ABSTRACT
The fluid resuscitation of patients with acute circulatory failure aims to increase systolic volume and consequently improve cardiac output for better tissue oxygenation. However, this effect does not always occur because approximately half of patients do not respond to fluids. The evaluation of fluid responsiveness before their administration may help to identify patients who would benefit from fluid resuscitation and avoid the risk of fluid overload in the others. The dynamic parameters of fluid responsiveness evaluation are promising predictive factors. Of these, the echocardiographic measurement of the respiratory variation in the inferior vena cava diameter is easy to apply and has been used in the hemodynamic evaluation of intensive care unit patients. However, the applicability of this technique has many limitations, and the present studies are heterogeneous and inconsistent across specific groups of patients. We review the use of the inferior vena cava diameter respiratory variation, measured via transthoracic echocardiography, to decide whether to administer fluids to patients with acute circulatory failure in the intensive care unit. We explore the benefits and limitations of this technique, its current use, and the existing evidence.
Keywords:
Inferior vena cava; Echocardiography; Fluid therapy; Intensive care
RESUMO
A ressuscitação hídrica do paciente em falência circulatória aguda tem como um de seus objetivos aumentar o volume sistólico e, consequentemente, o débito cardíaco, para melhor oxigenação dos tecidos. Contudo, isso não se verifica em cerca de metade dos pacientes, que são considerados não respondedores a fluidos. A avaliação da resposta a fluidos antes de sua administração pode selecionar os pacientes que devem ter benefício e evitar o risco de sobrecarga nos restantes. Os parâmetros dinâmicos de avaliação da resposta a fluidos têm se revelado promissores enquanto fatores preditores. Entre estes, a medição ecocardiográfica da variação respiratória do diâmetro da veia cava inferior é um método de fácil aplicação, que tem sido difundido na avaliação hemodinâmica em unidades de cuidados intensivos. No entanto, a aplicabilidade desta técnica tem muitas limitações, e os estudos, até à presente data, são heterogêneos e pouco consistentes em alguns grupos de pacientes. Realizamos uma revisão sobre a utilização da variação respiratória do diâmetro da veia cava inferior, medida por ecocardiografia transtorácica, na decisão de administrar fluidos ao paciente em falência circulatória aguda, em cuidados intensivos, incluindo potencialidades e limitações da técnica, de sua interpretação e a evidência existente.
Descritores:
Veia cava inferior; Ecocardiografia; Hidratação; Cuidados críticos
INTRODUCTION
Patients with acute circulatory failure who exhibit signs of organ hypoperfusion and tissue hypoxia are common in the intensive care unit (ICU). The initial fluid resuscitation of patients in shock is associated with reduced mortality, and this effect is well established among patients with septic shock.(11 Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest. 2014;146(4):908-15.) However, after the initial fluid resuscitation phase, fluid administration is not necessarily beneficial.(22 Ogbu OC, Murphy DJ, Martin GS. How to avoid fluid overload. Curr Opin Crit Care. 2015;21(4):315-21.) It might even be deleterious and lead to increased left ventricular filling pressure with pulmonary and tissue edema, which is associated with increased mortality and invasive mechanical ventilation (IMV) time.(33 Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43(1):68-73.)
Fluid therapy seeks to increase systolic volume (SV) and consequently improve cardiac output (CO) and oxygen transport to tissues. However, the fluid responsiveness of patients with shock is not linear because it depends on the contractile capacity of the myocardium (Figure 1). Since the contractile capacity is not directly measured and it is not possible to predict the configuration of the Frank-Starling curve of each patient, it is difficult to predict response to volume. Previous studies have shown that its administration does not result in increased CO in approximately 50% of patients.(44 Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract. 2016;3(2):G1-12.
5 ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-93.
6 Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000-8.-77 Long E, Oakley E, Duke T, Babl FE; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Does respiratory variation in inferior vena cava diameter predic fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47(5):550-9.)
Frank-Starling curve and its relationship with inferior vena cava variation among patients under invasive mechanical ventilation.
The relationship between preload and systolic volume: Frank-Starling curve. This figure shows the expected increase in systolic volume after the administration of fluids, which depends on cardiac function and the initial preload. For the same amount of fluids administered and for a similar initial preload, the variation in the resulting systolic volume depends on the cardiac function: (A) The Frank-Starling curve of a patient with normal cardiac function. In patients with normal cardiac function, the results of fluid administration only depend on the initial preload: If it is low (rising phase of the curve), then systolic volume significantly increases (≥ 10 - 15%, respondent patient), corresponding to a significant variation in the diameter of the inferior vena cava with the application of positive pressure to the thorax during inspiration in the ventilated patient; if it is elevated (flat phase of the curve), then no significant increase in systolic volume is observed (<10 - 15%, nonrespondent) leading to pulmonary overload, which corresponds to an inferior vena cava with little distension. (B) The Frank-Starling curve in a patient with decreased cardiac function. In this case, the administration of fluids, even with low initial preload, may result in pulmonary fluid overload without a significant increase in systolic volume. SV - systolic volume
Therefore, the evaluation of fluid responsiveness aims to estimate the potential for a significant increase in CO in response to volume expansion, thereby avoiding inappropriate administration. A patient is considered a responder when an increase in CO of greater than 10 or 15% is observed;(44 Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract. 2016;3(2):G1-12.,88 Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20:274.,99 Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.) this result denotes that the patient is in the ascending phase of the Frank-Starling curve. In this patient, the administration of fluids would likely lead to increased SV, CO and consequent better oxygen delivery to tissues.
Several static and dynamic parameters have been evaluated as possible predictors of fluid responsiveness. In clinical practice, no standard reference or gold standard has been defined to evaluate fluid responsiveness; however, a growing consensus exists in favor of dynamic parameters(22 Ogbu OC, Murphy DJ, Martin GS. How to avoid fluid overload. Curr Opin Crit Care. 2015;21(4):315-21.) because static parameters have shown no predictive value.(1010 Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-81.,1111 Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29(3):352-60.)
The dynamic parameters are based on two ways of varying CO without administering fluids to predict the clinical response.(44 Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract. 2016;3(2):G1-12.) One of these forms is through the lower limb elevation maneuver, which increases venous return and preload so that the CO variation is directly evaluated. The other form is based on the use of the lung-heart interaction. Variations in transpulmonary pressure with respiration induce CO variation, which is evaluated using one of the following: SV variation, variation in pulse pressure, variation in the diameter of the superior vena cava (SVC), or variation in the diameter of the inferior vena cava (IVC).
This article reviews the use of IVC respiratory variation to assess fluid responsiveness and its applicability among adults with acute circulatory failure in the ICU. We address the physiological principle, the technique, the clinical utility, the practical difficulties associated with its use and interpretation, and the underlying evidence.
METHODS
A search was performed using the PubMed database with the terms "fluid responsiveness", "inferior vena cava", "echocardiography", "hemodynamic assessment", and "intensive care". The references of the included articles were also searched when considered relevant by the authors. The selection strategy was restricted to articles focusing on the use of IVC assessment among adult ICU patients published before January 1, 2018. No restriction filters were used with regard to language.
DISCUSSION
Echocardiographic evaluation of the inferior vena cava in the intensive care unit: Physiological principles, technique, and clinical indications
The assessment of IVC using transthoracic echocardiography is a conventional element of the echocardiographic study of critical patients. The physiological principle behind it is the lung-heart interaction. The variation in transpulmonary pressure during respiration is transmitted to the right heart cavities, which varies the venous return and the IVC diameter. This relationship depends on the ventilatory mode and IVC compliance of the patient.(1212 Pinsky MR. Heart-lung interactions. Curr Opin Crit Care. 2007;13(5):528-31.
13 Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249-54.-1414 Ramos FJ, Azevedo LC. Assessment of fluid responsiveness in patients under spontaneous breathing activity. Rev Bras Ter Intensiva. 2009;21(2):212-8.)
In nonventilated patients or those under IMV with respiratory effort, there is a negative transpulmonary pressure at the beginning of inspiration that induces a variable degree of IVC collapse as a function of its compliance. For example, in patients with high right heart cavity pressure or elevated preload (during the flat phase of the Frank-Starling curve), IVC shows reduced compliance and limited collapse due to the negative transpulmonary pressure transmitted; in fact, collapse may be absent. Among patients with low right heart cavity pressure in hypovolemia (i.e., the ascending phase of the Frank-Starling curve), IVC compliance is high, and collapse is significant during inspiration.
By contrast, positive pressure can be applied to the thorax during inspiration among patients under IMV without respiratory effort (in the controlled mode). This pressure is transmitted to the right heart cavities and the IVC, which stretches as a function of its compliance. Among patients without cardiac reserve due to poor cardiac function and/or those with high preload (i.e., during the flat phase of the Frank-Starling curve), the IVC shows reduced compliance and limited distention, and its diameter may not vary. Conversely, the IVC of patients with cardiac reserve who potentially benefit from the administration of fluids shows significant distension during inspiration.
At the technical level, the IVC diameter should be measured with the patient in the supine position at the subcostal window using the long axis. Measurements should be performed in the two-dimensional mode distal to the hepatic vein (i.e., approximately 1 - 3cm from the IVC entrance in the right atrium; Figure 2).(1515 De Backer D, Fagnoul D. Intensive care ultrasound: VI. Fluid responsiveness and shock assessment. Ann Am Thorac Soc. 2014;11(1):129-36.
16 Evans D, Ferraioli G, Snellings J, Levitov A. Volume responsiveness in critically ill patients: use of sonography to guide management. J Ultrasound Med. 2014;33(1):3-7.-1717 Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.) The IVC diameter can also be measured in M mode, although a perfect probe alignment perpendicular to the long IVC axis is necessary. This measure implies the simultaneous use of the M and two-dimensional modes, with constant visualization of the IVC walls.
Inferior vena cava diameter measurement technique.
The inferior vena cava should be measured in two-dimensional mode at the subcostal window using the long axis distal to the hepatic vein (arrow), approximately 1 - 3cm from the entrance of the inferior vena cava in the right atrium (A). Measurements near the right atrium entrance or near the diaphragm should be avoided. Its diameter can also be measured in M mode simultaneously with the two-dimensional mode to ensure the perfect alignment of the probe, perpendicular to the long axis of the inferior vena cava. In patients under invasive mechanical ventilation, the diameter of the inferior vena cava at the end of inspiration (maximal diameter) and at the end of expiration (minimum diameter) is measured to calculate the distensibility index. The probe must be kept in a fixed position during the respiratory cycle. Image obtained with a GE Vivid T8 echocardiograph. IVC - inferior vena cava.
International recommendations(1717 Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.,1818 Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients - Part II: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-27.) suggest that the IVC be assessed to estimate the pressure in the right atrium of nonventilated patients because of its collapsibility during inspiration. An IVC diameter of < 21mm with a collapsibility of > 50% during inspiration suggests normal right atrium pressure (between 0 and 5 mmHg), whereas a diameter of > 21mm with collapsibility of < 50% suggests high pressure (between 10 and 20mmHg). A pressure between 5 and 10mmHg is considered intermediate; in this case, other parameters should be used to better characterize the pressure in the right atrium as normal or elevated, such as the size of the right atrium, hepatic flow, tricuspid regurgitation, and right ventricle function.(1717 Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.,1818 Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients - Part II: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-27.)
Inferior vena cava assessment can also be used to evaluate fluid responsiveness.(1818 Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients - Part II: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-27.,1919 Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795-815.) At the end of expiration, an IVC diameter of < 10mm is frequent at low blood volume states, which suggests a higher probability of response, whereas a diameter of > 25mm is frequent during states of high blood volume and suggests a low probability of fluid responsiveness.(2020 Orde S, Slama M, Hilton A, Yastrebov K, McLean A. Pearls and pitfalls in comprehensive critical care echocardiography. Crit Care. 2017;21(1):279.
21 Lee CW, Kory PD, Arntfield RT. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care. 2016;31(1):96-100.
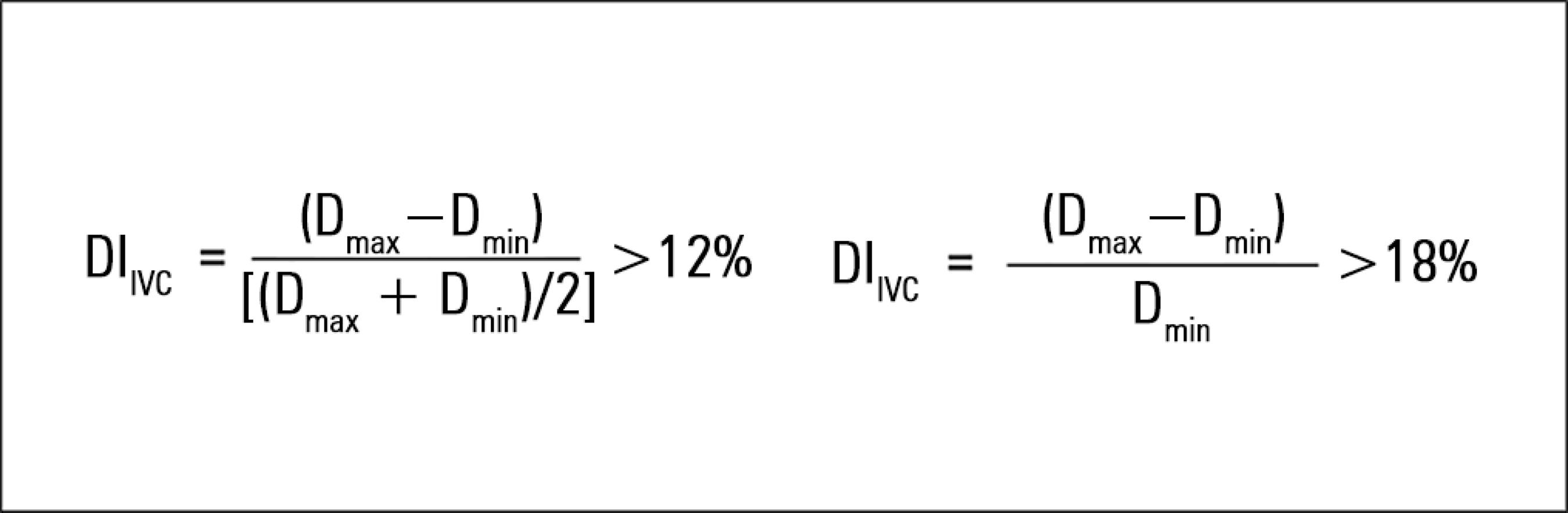
22 Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66.-2323 Schmidt GA, Koenig S, Mayo PH. Shock: ultrasound to guide diagnosis and therapy. Chest. 2012;142(4):1042-8.) However, these static values may not be relevant for the majority of patients, and their use is not indicated to predict fluid responsiveness because they do not show a reliable predictive value.(99 Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.,2424 Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-7.) On the other hand, the dynamic method of IVC evaluation, based on the variation in its diameter with respiration, enables the assessment of the potential benefit of fluid administration as a function of IVC compliance. However, the technique only showed a predictive value in a specific subgroup of patients: in IMV, in the controlled mode (without respiratory effort), with a tidal volume (TV) of ≥ 8mL/kg of ideal body weight.(2424 Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-7.,2525 Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-6.) IVC diameter is measured at the end of inspiration (maximum diameter [Dmax]) and the end of expiration (minimum diameter [Dmin]) using transthoracic echocardiography, and the distensibility index is calculated using one of two possible formulas (Figure 3).
In the first formula, the distensibility index is considered significant (i.e., it predicts fluid responsiveness) if it is > 12%.(2424 Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-7.) The second formula predicts fluid responsiveness if it is > 18%.(2525 Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-6.)
Respiratory variation in the inferior vena cava as a predictor of fluid responsiveness: Limitations to its clinical applicability and interpretation
The use of IVC respiratory variation is limited for patients who are obese, laparotomized, or show a poor echocardiographic window(1313 Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249-54.,2626 Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.) because of limitations inherent to this technique that includes the use of echocardiography via the subcostal window. Its use also requires a correct application of the ultrasound probe for a reliable measurement. An incorrect echocardiographic measurement technique can lead to false-positive or negative results. For example, the incorrect alignment of the probe or lateral deviation of the IVC due to the pressure exerted by the probe at the abdominal wall can lead to a wrong measurement.(2626 Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.)
Several limitations remain regarding the clinical applicability of this method; although not all of them have been studied, they should be considered.(2626 Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.) These limitations can be divided into factors that affect the variation in intrathoracic pressure, those that increase the right atrium pressure, and those that directly interfere with IVC compliance.
First, regarding the factors that affect intrathoracic pressure variation, attention must be paid to the positive end-expiratory pressure (PEEP) value and TV. A high PEEP value (e.g., during acute respiratory distress syndrome) elevates intrathoracic pressure, decreases the distensibility of the IVC, and leads to a false-negative result. Also, with a TV lower than 8 mL/kg, the pressure variations induced by IMV may not be sufficient to reliably vary the IVC diameter.(1313 Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249-54.)
The value of this technique is also questionable in ventilated patients with respiratory effort (IMV in the assisted or spontaneous mode). During the respiratory stimulus, a negative transpulmonary pressure exists that is the inverse to that caused by IMV. Therefore, it is not possible to control or predict the effective variation in the IVC diameter among these patients.(2626 Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.) In addition, in patients with respiratory stimuli (either ventilated or nonventilated), the respiratory pattern can vary, and the pressure variation in the chest will not be constant, leading to possible false positives or negatives.(2727 Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY; AzuRea group. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188.)
Second, in patients with cardiac pathologies that are obstacles to venous return, pressure increases in the right atrium and consequently distends the IVC. This state is not related to the blood volume state(2626 Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.) and occurs in patients with right ventricular dysfunction,(2828 Goldstein JA, Oak R. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40(5):841-53.) severe tricuspid regurgitation, or cardiac tamponade.
Finally, since the IVC is located in the abdominal cavity, it is subjected to intra-abdominal and chest pressure. Patients with increased intra-abdominal pressure will have IVCs with decreased compliance, which can lead to false negatives in patients under IMV. Other mechanical factors must be considered, such as thrombosis or extrinsic IVC compression.(2626 Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.) The same problem arises in individuals under extracorporeal membrane oxygenation (ECMO) with one of the cannula in the IVC. In these cases, transthoracic echocardiography serves only to monitor cardiac function and the position of the cannula.(2929 Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-53.)
Evidence regarding the use of the inferior vena cava respiratory variation to predict fluid responsiveness in patients with acute circulatory failure in intensive care units
Studies in adults under invasive mechanical ventilation without respiratory effort (controlled mode)
The use of the IVC to predict fluid responsiveness has only been validated in small groups of patients (Table 1) and under well-defined conditions. Feissel et al.(2424 Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-7.) showed a distensibility index (Dmax - Dmin)/[(Dmax + Dmin)/2] of > 12% among 12 patients with septic shock in controlled-mode IMV and a TV of ≥ 8mL/kg, with a negative predictive value of 92% and a positive predictive value of 93%. Barbier et al.(2525 Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-6.) found similar results in a similar group of patients (39 with septic shock under IMV with a TV of ≥ 8mL/kg), in which an IVC distensibility index (Dmax - Dmin)/Dmin of > 18% had high sensitivity and specificity values (90%) and an area under the curve (AUC) of 0.91 (95% confidence interval [95%CI] 0.84 - 0.98). Although these studies show that the characteristics of IVC variation make it a good predictive test, its applicability is limited by the lack of generalizability, given the small sample size and the specificity of the clinical context.
Major published studies regarding the use of IVC respiratory variation to predict fluid responsiveness in adult ICU patients with acute circulatory failure
Additional studies(3030 Charbonneau H, Riu B, Faron M, Mari A, Kurrek MM, Ruiz J, et al. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit Care. 2014;18(5):473.,3131 Theerawit P, Morasert T, Sutherasan Y. Inferior vena cava diameter variation compared with pulse pressure variation as predictors of fluid responsiveness in patients with sepsis. J Crit Care. 2016;36:246-51.) of patients in septic shock had less consistent results, showing discriminatory powers of AUC = 0.43 (95%CI 0.25 - 0.61) and AUC = 0.69 (95%CI 0.48 - 0.89), respectively. One potential explanation of this discrepancy compared with previous studies is related to the fact that Charbonneau et al.(3030 Charbonneau H, Riu B, Faron M, Mari A, Kurrek MM, Ruiz J, et al. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit Care. 2014;18(5):473.) found a higher percentage of patients receiving laparotomy (23% versus 9% in Barbier et al.(2525 Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-6.))), which might have conditioned the accuracy of the test casting doubts about its use among patients undergoing abdominal surgery. In the case of Theerawit et al.,(3131 Theerawit P, Morasert T, Sutherasan Y. Inferior vena cava diameter variation compared with pulse pressure variation as predictors of fluid responsiveness in patients with sepsis. J Crit Care. 2016;36:246-51.) patients with severe sepsis were included, who might have increased intra-abdominal pressure in that context. Intra-abdominal pressure was not monitored and may have biased the results.
More recently, a study with a larger and more heterogeneous sample revealed less promising results. Vignon et al.(3232 Vignon P, Repessé X, Bégot E, Léger J, Jacob C, Bouferrache K, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017;195(8):1022-32.) conducted a prospective, multicenter study of 540 patients with circulatory failure of any cause and under IMV. They compared the respiratory variation in SVS, IVC, and maximal aortic velocity with the test of lower limb elevation (i.e., the standard reference). In this study, only 42% of the patients responded to fluids, and the variation in SVS was the best discriminatory test. However, this finding implies the use of transesophageal echocardiography, which limits its applicability. The index of IVC variation exhibited a 55% sensitivity (95%CI 50 - 59) and a 70% specificity (95%CI 66 - 75). However, the discriminatory value considered was 8%, and the assessment of the IVC was only possible for 78% of patients due to the difficulties in image acquisition because of recent surgery (approximately 25% of the patients), which might have reduced its diagnostic acuity. Furthermore, most patients had a protective ventilatory mode with TV < 8mL/kg, which was contrary to previous studies. Despite these limitations, this sample was larger than those of other studies and included multiple causes of shock, which reflects usual clinical practice conditions and their inherent limitations. The authors concluded that the discriminatory power of these parameters was not sufficient to overcome clinical judgement and recommended fluid bolus if the risk is low and signs of hypoperfusion are present, even if the echocardiographic parameters predict a weak response.
Studies in nonventilated adults
Studies have shown high specificity but low sensitivity with regard to nonventilated patients in the ICU. Muller et al.(2727 Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY; AzuRea group. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188.) showed that in 40 nonventilated patients with hemorrhagic, hypovolemic or septic shock, an IVC collapsibility index of > 40% had a specificity of 80% and a sensitivity of 70%, with an AUC of 0.77 (95%CI 0.60 - 0.88); however, the test was not reliable concerning these patients because the lower limit of the 95%CI of the AUC was < 0.75. An IVC collapsibility index below 40% does not allow us to exclude fluid responsiveness, and the probability of response increases when the index is above 40%. Airapetian et al.(99 Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.) found similar results among 59 nonintubated, nonventilated patients, in which a collapsibility index of > 42% had a specificity of 97% and a positive predictive value of 90% but low sensitivity and negative predictive values, with an AUC of 0.62 (95%CI 0.49 - 0.74).
The main characteristics of the aforementioned studies are shown in table 1. These studies are highly heterogeneous; thus, comparisons are difficult. A standard reference (namely, the parameter considered [cardiac index, CO, or SV index], the maneuver used, the type of fluid administered, or its mode of administration) does not exist with regard to the definition of a fluid responder, which is a limiting factor in the study of this technique.
Meta-analyses of the use of inferior vena cava respiratory variation to predict fluid responsiveness in intensive care units, regardless of ventilatory mode or clinical context
In a 2014 meta-analysis(2929 Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-53.) of eight studies including 235 patients, either nonventilated or under IMV, the combined sensitivity was 76% (95% CI = 61 - 86) and the specificity was 86% (95%CI 69 - 95). The combined AUC was 0.84 (95%CI 0.79 - 0.89). The discriminatory value of IVC variation ranged between 12 and 40% across these studies. Of the patients under IMV, better sensitivity (81%; 95%CI 67 - 91) was found for similar specificity (87%; 95%CI 63 - 97). In a 2017 systematic review and meta-analysis(77 Long E, Oakley E, Duke T, Babl FE; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Does respiratory variation in inferior vena cava diameter predic fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47(5):550-9.) of 17 studies including 533 patients with circulatory failure, the combined sensitivity and specificity values of the IVC variation index to predict fluid responsiveness were 63% (95%CI 56 - 69) and 73% (95%CI 67 - 78), respectively, with a combined AUC of 0.79 (standard error = 0.05). The subgroup of ventilated patients (combined sensitivity = 67% [95% CI = 58 - 75]; specificity = 68% [95%CI 60 - 76]) presented with better results than nonventilated patients (combined sensitivity = 52% [95%CI 42 - 62]; specificity = 77% [95%CI 68 - 84]) as previously shown. The authors reported that the respiratory variation in the IVC diameter moderately predicted fluid responsiveness and that a negative test does not exclude fluid responsiveness; thus, its clinical usefulness is limited, particularly among nonventilated patients. Because these meta-analyses include original studies across varied clinical contexts (ICUs and emergency departments; type of circulatory shock and ventilation; mode of measurement of IVC; the considered discriminatory value or standard reference), their results should be valued accordingly. The value of the IVC test depends on the clinical context, which should be considered during assessment and interpretation.
Use of inferior vena cava respiratory variation to evaluate fluid responsiveness in clinical practice in intensive care units: advantages, disadvantages, and current outlook
The use of IVC variation is favored among the dynamic methods of fluid responsiveness assessment in the ICU because it is noninvasive, inexpensive, easy, and reproducible; moreover, it does not demand a high level of training.(3333 Au SM, Vieillard-Baron A. Bedside echocardiography in critically ill patients: a true hemodynamic monitoring tool. J Clin Monit Comput. 2012;26(5):355-60.,3434 Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-11.) In addition, complementary echocardiographic assessment, both quantitative and qualitative, contributes to a better overall clinical evaluation.(88 Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20:274.,1818 Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients - Part II: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-27.)
However, the use of IVC regarding the decision to administer fluids should be considered only if certain technical and clinical conditions are met, i.e., patients under IMV, in the controlled mode (without respiratory effort), TV ≥ 8mL/kg, normal intra-abdominal pressure, and without acute cor pulmonale or severe right ventricular dysfunction. Otherwise, the studies are too heterogeneous and unlikely to be generalized.
The specificity of these conditions restricts the use of IVC respiratory variation.(3535 Taniguchi LU, Zampieri FG, Nassar AP Jr. Applicability of respiratory variations in stroke volume and its surrogates for dynamic fluid responsiveness prediction in critically ill patients: a systematic review of the prevalence of required conditions. Rev Bras Ter Intensiva. 2017;29(1):70-6.
36 Mendes PV, Rodrigues BN, Miranda LC, Zampieri FG, Queiroz EL, Schettino G, et al. Prevalence of ventilatory conditions for dynamic fluid responsiveness prediction in 2 tertiary intensive care units. J Intensive Care Med. 2016;31(4):258-62.-3737 Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D; FENICE Investigators; ESICM Trial Group. Fluid challenges in intensive care: the FENICE study - a global inception cohort study. Intensive Care Med. 2015;41(9):1529-37. Erratum in: Intensive Care Med. 2015;41(9):1737-8.) The studies evaluating the prevalence of the ventilatory conditions required for the application of this technique in the ICU (i.e., the prevalence of patients under controlled-mode IMV with TV ≥ 8mL/kg) show that these are present only in a small percentage of patients. In these studies, the possibility of a transitory increase in TV only to perform the maneuver was not considered. The various aspects limiting the use of IVC respiratory variation to predict fluid responsiveness may be one of the reasons for its infrequent use in the ICU.(3737 Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D; FENICE Investigators; ESICM Trial Group. Fluid challenges in intensive care: the FENICE study - a global inception cohort study. Intensive Care Med. 2015;41(9):1529-37. Erratum in: Intensive Care Med. 2015;41(9):1737-8.) In the observational and multicenter study FENICE,(3737 Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D; FENICE Investigators; ESICM Trial Group. Fluid challenges in intensive care: the FENICE study - a global inception cohort study. Intensive Care Med. 2015;41(9):1529-37. Erratum in: Intensive Care Med. 2015;41(9):1737-8.) which evaluated the way that physicians apply volume expansion among critically ill ICU patients, hemodynamic variables were used to predict fluid responsiveness in only 57.3% of the patients, of whom only 9.3% corresponded to echocardiographic parameters.
CONCLUSION
Fluid therapy increases cardiac output in only approximately half of patients with acute circulatory failure. Ideally, patients with acute circulatory failure should be evaluated with regard to fluid responsiveness before its administration to avoid deleterious effects. In intensive care units, the use of inferior vena cava respiratory variation measured by transthoracic echocardiography may play a role in this evaluation; however, it is necessary to guarantee the conditions under which the technique is validated and to consider its limitations, depending on the clinical context, for correct interpretation. This technique has an unsatisfactory discriminatory power among nonventilated patients and those with respiratory effort because a negative test does not exclude fluid responsiveness.
The adequacy of resuscitation should be based on clinical judgment, considering the risk of fluid overload versus the potential benefit of fluid therapy, keeping in mind that not all responders need fluid administration. This practice must be individualized for each patient, integrating various clinical, echocardiographic, and biochemical parameters.
REFERÊNCIAS
-
1Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest. 2014;146(4):908-15.
-
2Ogbu OC, Murphy DJ, Martin GS. How to avoid fluid overload. Curr Opin Crit Care. 2015;21(4):315-21.
-
3Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43(1):68-73.
-
4Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract. 2016;3(2):G1-12.
-
5ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-93.
-
6Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000-8.
-
7Long E, Oakley E, Duke T, Babl FE; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Does respiratory variation in inferior vena cava diameter predic fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47(5):550-9.
-
8Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20:274.
-
9Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.
-
10Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-81.
-
11Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29(3):352-60.
-
12Pinsky MR. Heart-lung interactions. Curr Opin Crit Care. 2007;13(5):528-31.
-
13Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249-54.
-
14Ramos FJ, Azevedo LC. Assessment of fluid responsiveness in patients under spontaneous breathing activity. Rev Bras Ter Intensiva. 2009;21(2):212-8.
-
15De Backer D, Fagnoul D. Intensive care ultrasound: VI. Fluid responsiveness and shock assessment. Ann Am Thorac Soc. 2014;11(1):129-36.
-
16Evans D, Ferraioli G, Snellings J, Levitov A. Volume responsiveness in critically ill patients: use of sonography to guide management. J Ultrasound Med. 2014;33(1):3-7.
-
17Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.
-
18Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients - Part II: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-27.
-
19Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795-815.
-
20Orde S, Slama M, Hilton A, Yastrebov K, McLean A. Pearls and pitfalls in comprehensive critical care echocardiography. Crit Care. 2017;21(1):279.
-
21Lee CW, Kory PD, Arntfield RT. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care. 2016;31(1):96-100.
-
22Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66.
-
23Schmidt GA, Koenig S, Mayo PH. Shock: ultrasound to guide diagnosis and therapy. Chest. 2012;142(4):1042-8.
-
24Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-7.
-
25Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740-6.
-
26Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42(7):1164-7.
-
27Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY; AzuRea group. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188.
-
28Goldstein JA, Oak R. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40(5):841-53.
-
29Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-53.
-
30Charbonneau H, Riu B, Faron M, Mari A, Kurrek MM, Ruiz J, et al. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit Care. 2014;18(5):473.
-
31Theerawit P, Morasert T, Sutherasan Y. Inferior vena cava diameter variation compared with pulse pressure variation as predictors of fluid responsiveness in patients with sepsis. J Crit Care. 2016;36:246-51.
-
32Vignon P, Repessé X, Bégot E, Léger J, Jacob C, Bouferrache K, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017;195(8):1022-32.
-
33Au SM, Vieillard-Baron A. Bedside echocardiography in critically ill patients: a true hemodynamic monitoring tool. J Clin Monit Comput. 2012;26(5):355-60.
-
34Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-11.
-
35Taniguchi LU, Zampieri FG, Nassar AP Jr. Applicability of respiratory variations in stroke volume and its surrogates for dynamic fluid responsiveness prediction in critically ill patients: a systematic review of the prevalence of required conditions. Rev Bras Ter Intensiva. 2017;29(1):70-6.
-
36Mendes PV, Rodrigues BN, Miranda LC, Zampieri FG, Queiroz EL, Schettino G, et al. Prevalence of ventilatory conditions for dynamic fluid responsiveness prediction in 2 tertiary intensive care units. J Intensive Care Med. 2016;31(4):258-62.
-
37Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D; FENICE Investigators; ESICM Trial Group. Fluid challenges in intensive care: the FENICE study - a global inception cohort study. Intensive Care Med. 2015;41(9):1529-37. Erratum in: Intensive Care Med. 2015;41(9):1737-8.
Edited by
Publication Dates
-
Publication in this collection
27 June 2019 -
Date of issue
Apr-Jun 2019
History
-
Received
02 Apr 2018 -
Accepted
09 Oct 2018