßuorescent DCFH, which is trapped within the cells. DCFH is converted to fluorescent DCF by the action of peroxide rated by the presence of peroxidase (Halliwell & Whiteman, 2004; LeBel et al., 1992).
After each time point of exposure (24, 48 and 72 h), cells were treated with DCFH-DA (10 μM) for 60 min at 37°C. Fluorescence was measured at 488 nm excitation and 525 nm emission. All tests were performed in 96-well microplates, in quintuplicate for each of the samples that were tested, and the results were expressed as fluorescence intensity (nm).
Statistical analysis
The data were normally distributed. Therefore, data within each time point were submitted to one-way analysis of variance (ANOVA) followed by Dunnett's post-hoc test. ANOVA followed by Tukey's test was used to calculate overall time effects. The results with p < 0.05 were considered significant. The data were pooled from three independent experiments, and the results were expressed as the mean and standard deviation.
Results
MTT assay
The lymphocyte viability of broiler chickens was assessed in the presence of AFB1 using the MTT assay at 24, 48, and 72 h (Table 1). Compared with the control (0 µg/mL), this mycotoxin induced significant decrease of cell viability at the concentration of 10 µg/mL (p < 0.05) at 48 h, and at the concentrations of 10 and 20 µg/mL (p < 0.001) at 72 h of incubation.
PicoGreen fluorescent assay
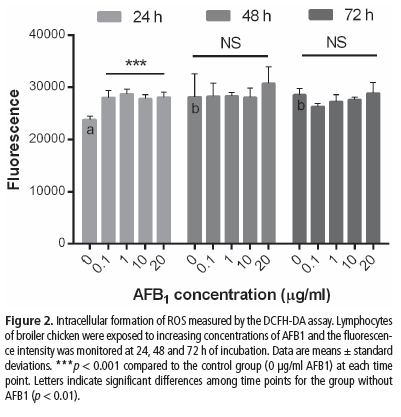
The effects of AFB1 on free dsDNA in lymphocytes of broiler chickens were evaluated using the PicoGreen fluorescent assay (Fig. 1). An increase in dsDNA was observed at the AFB1 concentrations of 1, 10, and 20 µg/mL at 24 h (p < 0.001). No significant differences from the control group (0 µg/mL) were observed at 48 and 72 h.
DCFH-DA assay
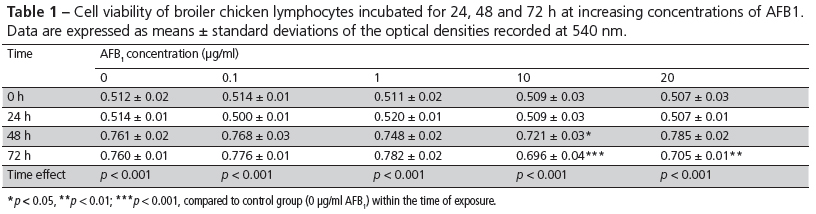
Intracellular ROS formation was examined using a fluorescence sensitive probe, through the DCFH-DA assay. When compared with the control (0 µg/mL), an increase in ROS concentrations was observed at 24 h of exposure to AFB1 at the concentrations of 0.1, 1, 10, and 20 µg/mL (p < 0.001) (Fig. 2). At 48 and 72 h, the formation of ROS in cultures of broiler chicken lymphocytes tended to stabilize.
Discussion
Immune function is a complex process comprising different elements of the immune system that must work together to elicit an effective immune response. Therefore, the ideal assessment of immune function requires a set of tests that measure several different components. Studies have shown that the immune system of birds is sensitive to environmental contaminants that can cause immunotoxicity (Yunus et al., 2011). Aiming at better understanding mycotoxin immunotoxicity in birds, we evaluated the in-vitro cytotoxicity of a wide range of AFB1 concentrations to peripheral lymphocytes of broiler chickens at different time points.
First, we investigated the role of AFB1 on lymphocyte viability by measuring the dehydrogenase enzyme activity. MTT assay is dependent on respiratory chain activity (Mosmann, 1983), which is responsible for energy production in the form of ATP and it is necessary to maintain system organization and cellular functions. Our results with the MTT assay showed a strong correlation of mycotoxin concentration with the time of exposure. AFB1 significantly reduced lymphocyte mitochondrial activity at 48 h and 72 h of incubation at the higher concentrations tested, possibly due to mitochondrial dysfunction (Bbosa et al., 2013). Consistent with this study, Taranu et al. (2010), in experiments with human and porcine lymphocytes, also found considerable reduction of lymphocyte cellular proliferation at doses 1 and 10 μg/mL of AFB1. Contradictory results were reported by Bernabucci et al. (2011), who found increased viability of bovine lymphocytes in the presence of 20 μg/mL of AFB1 on days 2 and 7 of incubation.
According to Fairbrother et al. (2004), reduced lymphoid cell count may suggest decreased immunological defense functions associated with those cells. On the other hand, circulating white blood cell counts may increase in response to infections, even in the presence of immunosupression. This possibility represents a limitation of the MTT assay, because when bioactive compounds, such as AFB1, areanalyzed, cellular metabolism can be altered. Moreover, the detection of cell-free DNA has emerged as an attractive tool in the early prognosis of several diseases. For this reason, we conducted the PicoGreen fluorescent assay to measure cell-free DNA circulating in cell supernatants, which would represent the extent of cell damage.
We observed a dose-dependent increase in dsDNA at 24 h of incubation. This result is consistent with the findings of Guengerich et al. (1998), who reported that highly reactive AFB1-exo-8,9-epoxide is able to form adducts with DNA that are directly proportional to AFB1 dose (Choy, 1993). DNA release may either stimulate or inhibit immune cell activation, depending on its concentration, sequence, and context (Scaffidi et al., 2002). Moreover, viable cells produce only limited amounts of extracellular DNA and necrotic cells produce little or none, while apoptotic cell death can be a significant source of extracellular DNA (Choi et al., 2004). Considering cell culture as a closed system, we hypothesized that apoptotic cell death was the source of dsDNA at 24 h, whereas immunostimulation can be related to the interference with regulatory mechanisms that induce direct and indirect consequences on proliferation and differentiation of lymphocytes, as observed at 24 and 48 h (MTT assay). Our DNA damage findings agree with the results of Awad et al. (2012), who demonstrated that diets contaminated with the mycotoxin deoxynivalenol at moderate levels, in combination with low-protein feed, are able to induce lymphocyte DNA damage in chickens. Frankic et al. (2006) also demonstrated DNA fragmentation in chicken splenocytes treated with T-2 toxin and deoxynivalenol.
In the present study, ROS levels increased in AFB1-treated lymphocytes of broiler chickens at 24 h of exposure at all the evaluated concentrations. It was shown that mycotoxin interaction in the oxidative stress induction is an early effect, which might be related to AFB1 immunotoxicity. AFB1 requires metabolic activation by CYP-450 to exert its cytotoxic and carcinogenic effects (Bbosa et al., 2013). CYP-450 activity itself is associated with electron leakage, and it is also one of the important sources of intracellular ROS generation (Shen et al., 1996). Therefore, when AFB1 is bioactivated by this enzymatic complex, such process is expected to increase ROS production. Bischoff & Ramaiah (2007) described that the reactive species generated result in lipid peroxidation of membranes and oxidative modification of proteins and DNA in lesions. Increased ROS levels were also reported by Kouadio et al. (2005) in the human intestinal cell line Caco-2 following exposure to the mycotoxins fumonisin B1 (FB1), deoxynevalenol and zearalenone, and in rat spleen mononuclear cells treated with AFB1 and FB1.
In conclusion, the results of this study showed that the AFB1 effects on broiler chicken immune system can be cytotoxic, depending on exposure time and dose. In addition, it was demonstrated that AFB1 is able to affect the oxidative status of broiler chicken lymphocytes by increasing ROS levels, and to cause biomolecular oxidative damage, as evidenced by the PicoGreen assay. This study contributes to the use of the immune function as a biological marker of contamination effects in avian species, because immune cells such as lymphocytes can be important mediators of immunotoxic responses.
Conflicts of Interest Statement
There are no actual or potential conflicts of interest.
Acknowledgements
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. R. A. Z. acknowledges FAPERGS for the grant.
Submitted: October/2013
Approved: may/2014
- Abbès S, Ben Salah-Abbès J, Abdel-Wahhab MA, Ouslati R. Immunotoxicological and biochemical effects of aflatoxins in rats prevented by Tunisian montmorillonite with reference to HSCAS. Immunopharmacology and Immunotoxicology 2010;32(3):514-522.
- Awad WA, Ghareeb K, Dadak L, Gille L, Staniek K, Hess M, Böhm J. Genotoxic effects of deoxynivalenol in broiler chickens fed low-protein feeds. Poultry Science 2012;91(3):550-5.
- Azzam AH, Gabal MA. Aflatoxin and immunity in layer hens. Avian Pathology 1998; 27(6):570-7.
- Bbosa GS, Kitya D, Lubega A, Ogwal-Okeng J, Anokbonggo WW, Kyegombe DB. Review of the biological and health effects of aflatoxins on body organs and body systems. In: Mehdi Razzaghi-Abyaneh M. Aflatoxins - recent advances and future prospects. InTech; 2013. chap. 12.
- Bernabucci U, Colavecchia L, Danieli PP, Basirico L, Lacetera N, Nardone A, Ronchi B. Aflatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells. Toxicology in vitro 2011;25(3):684-691.
- Bischoff K, Ramaiah K. Liver toxicity. In: Gupta RC, editor. Veterinary toxicology basic and clinical principles: New York: Elsevier Academic Press; 2007. p. 145-160.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scandinavian Journal of Clinical and Laboratory Investigation 1968; 97:77-89.
- Brasil. Ministério da Agricultura do Abastecimento e da Reforma Agrária; 2000. Instrução Normativa nº 3 de 17 de janeiro de 2000. Aprovar o Regulamento Técnico de Métodos de Insensibilização para o Abate Humanitário de Animais de Açougue. Brasília, DF; 2000. (corrigir no texto de Mapa 2000 para Brasil 2000)
- Choi JJ, Reich III CF, Pisetsky DS. Release of DNA from dead and dying lymphocyte and monocyte cell lines in vitro. Scandinavian Journal of Immunology 2004;60(1-2):159-166.
- Choy WN. A review of the dose-response induction of DNA adducts by aflatoxin B1 and its implications to quantitative cancer-risk assessment. Mutation Research 1993;296(3):181-198.
- Fairbrother A, Smits J, Grasman KA. Avian immunotoxicology. Journal of Toxicology and Environmental Health Part B: Critical Reviews 2004;7(2):105-37.
- Frankic T, Pajk T, Rezar V, Levart A, Salobir J. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food and Chemical Toxicology 2006;44(11):1838-44.
- Gabal MA, Azzam AH. Interaction of aflatoxin in the feed and immunization against selected infectious diseases in poultry. II. Effect on one-day-old layer chicks simultaneously vaccinated against Newcastle disease, infectious bronchitis and infectious bursal disease. Avian Pathology 1998;27(3):290-295.
- Guengerich FP, Johnson WW, Shimada T, Ueng YF, Yamazaki H, Langouët S. Activation and detoxication of aflatoxin B1 Mutation Research 1998;402(1-2):121-8.
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology 2004;142(2):231-255.
- Hamid AS, Tesfamariam IG, Zhang Y, Zhang ZG. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncology Letters 2013;5(4):1087-1092.
- Kalpana S, Aggarwal M, Srinivasa Rao G, Malik JK. Effects of aflatoxin B1 on tissue residues of enrofloxacin and its metabolite ciprofloxacin in broiler chickens. Environmental Toxicology and Pharmacology 2012;33(2):121-6.
- Kouadio JH, Théophile AT, Baudrimont I, Moukha S, Dano SD, Creppy EE. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005;213(1-2):56-65.
- LeBel C, Ischiropoulos H, Bondy S. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical Research in Toxicology 1992;5(2):227-231.
- Lewis C, Smith J, Anderson J, Freshney R. Increased cytotoxicity of food-borne mycotoxins toward human cell lines in vitro via enhanced cytochrome p450 expression using the MTT bioassay. Mycopathologia 1999;148(2):97-102.
- Marai IFM, Asker AA. Aflatoxins in rabbit production: hazards and control. Tropical and Subtropical Agroecosystems 2008;8(1):1-28.
- Mehrzad J, Klein G, Kamphuesd J, Wolf P, Grabowski N, Schuberth HJ. In vitro effects of very low levels of aflatoxin B1 on free radicals production and bactericidal activity of bovine blood neutrophils. Veterinary Immunology and Immunopathology 2011;141:16-25.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 1983;65(1-2):55-63.
- Nathanson RM. Transformation of chicken lymphocytes stimulated by concanavalin A: response parameters. Canadian Journal of Comparative Medicine 1982;46(1):57-59.
- Oliveira CAF, Kobashigawa E, Reis TA, Mestieri L, Albuquerque R, Corrêa B. Aflatoxin B1 residues in eggs of laying hens fed a diet containing different levels of the mycotoxin. Food Additives & Contaminants: Part A 2000;17(6):459-462.
- Santurio JM. Mycotoxins and mycotoxicosis in poultry. Revista Brasileira de Ciência Avícola 2000;2(1):01-12.
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418(6894):191-5.
- Shen H, Shi C, Shen YI, Ong C. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1 Free Radical Biology & Medicine 1996;21(2):139-146.
- Sur E, Celik I. Effects of aflatoxin B1 on the development of the bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken. British Poultry Science 2003;44(4):558-566.
- Swarup V, Srivastava AK, Padma MV, Rajeswari MR. Quantification of circulating plasma DNA in Friedreich's ataxia and spinocerebellar ataxia types 2 and 12. DNA Cell Biology 2011; 30(6):389-394.
- Taranu I, Marina D, Burlacu R, Pinton P, Damian V, Oswald IP. Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Archives of Animal Nutrition 2010;64(5):383-393.
- Williams J, Phillips T, Jolly P, Stiles J, Jolly C, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American Journal of Clinical Nutrition 2004;80(5):1106–22.
- Yunus AW, Razzazi-Fazeli E, Bohm J. Aflatoxin B1 in affecting broiler's performance, immunity, and gastrointestinal tract: a review of history and contemporary issues. Poultry Science 2011;90:1683-89.
Publication Dates
-
Publication in this collection
01 Oct 2014 -
Date of issue
Sept 2014
History
-
Received
Oct 2013 -
Accepted
May 2014