Abstracts
Various studies have demonstrated the impact of omega-3 fatty acids on the concentration of C reactive protein (CRP), pro-inflammatory eicosanoids, cytokines, chemokines and other inflammatory mediators. Therefore, the supplementation of these types of lipids may represent additional option treatment for chronic systemic diseases, such as Systemic Lupus Erythematous and other rheumatic diseases. The role of these lipids has not been well established, yet. However, it seems there is a direct relationship between its intake and the decrease of the disease clinical manifestations as well as of the inflammatory status of the patients. Thus, the aim of this manuscript is to present a thorough review on the effects of omega-3 fatty acids in patients with SLE. Bibliographic data set as the Medical Literature Analysis and Retrieval System Online (MEDLINE) and Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS) were searched using as key words: systemic lupus erythematous (SLE), polyunsaturated fatty acids omega-3, eicosapentanoic acid (EPA), docosahexanoic acid (DHA), antioxidants and diet. Manuscripts published up to September 2013 were included. There were 43 articles related to the topic, however only 15 pertained human studies, with three review articles and 12 clinical studies.
Systemic lupus erythematous (SLE); Polyunsaturated fatty acids omega-3; Eicosapentanoic acid (EPA); Docosahexanoic acid (DHA); Antioxidants and diet
Diversos estudos têm demonstrado a habilidade dos ácidos graxos ômega-3 em reduzir asconcentrações de proteína C-reativa (PCR), eicosanoides pró-inflamatórios, citocinas, quimiocinas e de outros biomarcadores da inflamação. Por essas propriedades, a suplementação com essa classe de lipídeos pode representar terapia adicional ao tratamento de doençasinflamatórias crônicas sistêmicas, como o lúpus eritematoso sistêmico (LES) e outrasdoenças reumáticas. O papel dessa classe de lipídeos no LES ainda não está bem estabelecido. No entanto, parece haver relação entre o consumo deste tipo de gordura e a diminuiçãodas manifestações e da atividade inflamatória da doença. Sendo assim, este artigo apresentarevisão da literatura científica sobre os efeitos dos ácidos graxos ômega-3 em pacientescom LES. Realizou-se levantamento bibliográfico junto aos bancos de dados Medical Literature Analysis and Retrieval System Online (MEDLINE) e Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS), utizando-se como palavras-chave: lúpus eritematoso sistêmico (LES), ácidos graxos poli-insaturados ômega-3, ácido eicosapentaenoico(EPA), ácido docosahexaenoico (DHA), antioxidantes e dieta. Foram incluídos artigos publicados até setembro de 2013. Quarenta e três artigos relacionados ao tema foram encontrados. Após limitar a busca apenas para estudos realizados em seres humanos foram encontrados 15 artigos, sendo três de revisão e 12 ensaios clínicos.
Lúpus eritematoso sistêmico (LES); Ácidos graxos poli-insaturados; ômega-3; Ácido eicosapentaenoico (EPA); Ácido docosahexaenoico (DHA); Antioxidantesr
Introduction
Beneficial effects of omega-3 polyunsaturated fatty acids have been extensively studied, mostly those acting on the cardiovascular system.11 Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55.–44 Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M,Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acidsin obesity, metabolic syndrome, and cardiovascular diseases:a review of the evidence. J Physiol Biochem. 2013;69:633–51 Over the last few years, the interest in the role of this nutrient in reducing inflammation has grown.55 Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013;2013:743171.
These fatty acids are considered essential and should be provided by the diet or from supplements. They compete with the arachidonic acid (AA), a member of the omega-6 family through the same enzymatic pathway and stimulate series 3 prostaglandins and series 5 leukotrienes, which have a lower inflammatory action than those AA-derived eicosanoids.66 Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Aandres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. JCEM. 2006;91:439–46.
Several studies have demonstrated that omega-3 fatty acids can reduce the concentrations of C reactive protein (CRP), proinflammatory eicosanoids, cytokines, chemokines, and other inflammatory biomarkers.77 Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–20.–1010 Wallace FA, Miles EA, Calder PC. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Brit J Nutr. 2003;89:679–89. In addition, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both members of omega-3 family, are precursors of the lipid mediators resolvins and protectins, which have anti-inflammatory and immunomodulatory characteristics.1111 Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammatory signals. J Exp Med. 2002;196:1025–37.–1313 Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74.
In view of these properties, supplements with this class of lipids might represent an additional therapy of systemic chronic inflammatory diseases, such as systemic lupus erythematosus (SLE) and other rheumatic conditions. Studies conducted in subjects with rheumatoid arthritis report an improvement in general physical evaluation, pain, morning stiffness and reduced anti-inflammatory agent use following supplementation with omega-3.1414 Lau CS, Morley KD, Belch JJ. Effects of fish oil supplementation on non-steroidal anti-inflammatory drug requirement in patients with mild rheumatoid arthritis - a double-blind placebo controlled study. Br J Rheumatol. 1993;32:982–9.–1616 Lee YH, Bae SC, Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012;43:356–62.
The role of these fatty acids in SLE is not fully established yet. However, the intake of this kind of fat seems to be related to the reduction in disease manifestations and inflammatory activity.1717 Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, Falardeau P. Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int. 1989;36:653–60.–2525 Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, Magder LS, Petri M. Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatol Int. 2013. Epub ahead of print.
The authors present a literature review on the effects of omega-3 fatty acids on patients with SLE.
Method
A literature search on MEDLINE and LILACS databases was performed with the following keywords: systemic lupus erythematosus (SLE), omega-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), antioxidants, and diet. Articles published up to September 2013 were included. Forty-three articles related to the topic have been identified. After the search was limited to studies conducted in humans, 15 articles were found, with three of them being review articles and 12 clinical trials.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune inflammatory disease with a large spectrum of manifestations that is clinically characterized by relapses and remission periods with a varying course and outcome. The disease severity ranges from mild to severe. However, complete and prolonged remission is rare.2626 Vasudevan A, Ginzler E. Clinical features of systemic lupus erythematosus. In: Hochberg M, Silman A, Smolen J, Weinblatt M, Weisman M, editors. Rheumatology. Philadelphia: Mosby Elsevier; 2011.
Although SLE etiology is not known, an interaction of genetic, hormonal and environmental factors are probably involved in the disease development, thus leading to a loss of cell immunoregulation balance.2727 Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. J Clin Epidemiol. 2002;55:982–9. This immune disturbance occurs through loss of tolerance to nuclear antigens, poorly regulated activation of B- and T lymphocytes and subsequent B-lymphocyte polyclonal activation, with large numbers of reactive self-antibodies being produced and immune complex being formed. The immune complexes, along with self-antibodies, are primarily responsible for tissue lesion and organ damage.2828 Yu SL, Kuan WP, Wong CK, Li EK, Tam LS. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:715190.
This complex process involves interaction of various cytokines, chemokines, adhesion molecules, and pattern-recognition receptors (PRRs) triggering cell activation.2929 Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC,Harley JB, JA J. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56:2344–51.
T lymphocytes with a TH1 functional response are stimulated by interleukin (IL)-12 to produce tumor necrosis factor alpha (TNF-), interferon gamma (IFN-), and IL-2. On the other hand, TH2 lymphocytes produce IL-4, IL-5, and IL-13 when stimulated by IL-4. A significant cytokine elevation resulting from the TH1 response is observed in the serum of patients with SLE, thus indicating a disturbance in TH1/TH2 response pattern is likely.2828 Yu SL, Kuan WP, Wong CK, Li EK, Tam LS. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:715190. The TH17 functional response and serum levels of other cytokines, such as IL-23, IL-18, IL-21, and IL-23 are also elevated, but their role in the disease pathogenesis is still under investigation.2828 Yu SL, Kuan WP, Wong CK, Li EK, Tam LS. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:715190. Many of these cytokines are elevated in the serum of patients with SLE and are associated with a higher disease activity or certain clinical manifestations.2929 Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC,Harley JB, JA J. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56:2344–51.–3232 Su DL, Lu ZM, Shen MN, Li X, Sun LY. Roles of pro and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotechnol. 2012;2012:34714.
Few studies analyzed the role of nutrition in SLE pathogenesis to this moment. Omega-3 polyunsaturated fatty acids might have beneficial clinical effects on SLE because of their anti-inflammatory actions, which would warrant their use as another treatment option for the disease.1313 Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74.
Omega-3 polyunsaturated fatty acids
Fatty acids may be classified as saturated (no double bonds between individual carbon atoms) fatty acids and mono- or polyunsaturated fatty acids according to the number of double bonds in the chain. The most common saturated fatty acids in human food are: lauric, myristic, palmitic, and stearic acids (ranging from 12 to 18 carbon atoms). Unsaturated fatty acids are classified into two main categories: polyunsaturated fatty acids, represented by omega-6 fats (with main representatives being linoleic and arachidonic acids) and omega-3 fats (with main representatives being -linolenic, eicosapentaenoic [EPA], and docosahexaenoic [DHA] acids) or monounsaturated fatty acids, represented by the omega-9 (omega-9 – oleic) fats. Omega-3 and omega-6 fatty acids are considered essential, as they are not synthesized by the human body. The linoleic acid (18:2 omega-3) is the precursor of the other polyunsaturated fatty acids in omega-6 fats, which has soy, corn, and sunflower vegetable oils as their main food sources. In the omega-3 family, the -linolenic acid (18:3 omega-3) is found in a number of vegetables, such as canola and linseed, whereas EPA (20:5 omega-3) and DHA (22:6 omega-3) are found in deep sea cold water fish (mackerel, sardines, salmon, herring). On the other hand, oleic acid (18:1 omega-9) can be synthesized by the body and its main diet sources are olive oil, canola oil, olives, avocado and nuts (peanuts, chestnuts, walnuts, almonds).3333 Bhangle S, Kolasinski SL. Fish oil in rheumatic diseases. Rheum Dis Clin N Am. 2011;37:77–84.
Mechanisms of action of omega-3 fatty acids
The first data suggesting a possible anti-inflammatory role for omega-3 fatty acids are derived from an epidemiological study in Greenland Eskimos conducted by Kronmann and Green in 1980. The authors found that the prevalence of diseases with an inflammatory component, such as acute myocardium infarction, diabetes mellitus (DM), multiple sclerosis, bronchial asthma, and thyrotoxicosis, was lower in the study population than in Western country populations. Eskimos diet have a large amount of seafood, which is a source of polyunsaturated fatty acids.3434 Kronmann N, Green A. Epidemiological studies in the Upernavik district, Greenland: incidence of some chronic diseases 1950-1974. Acta Med Scand. 1980;208:401–6. Thereafter, several studies were conducted to find the role of these lipids in many chronic diseases in which inflammation plays a major pathogenetic role.
The omega-3 and omega-6 families, through their members EPA, DHA, and arachidonic acid (AA), are the essential lipid classes for eicosanoid synthesis. Eicosanoids are among the major inflammation mediators and regulators. They include prostaglandins (PG), leukotrienes (LT), thromboxanes, and other oxidized derivatives. They are produced by oxidative pathways of the enzymes cyclooxygenase (COX) and lipoxygenase (LOX).3535 Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest.2001;108:15–23.
Due to their molecular similarity, linoleic acid and -linolenic acid compete for the same enzymes to synthesize derivatives with 20 carbon atoms: AA (20:4 omega-6), eicosapentaenoic acid (EPA) (20:5 omega-3) e eicosatrienoic acid (ETA) (20:3 omega-9). The -linolenic acid has a 22-carbon atom derivative, the docosahexaenoic acid (DHA) (22:6 omega-3)3333 Bhangle S, Kolasinski SL. Fish oil in rheumatic diseases. Rheum Dis Clin N Am. 2011;37:77–84.,3636 Bistrian BR. Clinical aspects of essential fatty acid metabolism: Jonathan Rhoads lecture. JPEN. 2003;27:168–75.,3737 Leonard AE, Pereira SL, Sprecher H, Huang HS. Elogation of long-chain fatty acids. Prog Lipid Res. 2004;43:36–54. (Fig. 1).
Omega-3 and omega-6 fatty-acid metabolism Adapted from Leonard et al., 2004.3737 Leonard AE, Pereira SL, Sprecher H, Huang HS. Elogation of long-chain fatty acids. Prog Lipid Res. 2004;43:36–54..
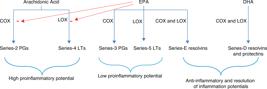
A high intake of linoleic acid favors an increased AA content in membrane phospholipids, thus increasing the production of series 2 and 4 eicosanoids (prostaglandin E2 and leukotriene B4) through cyclooxygenase and lipoxygenase enzymatic pathways, respectively. A high production of eicosanoids is related to the occurrence of immune disorders, cardiovascular and inflammatory diseases. On the other hand, the intake of omega-3 family fatty acids, such as -linolenic acid, EPA or DHA, which compete with AA for the same enzymatic pathways, competitively inhibits the arachidonic acid oxidation by cyclooxygenase (COX) into prostaglandins and their conversion into leukotrienes (LTs) via 5-lipoxygenase (LOX) pathway.66 Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Aandres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. JCEM. 2006;91:439–46.,3333 Bhangle S, Kolasinski SL. Fish oil in rheumatic diseases. Rheum Dis Clin N Am. 2011;37:77–84. The omega-6 fatty acid leads to proinflammatory series 2 and 4 eicosanoid production [PGE2, thromboxane A2 (TXA2) and leukotriene B4 (LTB4)]. PGE2 induces a number of proinflammatory effects, such as increased vascular permeability, vasodilation, hyperemia, and hiperalgesia.3838 Samuelsson B. Leukotrienes: mediators of immediate hyersensitivity reactions and inflammation. Science. 1983;220:568–75.–4040 Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107:S171–84. TXA2 promotes the synthesis of inflammatory cytokines, IL-1β and TNF-α by mononuclear phagocytes.3939 Salmmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43:285–96.,4141 Caughey GE, Pouliot M, Cleland LG, James MJ. Regulation of tumor necrosis factor-alpha and IL-1 beta synthesis by thromboxane A2 in nonadherent human monocytes. J Immunol. 1997;158:351–8. On the other hand, LTB4, in addition to causing increased vascular permeability and hyperemia, is a powerful leukocyte chemotactic agent inducing the production of lysosomal enzymes and increasing the production of reactive oxygen species and cytokines, such as TNF-, IL-1, and IL-6 (Fig. 2).3939 Salmmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43:285–96.,4040 Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107:S171–84.
Syntheses and actions of lipid mediators produced by AA, EPA, and DHAAA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docoxahexaenoic acid; COX, cyclooxygenase; LOX, lipoxygenase;PG, prostaglandins; LT, leukotrienes Adapted from Calder et al., 2010.1313 Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74..
Thus, the omega-3 fatty acid acts by reducing the formation of eicosanoids with inflammatory characteristics, since it competes with omega-6 fatty acids for the same enzymatic pathway, leading to the inhibition of TNF-, IL-1 and IL-6 synthesis and reducing the intercellular adhesion molecule-1 (ICAM-1) expression.4242 Hugues DA, Pinder AC. n-3 polynsaturated fatty acids inhibit the antigen-presenting function of human monocytes. Am J Clin Nutr. 2000;71:357S–60S. It is also a substrate for the synthesis of series 3 and 5 eicosanoids, which have less inflammatory characteristics (Fig. 2).66 Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Aandres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. JCEM. 2006;91:439–46.
Studies in cultured cells have further demonstrated that EPA and DHA can inhibit the production of classic inflammatory cytokines, such as TNF-, IL-1, IL-6, and others.4040 Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107:S171–84.,4343 De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic an proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–36.–4545 Zhao Y, Joshi-Barve S, Barve S, Chen LH, Barve S, Chen LH. Eicosapentaenoic acid prevents LSP-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–8. In healthy subjects, reduced production of TNF-, IL-1 and IL6 by monocytes and mononuclear cells has already been demonstrated following fish oil supplementation,99 Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, Ballinger AB, Thompson RL, Calder PC. Inhibition of tumour necrosis factor-α and interleukin-6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidantco-supplementation. Brit J Nutr. 2003;90:405–12.,1010 Wallace FA, Miles EA, Calder PC. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Brit J Nutr. 2003;89:679–89.,4646 Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older woman. J Nutr. 1991;121:547–55. although other studies have not confirmed those findings.88 Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in health adults. Lipids. 2001;36:1183–93.,4747 Kew S, Banerjee T, Minihane AM, Finnegan YE, Muggli R, Albers R, Williams CM, Calder PC. Lack of effect of food senriched with plan- or marine-derived n-3 fatty acids on human immune function. Am J Clin Nutr. 2003;77:1287–95.,4848 Miles EA, Banerjee T, Dooper MM, M’Rabet L, Graus YM,Calder PC. The influence of different combinations of gamma-linolenic acid, stearidonic acid and EPA on immune function in healthy young male subjects. Brit J Nutr. 2004;91:893–903. These differences might have occurred because of the different doses used, the duration of treatment, and non-representative samples.
T-cell proliferation inhibition and IL-2 production were also found in cultured cells following supplementation with EPA and DHA.4949 Calder PC, Newsholme EA. Polyunsaturated fatty acids suppress human peripheral blood lymphocyte proliferation and interleukin-2 production. Clin Sci. 1992;82:695–700. However, these findings are inconsistent in human studies.
In addition, EPA and DHA are precursors of the lipid mediators resolvins and protectins. The pathway for production of theses mediators also involves the enzymes cyclooxygenase and lipoxygenase (Fig. 2). In cultured-cell and animal studies, both resolvins and protectins demonstrated to have anti-inflammatory and immunomodulating actions.1111 Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammatory signals. J Exp Med. 2002;196:1025–37.–1313 Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74.,5050 Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Rev Immunol. 2008;8:349–61. Resolvins E1 and D1 and protectin D1 can inhibit neutrophil transendothelial migration, thus preventing the infiltration of immune cells at the inflammation site. Resolvin E1 can further inhibit IL-1 production and protectin E1 inhibits IL-1 and TNF- production.5050 Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Rev Immunol. 2008;8:349–61. Thus, these lipid mediators can play a role in the resolution phase of inflammation and in limiting tissue damage.
Thus, the ratio between daily intake of omega-6 and omega-3 fatty-acid sources assumes great importance in human nutrition.3636 Bistrian BR. Clinical aspects of essential fatty acid metabolism: Jonathan Rhoads lecture. JPEN. 2003;27:168–75. However, the Western diet is characterized by a high omega-6 polyunsaturated fatty acid intake and high omega-6/omega-3 ratio, which favors pathogenesis in a number of conditions, including cardiovascular, inflammatory, and autoimmune diseases, as well as cancer. The omega-6/omega-3 ratio in Western diet is estimated to be 15-20:1. On the other hand, an increased omega-3 intake and a reduced ratio between these fatty acids as a consequence seems to exert a suppressive effect on disease pathogenesis.5151 Simopoulos AP. Ômega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev Int. 2004;20:36–54.
Omega-3 and Systemic Lupus Erythematosus
Few studies investigating omega-3 fatty acid effects on subjects with SLE have been conducted to this moment. However, a few trials suggest that supplementation with this class of lipids can represent an additional therapy to the standard pharmacological treatment of this disease due to their anti-inflammatory properties.1717 Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, Falardeau P. Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int. 1989;36:653–60.–2525 Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, Magder LS, Petri M. Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatol Int. 2013. Epub ahead of print.
A difference in erythrocyte and plasma fatty acid level between individuals with SLE and individuals without SLE seems to exist. However, it is not known whether this difference occurs due to an inappropriate diet or the disease itself. Aghdassi et al. (2011) compared the content of red cell and plasma fatty acids between women with SLE and controls, between patients with SLE with or without a history of cardiovascular disease (CVD) and between patients taking or not prednisone. Lower levels of omega-3 (EPA+DHA) and EPA alone were found in red-cell membranes of individuals with SLE compared with healthy individuals. On the other hand, no significant differences for fatty acid content between patients and controls were found in plasma. In addition, patients with SLE and a history of CVD had higher levels of plasma monounsaturated fatty acids and higher levels of omega-6 family polyunsaturated fatty acids than those without CVD. Patients taking prednisone had higher levels of omega-3 and lower AA/EPA ratios compared with those not taking prednisone. However, these differences were not statistically significant.2323 Aghdassi E, L. Ma DW, Morrison S, Hillyer LM, Clarke S, Gladman DD, Urowitz MB, Fortin PR. Alterations in circulating fatty acid composition in patients with systemic lupus erythematosus: a pilot study. JPEN. 2011;35:198–208.
Low levels of omega-3 could contribute to worsen the inflammation already present in these patients, thus indicating that the nutrient supplementation, on its turn, would contribute to improve the inflammatory profile and, consequently, the disease activity, as substantiated by Elkan et al. (2012) in a study conducted in 114 patients with SLE and 122 subjects without the disease. The authors found that higher levels of EPA and DHA in fat cells of patients with SLE were negatively associated with disease activity (measured by Systemic Lupus Erythematosus Disease Activity Index – SLEDAI) and the presence of atherosclerotic plaques. On the other hand, higher omega-6 levels were positively associated with a higher damage index (evaluated by the Systemic Lupus International Collaboration Clinics/American College of Rheumatology Damage Index – SLICC/ACR) and the presence of atherosclerotic plaques. In this study, food intake evaluation identified that subjects with SLE described a higher carbohydrate intake and a lower fiber and polyunsaturated fatty acid (omega-6 and omega-3) intake than controls. Carbohydrate intake was positively associated with the amount of omega-6 fatty acids present in adipose tissue. An association between carbohydrate, fiber, saturated and monounsaturated fatty acid intake and disease activity or damage index could not be found.2424 Elkan AC, Anania C, Gustafsson T, Jogestrand T, Hafstrõm I, Frostegard J. Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and atherosclerosis. Lupus. 2012;21:1405–11.
The first study evaluating the effect of omega-3 fatty acids on patients with SLE taking fish oil supplementation was reported in 1989 by Clark et al. The authors analyzed the effects of omega-3 (EPA and DHA) on 12 subjects with SLE and nephritis and concluded that diet supplementation with fish oil can affect the mechanisms involved in inflammation and atherosclerosis. The patients were given a supplementation over five weeks with daily doses of 6 g of fish oil, followed by a period of five weeks without the supplementation and then five more weeks with 18 g of fish oil daily. A rise in EPA and DHA levels in membrane phospholipids and reduced AA incorporation were found. These changes were associated with reduced platelet aggregation and blood viscosity and with an increase in red cell flexibility. LTB4 production by neutrophils was also significantly reduced. The higher fish oil dose induced 38% reduction in triglyceride levels and a 39% reduction in VLDL-cholesterol, associated with a 28% increase in HDL-cholesterol levels. The effects on the anti-DNA antibodies title and albuminuria was not verified.1717 Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, Falardeau P. Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int. 1989;36:653–60.
On the other hand, in another trial conducted by the same group of investigators, different results were found. In 21 patients with a stable lupus nephritis who were given a daily dose of 15 g fish oil or placebo containing olive oil over one year, an improvement in kidney function or in the disease activity was not found in the fish oil group compared with the placebo group. As for blood lipids, only VLDL-cholesterol and triglycerides had a significant reduction following the treatment with fish oil, whereas LDL and HDL fractions remained unchanged.1919 Clark WF, Parbtani A, Naylor CD, Levinton CM, Muirhead N, Spanner E, Huff MW, Philbrick DJ, Holub BJ. Fish oil in lupus nephritis: clinical findings and methodological implications. Kidney Int. 1993;44:75–86.
Westberg et al. (1990) evaluated the effects of omega-3 fatty acids supplementation with capsules containing fish oil or capsules of olive oil as a placebo in 60 patients with moderately active SLE. Over the first three months, the patients receiving omega-3 supplementation had significant improvement in clinical and laboratory parameters. However, after a six-month supplementation, no differences between groups could be detected, which suggests, according to the authors, the supplement effects are short-lived.1818 Westberg G, Tarkowski A. Effect of MaxEPA in patients with SLE. A double-blind, crossover study. Scand J Rheumatol. 1990;19:137–43.
In 2004, Duffy et al. published another trial evaluating the effect of omega-3 supplements and/or copper in patients with SLE. Fifty-two patients were divided into four treatment groups. One of the groups was given 3 g of fish oil in capsules (EPA 540 mg+DHA 360 mg/capsule) and 3 mg of copper; the second group was given 3 g of fish oil and copper as a placebo; the third group received a supplement with 3 mg of copper and fish oil placebo; the fourth group received placebo instead of both nutrients. After a six-month supplementation, a reduced disease activity from 6.12 to 4.69 (P < 0.05), measured by the Systemic Lupus Activity Measure (SLAM) score, was found. The integument, neuromotor, and laboratory domains of SLAM-R were most affected by supplementation.2020 Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL. The clinical effect of dietary supplementation with omega-3 fish oils and copper in systemic lupu serythematosus. J Rheumatol. 2004;31:1551–6.
Omega-3 beneficial effects on reducing lupus nephritis activity were evaluated by Nakamura et al. (2005). The authors examined the effect of daily supplementation with 1.8 g of purified EPA in six patients with lupus nephritis diagnosed by kidney biopsy. After a 3-month supplementation, reduced AA levels and increased levels of EPA in plasma phospholipids were detected, in addition to reduced urinary levels of 8-isoprostane (from 550 113 pg/mg CR to 235 49 pg/mg CR, P=0.02). In the authors’ opinion, these findings indicate that EPA can promote decreased oxidative stress, with consequent benefits for lupus nephritis treatment. On the other hand, when immune parameters were evaluated, no significant differences were found in anti-DNA and serum complement values after omega-3 treatment.2121 Nakamura N, Kumasaka R, Osawa H, Yamabe H, Shirato K, Fujita T, Murakami R, Shimada M, Nakamura M, Okumura K, Hamazaki K, Hamazaki T. Effects of eicosapentaenoic acids on oxidative stress and plasma fatty acid composition in patients with lupus nephritis. In vivo. 2005;19:879–82.
Wright et al. (2008) compared the effects of daily intake of 3 g of omega-3 (1.200 mg of DHA+1.800 mg of EPA) with placebo of olive oil in 60 patients with SLE over a 24-week period. They noted that the omega-3 supplementation in patients with SLE could not only have a therapeutic effect on the disease activity measured through the Systemic Lupus Activity Measure (SLAM-R) and British Isles Lupus Assessment Group (BILAG) indexes, but also promote improvement in endothelial function and reduced oxidative stress, thus conferring cardiovascular benefits.2222 Wright SA, O´Prey FM, McHenry MT, Leahey WJ, Devine AB, Duffy EM, Johnston DG, Finch MB, Bell AL, McVeigh GE. A randomised interventional trial of w-3 polyunsaturated acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841–8.
Recently, Bello et al. (2013) have published a double-blind, placebo-controlled trial in which 85 patients with SLE were randomized to receive either 3 g of omega-3 supplements or placebo. After a 12-week supplementation, no significant differences between the groups were found for disease activity, serum levels of inflammatory markers (ICAM-1, VCAM-1, and IL-6), and endothelial function. As for the lipid profile, patients receiving omega-3 had a significant increase in LDL cholesterol levels, whereas those in the placebo group had a significant reduction of this lipoprotein.2525 Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, Magder LS, Petri M. Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatol Int. 2013. Epub ahead of print.
Studies evaluating the effect of omega-3 fatty acids on SLE are listed in Table 1.
Interventional studies with omega-3 fatty acids in patients with systemic lupus erythematosus (SLE).
Final remarks
Clinical studies suggest that supplementation with omega-3 polyunsaturated fatty acids can represent an additional option to SLE standard pharmacological treatment because of their anti-inflammatory properties.1717 Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, Falardeau P. Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int. 1989;36:653–60.–2525 Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, Magder LS, Petri M. Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatol Int. 2013. Epub ahead of print. This lipid class is related to the production of eicosanoids with a lower inflammatory action than those produced by fatty acids pertaining to the omega-6 family, additionally reducing serum levels of inflammatory cytokines and T lymphocyte activation.
Clinical trials conducted used very different doses, ranging from 1.8 g to 18 g of fish oil, and different treatment lengths, ranging from five weeks to 12 months. Whether positive effects are caused by EPA intake, DHA intake or by a combined action of both fatty acids is questionable. In addition, most studies have small numbers of subjects included, therefore leading to limited conclusions.
Thus, further studies with a larger number of subjects are needed to evaluate the real effect of these fatty acids in patients with SLE, the effective dose, and the duration of treatment.
-
☆
Institution: From the Clinic of Rheumatology, Hospital das Clínicas, UFMG/Department of Musculoskeletal System and Department of Surgery, Faculty of Medicine, UFMG, Post-graduation Program in Sciences Applied to Adult Health, Faculty of Medicine, UFM, major area in Clinical Sciences.
-
FundingThis research project received funding from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig).
Referências
-
1Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55.
-
2Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. N−3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17.
-
3Kromhout D, Giltay EJ, Geleijnse JM. N−3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26
-
4Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M,Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acidsin obesity, metabolic syndrome, and cardiovascular diseases:a review of the evidence. J Physiol Biochem. 2013;69:633–51
-
5Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013;2013:743171.
-
6Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Aandres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. JCEM. 2006;91:439–46.
-
7Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–20.
-
8Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in health adults. Lipids. 2001;36:1183–93.
-
9Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, Ballinger AB, Thompson RL, Calder PC. Inhibition of tumour necrosis factor-α and interleukin-6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidantco-supplementation. Brit J Nutr. 2003;90:405–12.
-
10Wallace FA, Miles EA, Calder PC. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Brit J Nutr. 2003;89:679–89.
-
11Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammatory signals. J Exp Med. 2002;196:1025–37.
-
12Hong S, Gronert K, Devchand P, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autocoids in anti-inflammation. J Biol Chem. 2003;278:14677–87.
-
13Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74.
-
14Lau CS, Morley KD, Belch JJ. Effects of fish oil supplementation on non-steroidal anti-inflammatory drug requirement in patients with mild rheumatoid arthritis - a double-blind placebo controlled study. Br J Rheumatol. 1993;32:982–9.
-
15Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21:131–6.
-
16Lee YH, Bae SC, Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012;43:356–62.
-
17Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, Falardeau P. Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int. 1989;36:653–60.
-
18Westberg G, Tarkowski A. Effect of MaxEPA in patients with SLE. A double-blind, crossover study. Scand J Rheumatol. 1990;19:137–43.
-
19Clark WF, Parbtani A, Naylor CD, Levinton CM, Muirhead N, Spanner E, Huff MW, Philbrick DJ, Holub BJ. Fish oil in lupus nephritis: clinical findings and methodological implications. Kidney Int. 1993;44:75–86.
-
20Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL. The clinical effect of dietary supplementation with omega-3 fish oils and copper in systemic lupu serythematosus. J Rheumatol. 2004;31:1551–6.
-
21Nakamura N, Kumasaka R, Osawa H, Yamabe H, Shirato K, Fujita T, Murakami R, Shimada M, Nakamura M, Okumura K, Hamazaki K, Hamazaki T. Effects of eicosapentaenoic acids on oxidative stress and plasma fatty acid composition in patients with lupus nephritis. In vivo. 2005;19:879–82.
-
22Wright SA, O´Prey FM, McHenry MT, Leahey WJ, Devine AB, Duffy EM, Johnston DG, Finch MB, Bell AL, McVeigh GE. A randomised interventional trial of w-3 polyunsaturated acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841–8.
-
23Aghdassi E, L. Ma DW, Morrison S, Hillyer LM, Clarke S, Gladman DD, Urowitz MB, Fortin PR. Alterations in circulating fatty acid composition in patients with systemic lupus erythematosus: a pilot study. JPEN. 2011;35:198–208.
-
24Elkan AC, Anania C, Gustafsson T, Jogestrand T, Hafstrõm I, Frostegard J. Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and atherosclerosis. Lupus. 2012;21:1405–11.
-
25Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, Magder LS, Petri M. Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatol Int. 2013. Epub ahead of print.
-
26Vasudevan A, Ginzler E. Clinical features of systemic lupus erythematosus. In: Hochberg M, Silman A, Smolen J, Weinblatt M, Weisman M, editors. Rheumatology. Philadelphia: Mosby Elsevier; 2011.
-
27Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. J Clin Epidemiol. 2002;55:982–9.
-
28Yu SL, Kuan WP, Wong CK, Li EK, Tam LS. Immunopathological roles of cytokines, chemokines, signaling molecules, and pattern-recognition receptors in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:715190.
-
29Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC,Harley JB, JA J. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56:2344–51.
-
30Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res. 2011;13:228.
-
31Ohl K, Tenbrock K. Inflammatory cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:432595.
-
32Su DL, Lu ZM, Shen MN, Li X, Sun LY. Roles of pro and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotechnol. 2012;2012:34714.
-
33Bhangle S, Kolasinski SL. Fish oil in rheumatic diseases. Rheum Dis Clin N Am. 2011;37:77–84.
-
34Kronmann N, Green A. Epidemiological studies in the Upernavik district, Greenland: incidence of some chronic diseases 1950-1974. Acta Med Scand. 1980;208:401–6.
-
35Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest.2001;108:15–23.
-
36Bistrian BR. Clinical aspects of essential fatty acid metabolism: Jonathan Rhoads lecture. JPEN. 2003;27:168–75.
-
37Leonard AE, Pereira SL, Sprecher H, Huang HS. Elogation of long-chain fatty acids. Prog Lipid Res. 2004;43:36–54.
-
38Samuelsson B. Leukotrienes: mediators of immediate hyersensitivity reactions and inflammation. Science. 1983;220:568–75.
-
39Salmmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43:285–96.
-
40Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107:S171–84.
-
41Caughey GE, Pouliot M, Cleland LG, James MJ. Regulation of tumor necrosis factor-alpha and IL-1 beta synthesis by thromboxane A2 in nonadherent human monocytes. J Immunol. 1997;158:351–8.
-
42Hugues DA, Pinder AC. n-3 polynsaturated fatty acids inhibit the antigen-presenting function of human monocytes. Am J Clin Nutr. 2000;71:357S–60S.
-
43De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic an proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–36.
-
44Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in bitro human endothelial cell production of interleukin-6. Adv Exp Biol Med. 1997;400:589–97.
-
45Zhao Y, Joshi-Barve S, Barve S, Chen LH, Barve S, Chen LH. Eicosapentaenoic acid prevents LSP-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–8.
-
46Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older woman. J Nutr. 1991;121:547–55.
-
47Kew S, Banerjee T, Minihane AM, Finnegan YE, Muggli R, Albers R, Williams CM, Calder PC. Lack of effect of food senriched with plan- or marine-derived n-3 fatty acids on human immune function. Am J Clin Nutr. 2003;77:1287–95.
-
48Miles EA, Banerjee T, Dooper MM, M’Rabet L, Graus YM,Calder PC. The influence of different combinations of gamma-linolenic acid, stearidonic acid and EPA on immune function in healthy young male subjects. Brit J Nutr. 2004;91:893–903.
-
49Calder PC, Newsholme EA. Polyunsaturated fatty acids suppress human peripheral blood lymphocyte proliferation and interleukin-2 production. Clin Sci. 1992;82:695–700.
-
50Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Rev Immunol. 2008;8:349–61.
-
51Simopoulos AP. Ômega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev Int. 2004;20:36–54.
Publication Dates
-
Publication in this collection
Nov-Dec 2014
History
-
Received
16 Oct 2013 -
Accepted
10 Dec 2013