ABSTRACT
The biochar, product of pyrolysis of organic waste, has been used as a soil conditioner and alternative on solid waste management. However, the raw material and pyrolysis temperature used influence the quantity and dynamics of release of nutrients and contaminants from the biochar. The objective was to evaluate the use of waste sugarcane bagasse, eucalyptus and sewage sludge for production of biochar and determine the chemical, physical, mineralogical properties and acid extraction of these materials produced at 350 °C and 500 °C. Were evaluated the proportion of C, H, N, O; ashes; macro and micronutrients, plus some contaminants; characterization of mineral phases by diffractometry of X- rays; functional groups by infrared absorption spectroscopy (FTIR). Moreover, it was determined the release of nutrients and contaminants for the extraction in increasing concentration of HNO3 (0,01 - 2,0 mol L-1). The O/C and H/C relations decreased with increasing temperature of pyrolysis, which define a greater stability of the C of biochars. Sewage sludge biochar (BC-L) had the highest nutrient release rates and contaminant metals (Cd, Cr, Ni and Pb). Acid extraction of other biochars was very low (<20% of the total content). The results indicate that the carbon fraction of biochar contributes to the low rate of release of the elements in acid place.
Key words:
Biochar; Waste; Acid extraction
RESUMO
O biocarvão, produto da pirólise de resíduos orgânicos, vem sendo utilizado como condicionador de solo e como alternativa na gestão de resíduos sólidos. O objetivo deste trabalho foi avaliar o efeito da temperatura de pirólise nas propriedades químicas, físicas, mineralógicas e a extração ácida de metais em biocarvões produzidos a partir de resíduos de bagaço da cana-de-açúcar, eucalipto e lodo de esgoto. Foram avaliados os teores de C, H, N, O; cinzas; macro e micronutrientes, além de alguns contaminantes; relações molares O/C e H/C; difratometria de raios X; grupos funcionais por FTIR. Além disso, foi determinada a liberação de nutrientes e contaminantes pela extração em concentrações crescentes de HNO3 (0,01-2,0 mol L-1). As relações O/C e H/C diminuíram com o aumento da temperatura de pirólise, o que caracteriza uma maior estabilidade do C dos biocarvões. O biocarvão de lodo de esgoto (BC-L) apresentou as maiores taxas de liberação de nutrientes e metais contaminantes (Cd, Cr, Ni e Pb). A extração ácida dos demais biocarvões foi muito baixa (< 20% do teor total). Os resultados indicam que a fração carbonosa do biocarvão contribui para a baixa taxa de liberação dos elementos em meio ácido.
Palavras-chaves:
Biocarvão; Resíduos; Extração ácida
INTRODUCTION
There is currently an imbalance in the carbon cycle, with growing CO2 emissions to the atmosphere and lower retention of C by environmental compartments, making it necessary to develop methods to sequester carbon for a longer time. Agricultural, forestry, and industrial residues, poultry manure, urban waste, and sewage sludge are potential sources of biomass and materials that can be used as renewable energy sources and can contribute to significant environmental benefits through the use of the by-product, charcoal or biochar (BC)(ZHANG; CHUNBAO; XU, 2010ZHANG, L., CHUNBAO, C., XU, P.C. Overview of recent advances in thermo-chemical conversion of biomass. Energy Conversion and Management, v.51, n. 5, p. 969-982, 2010.).
Studies have shown that applications of BCs in soils have led to positive results, especially in regard to physical, chemical, and biological properties (GRAY et al., 2014GRAY, M. et al. Water uptake in biochars: the roles of porosity and hydrophobicity. Biomass and Bioenergy, v. 61, p. 196-205, 2014.; LEHMANN et al., 2011LEHMANN, J. et al. Biochar effects on soil biota:a review. Soil Biology and Biochemistry, v. 43, n. 9, p. 1812-1836, 2011.;MITCHELL et al., 2015MITCHELL, P.J. et al. Shifts in microbial communityand water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biology and Biochemistry, v. 81, p. 244-254, 2015.) and can also control mobilities of environmental pollutants, such as heavy metals (CASE et al., 2015CASE, S.D.C.et al. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biology and Biochemistry, v. 81, p. 178-185, 2015.; UCHIMIYA; CHANG, 2011UCHIMIYA, M.; CHANG, S.C. Screening biochars for heavy metal retention in soil: role of oxygen functional groups. Journal of Hazardous Materials,v. 190, n. 1/3, p. 432-441, 2011.).
The properties of BC, such as elemental composition, structure, and chemical stability, depend on the raw material and the temperature of pyrolysis. At higher pyrolysis temperatures, the specific surface, porosity, and stability of C are greater, and the functional groups are gradually lost, leaving the material more recalcitrant, with polycyclical aromatic structure and a high degree of condensation (SONG; GUO, 2012SONG, W.; GUO, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 94, p. 138-145, 2012. ).
Pyrolysis of different types of residues has been suggested as a form of management, especially for sewage sludge, which is one of the main sources of environmental pollution (HWANG et al., 2007HWANG, I.H. et al. Characteristics of leachate from pyrolysis residue of sewage sludge. Chemosphere, v. 68, n. 10, p. 1913-1919, 2007.). BC from sewage sludge has the potential to improve the soil through release of P and micronutrients (HUANLIANG et al., 2013HUANLIANG, L. et al. Characterization of sewage sludge-derived biochar from different feedstocks and pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 102, p. 137-143, 2013.); it also reduces the volume of the solid residue, eliminates pathogens and organic compounds, and may also be used as a fertilizer or in remediation of contaminated soils (HOSSAIN et al., 2011HOSSAIN, M.K. et al. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, v. 92, n. 1, p. 223-228, 2011.).
Thus, the use of residues for BC production can represent a significant contribution in control of contamination of soil and water resources, as well as an effective form of C retention. Nevertheless, more studies are necessary regarding the chemical characteristics of BC that can affect the processes involved in geoavailability of chemical elements, which allows development of measures to decrease leaching and immobilization, especially of heavy metals. Thus, the aim of this study was to evaluate the chemical properties and release rate of chemical elements in the acidic medium of the biochars produced from residues of sugarcane, eucalyptus, and sewage sludge.
MATERIALS AND METHODS
The BCs were produced from residues of sugarcane bagasse, eucalyptus (Eucalyptus globulus) bark, and sewage sludge. The sugarcane bagasse was collected from a sugar and alcohol production unit located in the municipality of Urucânia, MG, Brazil. Eucalyptus bark was provided by the Wood Panel and Energy Laboratory of the Universidade Federal de Viçosa, Viçosa, MG, Brazil. The sewage sludge samples were collected in a household sewage treatment plant in Viçosa, MG, and dried at ambient temperature, and physical impurities were removed.
The sugarcane bagasse and sewage sludge were ground and then passed through a 2 mm mesh sieve; the eucalyptus bark was cut up into 2 x 3 cm pieces. After that, all the samples were dried in a forced air circulation oven at 105 °C for 24 h. The pyrolysis process was carried out in a muffle furnace, with an opening at the top part to release volatile substances, thus restricting the entrance of new oxygen within, at pyrolysis temperatures of 350 and 500 °C, with a heating rate of 25 °C min-1 for 30 minutes. After reduction of internal temperature of the muffle furnace to around 100 °C, the charcoal was removed from within the crucible. The charcoal produced was stored in a desiccator until all analyses were made. This procedure is necessary because charcoal undergoes oxidation reactions in contact with the atmosphere, changing the number of surface active groups (charges) (BOEHM, 2008BOEHM, H.-P. Surface chemical characterization of carbons from adsorption studies. In: BOTTANI, E.J.; TASCÓN, J.M.D. (Ed.). Adsorption by carbons. Amsterdam: Elsevier, 2008. p. 301-328.).
The gravimetric yield of the BCs of sugarcane bagasse (BC-C), eucalyptus bark (BC-E), and sewage sludge (BC-S)was calculated from the original weight and the weight of the BC produced after conclusion of pyrolysis. The C, H, and N concentrations were determined in an elemental analyzer (Perkin Elmer 2400 Series II CHNS/O); the O concentration was calculated by the difference, discounting the ash content. For purposes of comparison, the C concentration was also determined by the Walkley-Black method (MENDONÇA; MATOS, 2005MENDONÇA, E.S; MATOS, E.S. Matéria orgânica do solo: métodos de análise. Viçosa, MG: UFV, 2005. 107 p.). Quantification of the C concentration by this method is in agreement with the studies of Chen et al. (2016)CHEN, D. et al. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresource Technology, v. 218, p. 1303-1306, 2016., who consider K2Cr2O7to be a promising indicator of chemical stability of oxidation of biochars, given the lack of availability of methods reported in the literature.
Functional groups were identified after spectrophotometry analyses in the infrared region, with Fourier transform infrared (FTIR) spectroscopy (Jasco FTIR 4100). Spectra were obtained with 60 scans with wave numbers from 4000 to 400 cm-1and resolution from 4 cm-1 in KBr pellets. For determination of crystalline mineral phases, powder X-ray diffraction (PXRD) was carried out in a cobalt tube and nickel filter in the interval from 4 to 50 o2θ and scanning speed of 10 o2θ per minute, with voltage of 40 kV and current of 30 mA.
For determination of total contents of nutrients and contaminants in the raw materials and in the BCs, 1 g of each sample of these materials was calcined in open crucibles in a muffle furnace with a heating rate of 3 °C/min until reaching 550 °C for 6 hours. After incineration, the ashes were weighed and they were solubilized in 20 mL of 2 mol L-1 HNO3. After slow filtering, the extracts were read in ICP-OES. The pH in water was determined at the ratio of 1:10 mL.
Cation exchange capacity (CEC) was quantified according to Song and Guo (2012)SONG, W.; GUO, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 94, p. 138-145, 2012. , with modifications. A quantity of 40 mL of 1 mol L-1 ammonium acetate (NH4OAc) was added to 0.5 g of BC, followed by shaking for 30 minutes; the BC retained in the slow filter was washed with 40 mL of1 mol L-1 NH4OAc, followed by 90 mL (three portions of 30 mL) of isopropanol. After these washings, 30 mL of 1 mol L-1KCl was added to the samples retained in the filter,which were shaken for 1 hour. This was followed by reading of NH4+by distillation (Kjeidahl). Analyses were made in triplicate.
Extraction in acid medium was carried out to estimate the release curve of the elements Ca, Mg, K, P, Al, Fe, Zn, Cu, Cd, Cr, Co, Mn, Ni, and Pb according to the methodology of Amaral Sobrinho et al. (1992). Only the BC samples obtained at 500 °C were studied, due to their chemical characteristics; this contributed to greater applicability of these materials in the environment. Therefore, 20 mL of HNO3 was added at concentrations of 0.01, 0.05, 0.1, 0.5, 1.0, and 2.0 mol L-1 in 1 g of BC sample, in triplicate. The mixtures were shaken horizontally for 24 h, followed by filtering for determination of chemical elements in ICP-OES. Descriptive statistics were carried out, using mean values and standard deviation.
RESULTS AND DISCUSSION
The mean gravimetric yield of the BCs produced from residues of sugarcane bagasse (BC-C), eucalyptus bark (BC-E), and sewage sludge (BC-S) at the pyrolysis temperature of 500 °C decreased around 24, 34, and 15%, respectively, in comparison to yield at 350 ºC. BC-Shad greater yield, of 74.6% at 350 °C and 63.1% at 500 °C, in relation to the weight of raw material (Table 1). This difference in yield in relation to other raw materials is due to the high mineral fraction present in sewage sludge, as can be observed by its high ash content and low C content. This generates a BC with more inorganic than organic characteristics. Other authors have found similar results, with yield of 35% for charcoal derived from eucalyptus (GARCIA-PEREZ et al., 2008GARCIA-PEREZ, M. et al. Fast pyrolysis of oil mallee woody biomass:effect of temperature on the yield and quality of pyrolysis products. Industrial & Engineering Chemistry Research, v. 47, n. 6, p. 1846-1854, 2008.) and 58% in BC derived from sewage sludge at 500 ºC (HOSSAIN et al., 2011HOSSAIN, M.K. et al. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, v. 92, n. 1, p. 223-228, 2011.). This loss of weight occurs due to release of water vapor and carbon monoxide and to decomposition of hemicelluloses, depolymerization of cellulose, and rupture of lignin, which occur as of 200 °C (LEHMANN et al., 2011LEHMANN, J. et al. Biochar effects on soil biota:a review. Soil Biology and Biochemistry, v. 43, n. 9, p. 1812-1836, 2011.). Pyrolysis of sewage sludge between 300 and 600 ºC results in considerable loss of weight and generates a product with an amorphous C matrix, which may be due to the conversion of the organic matter in sewage sludge during pyrolysis into volatile gases (HUANLIANG et al., 2013HUANLIANG, L. et al. Characterization of sewage sludge-derived biochar from different feedstocks and pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 102, p. 137-143, 2013.).
Characterization of biochars (BC) produced from different biomasses and pyrolysis temperatures
The charcoals produced from residues of grain hulls, husk, straw, and manure generally produce BCs with high ash content, which may be a result of the silica and of the mineral content of the raw material and of gradual loss of C, H, and O during processing (DEMIRBAS, 2004DEMIRBAS, A. Determination of calorific values of biochars and pyrolysis from pyrolysis of beech trunkbarks. Journal of Analytical and Applied Pyrolysis, v. 72, n. 2, p. 215-219, 2004.). Zhang, ChunbaoandXu (2010)ZHANG, L., CHUNBAO, C., XU, P.C. Overview of recent advances in thermo-chemical conversion of biomass. Energy Conversion and Management, v.51, n. 5, p. 969-982, 2010. and Hossain et al. (2011)HOSSAIN, M.K. et al. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, v. 92, n. 1, p. 223-228, 2011., observed ash concentrations of 7 and 9% in BC obtained from sugarcane bagasse and of 2 and 4% for BC of pine wood produced at 525 °C and 650 °C, respectively, which are compatible with the results obtained in this study.
The increase in pyrolysis temperature leads to loss of oxygenated groups and, consequently, increases the concentration of C in the BCs. This was observed in BC-C and BC-E as a result of increase in pyrolysis temperature from 350 ºC to 500 ºC (Table 1). However, the same was not observed for BC-S, such that there was a decrease in C concentration with the increase in temperature. BCs of sewage sludge can range from 15.24 to 33.18% of C (HUANLIANG et al., 2013HUANLIANG, L. et al. Characterization of sewage sludge-derived biochar from different feedstocks and pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 102, p. 137-143, 2013.).
The C concentration by the Walkley-Black method, which, corresponding to the easily oxidizable carbon (CO), decreased considerably in BC-C and in BC-S with the increase in pyrolysis temperature (Table 1). This result suggests greater recalcitrance of C in BCs produced at higher pyrolysis temperatures. At 500 ºC,the aliphatic radicals rich in oxygen from lignin and hemicellulose are nearly completely volatilized; the carbon chains that remain reorganize, forming polycondensed aromatic structures (LEHMANN et al., 2011LEHMANN, J. et al. Biochar effects on soil biota:a review. Soil Biology and Biochemistry, v. 43, n. 9, p. 1812-1836, 2011.). The CO concentration in BC-C corresponded to 50 and 25% of the total C content when produced at 350 and at 500 °C, respectively.
In contrast, BC-E has a much stabler carbon fraction and the CO content represented around 18% (350 °C) and 13% (500 °C) of total C, whereas in BC-S, almost all the C was oxidized by dichromate, which suggests low stability of C in this BC, regardless of the pyrolysis temperature evaluated. These values are important for evaluating the C stability and storage capacity of these materials in the soil. BCs rich in C and produced at high temperature (above 500 °C) can have higher values of C retained in the soil than biochars from low temperature (NGUYEN et al., 2010NGUYEN, B.T. et al. Temperature sensitivity of black carbon decomposition and oxidation. Environmental Science & Technology, v. 44, n. 9, p. 3324-3331, 2010.).
The degree of C stability can also be indicated by the molar ratios O/C and H/C. These ratios decreased with the increase in pyrolysis temperature, which indicates a higher degree of condensation and structures with aromatic rings, largely responsible for their chemical stability. Pyrolysis results in loss of O and H and the condensation of carbon chains, thus increasing resistance to microbial degradation in the soil (KOOKANA et al., 2011KOOKANA, R.S. et al. Biochar application to soil:agronomic and environmental benefits and unintended consequences. Advances in Agronomy,, v. 112, p. 103-143, 2011.). The greater stability of C may be due to the secondary reactions that occur in very slow speed carbonization, which results in more recalcitrant formation of BC. BC-S also exhibited a low H/C ratio, but a higher O/C ratio compared to the BCs of the biomasses, which may lead to an increase in functional groups and a lower proportion of aromatic rings. The C concentration and the H/C, O/C, and C/N ratios control the formation of surface functional groups and have a big influence on metal sorption (UCHIMIYA; CHANG, 2011UCHIMIYA, M.; CHANG, S.C. Screening biochars for heavy metal retention in soil: role of oxygen functional groups. Journal of Hazardous Materials,v. 190, n. 1/3, p. 432-441, 2011.). In general, this is related to the increase of O in the surface functional groups and, consequently, the power of adsorption of metals in the structure of the BC itself.
The N concentration was low in BC-C and BC-E, and was higher only in BC-S (Table 1). For BC-E, the N concentration increased from 0.46 to 0.66% with the increase in pyrolysis temperature, which may indicate the presence of nitrogen compounds with structures not easily decomposed at 350 °C. The C/N ratio ranged from 33 to 121 in BC-E and BC-C, whereas in BC-S, it was near 7. The lower values in BC-S may show faster decomposition of the compounds present in this material. The loss of nitrogen with the increase in pyrolysis temperature is dependent on the composition of the raw material used. The high pyrolysis temperature led to an even greater reduction in the C and N concentrations of the BCs from sewage sludge, but the opposite tendency was found in the BCs from plants since the C concentration is greater (GASKIN et al., 2008GASKIN J.W. et al. Effect of low temperature pyrolysis conditions on biochars for agricultural use. Transactions of the Asabe, v. 51, n. 6, p. 2061-2069, 2008.).
The values of pH in water in BC-S were low compared to the other BCs. Hossain et al. (2011)HOSSAIN, M.K. et al. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, v. 92, n. 1, p. 223-228, 2011. reported that the BCs produced from sewage sludge at low temperatures (300 and 400 °C) were acidic whereas at high temperatures (500 and 600 °C), they were alkaline. This suggests that the use of BCs may be suitable for improving acid and alkaline soils by controlling pyrolysis temperature. The increase in pH is associated with progressive loss of the acid surface of functional groups (REEVES et al., 2007REEVES, J.B. et al. Near infrared spectroscopic examination of charred pine wood, bark, cellulose and lignin: implications for the quantitative determination of charcoal in soils. Journal Near Infrared Spectroscopy,v. 15, p. 307-315, 2007.).
The CEC values were higher in BC-C, at 11.86 cmolc kg-1, followed by BC-S, at 10.3 cmolc kg-1. These values were higher than those identified by Song and Guo (2012)SONG, W.; GUO, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 94, p. 138-145, 2012. in the BCs of peanut shells and pine bark at 500 °C, with values of 4.5 and 6.0 cmolc kg-1, respectively.
Most of the elements analyzed (nutrients or contaminants) concentrated in the BC in relation to the original raw material (Table 2). In relation to the biomasses, eucalyptus had lower concentrations of the elements, with only Ca and Mn being higher than in sugarcane bagasse and sewage sludge. The most abundant elements in sugarcane bagasse were P, K, Mg, Zn, Cu, Si, and Al.In contrast, sewage sludge had high levels of P (46 g kg-1), Fe (19 g kg-1), Zn (448 mg kg-1), and Cu (460 mg kg-1) and also significant concentrations of Al (83.5 g kg-1), Ni (31.2 mg kg-1), Cr (55.8 mg kg-1), and Pb (65.5 mg kg-1). The values found by Oliveira (2003)OLIVEIRA, C. et al. Solubilidade de metais pesados em solos tratados com lodo de esgoto enriquecido. Revista Brasileira da Ciência do Solo, v. 27, n. 1, p. 171-181, 2003. were similar for P (13.8 to 26.9 g kg-1) and Cu (379 to 2404 mg kg-1), and lower for Zn (683 to 4327 mg kg-1), Pb (119 to 835 mg kg-1), and Cr (1545 to 2227 mg kg-1) in sewage sludge.The high nutrient concentrations and relatively low heavy metal concentrations in sewage sludge and its respective BC show the applicability of this material to agricultural use.
Concentration of the elements in the raw materials and in the biochars of sugarcane bagasse, eucalyptus, and sewage sludge pyrolyzed at 500 ºC
Comparing the difference of the elements in the BCs to the initial concentrations of the elements in the raw materials, a differential response is observed; some concentrations increased and others decreased during the pyrolysis process. In all the BCs, the Ca, Mg, and Al elements showed much higher concentrations than in the raw material. Sugarcane bagasse has a higher concentration of P compared to its BC and the concentrations of Fe and Si were less affected by pyrolysis. BC-E had the highest concentrations of the elements compared to those originating from eucalyptus bark.
BC-Sex hibited a reduction in the concentration of K, Cu, Co, Ni, and Pb in relation to raw sewage sludge. The Ni and Pb had a reduction of approximately 50% from the initial concentration of the raw material. The high contents of nutrients contained in BC-S can be beneficial if applied to the soil; however, care is necessary due to toxic elements. The concentrations of the metals, both in the sewage sludge and in the BC produced from it were below the mean concentrations in sewage sludge found in Brazil, which for the following elements are (in mg kg-1): Cr, 545-2.227; Cu, 379-2404; Fe, 34.954-170.955; Mn, 54-80; Ni, 378-1331; Pb, 119-835; and Zn, 683-4327 (OLIVEIRA et al., 2003OLIVEIRA, C. et al. Solubilidade de metais pesados em solos tratados com lodo de esgoto enriquecido. Revista Brasileira da Ciência do Solo, v. 27, n. 1, p. 171-181, 2003.). Huanliang et al. (2013)HUANLIANG, L. et al. Characterization of sewage sludge-derived biochar from different feedstocks and pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 102, p. 137-143, 2013. reported that the BC samples produced from sewage sludge showed high concentrations of Cu, Zn, Cr, and Ni. Song and Guo (2012)SONG, W.; GUO, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 94, p. 138-145, 2012. , for their part,identified that the trace elements were well below the levels of investigation (<0.5 mg kg-1) in BC derived from sewage sludge.
The concentrations of heavy metals in the sewage sludge obtained in the present study are much below the thresholds established by Conama Resolution 375 for agricultural use, which makes the BCs evaluated safe for application to the soil in relation to the elements Cu, Co, Ni, and Pb. It should be noted that pyrolysis can eliminate pathogenic organisms and organic components, which have a negative impact, above all in the use of sewage sludge in agricultural areas.
Characterization of the biochars by PXRD and FTIR
In diffractometry of BC-C at 350 °C, it was possible to detect peaks of kaolinite (7.14, 3.58, 2.34 Å), quartz (3.33, 4.27, 2.28, and 1.82 Å), and hematite (1.44 and 1.83 Å), while in the same BC at 500 °C, only quartz was identified. The BCs of eucalyptus (350 and 500 °C) were essentially amorphous. BC-S at 350 and 500 °C showed peaks characteristic of kaolinite (7.10, 4.32, 3.56, 2.32 Å), goethite (3.38, 4.20, 2.45, 2.14 Å), calcite (3.80, 2.28, and 2.10 Å), and quartz (4.36, 3.34 Å). Influence of the soil components in the materials of sugarcane and sewage sludge is perceived, which contributes to a greater affect on the reactivity of the biochars.
Carbonization is a process that involves an increase in aromatic structures and polymerization, and may also contribute to the force for retaining metals in the BC structure. The molecular structure was similar in the BCs produced, with absorbance in the wave numbers of 3600, 2360, 1700, 1600, 1300, 1200, and 1030 cm-1, characteristic of the C=C, C=O, and C-Obonds (Figure 1).
Molecular absorption in the infrared region with Fourier transform infrared (FTIR) spectroscopy of the biochars of eucalyptus (BC-E), sugarcane bagasse (BC-C), and sewage sludge (BC-S) produced at 350 °C and 500 °C.
In BC-C at 350 °C, there was greater absorbance from 1700 to 1740 cm-1 in reference to the stretching of the carboxyl group (C=O) and the C=C molecular bonds of the aromatic compounds (1600 to 1475 cm-1) compared to the same BC produced at 500 °C. At the temperature of 500 °C, molecular vibration of C=O was identified, which characterizes the ketone group (2300 cm-1). Other vibrations of elongation in these samples are from 1300 to 1000 cm-1 attributed to the C-O and C-H bonds, indicating the formations of the alcohol and ester group and vibrations typical of the aromatic groups. In studies of wood and grass BC, Keiluweit et al. (2010)KEILUWEIT, M.et al. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environmental Science & Technology, v. 44,n. 4, p. 1247-1253, 2010. identified elongation of the vibration 1600 cm-1 of the C=C bond of the aromatic group as the dominant characteristic of these materials. The strongest evidence of aromatic C is provided by stretching of the heterocyclic rings of C/H and N/O.
In BC-E, the wave number corresponding to the highest absorbance was 2300 cm-1of the C=O bond of the ketone group and of the stretching of the aromatic groups (1600 to 1475 cm-1). It can be observed that these bonds became less evident in the same BC at 500 °C. In the samples of BC from sewage sludge produced at 350 °C and 500 °C, we identified the wave number 1600 cm-1, attributed to the C=C aromatic group, 2400 cm-1 of the C=O ketone group, and near 1000 cm-1 of the C-O bond of the alcohol and ether group. In the sample of this BC-Sat 500 °C, stretching from3700 to 3600 cm-1of the O-H bondscould also be observed, which may belong to the phenol, alcohol, carboxylic acid, and water groups.
In general, the presence of organic components with chemical responses and distinct structures in the BCs produced is perceived, which will have an effect on the reactivity of these materials. The biochars of sugarcane bagasse and sewage sludge are composed of the carboxylic acid, ketone, alcohol, and ester functional groups, while, in addition, BC-S has the ether and phenol groups. BC-E exhibited only the ketone group, and aromatic compounds were identified in all the BCs produced.
The functional groups, especially the carboxyl and phenol groups, can be ionized and influence the formation of soil charges. Another point to consider is that the persistence of charcoal in the environment for a long time is due to its polycyclic aromatic structure (GLASER et al., 2001GLASER, B. et al. The Terra Preta phenomenon - a model for sustainable agriculture in the humid tropics. Naturwissens chaften. v. 88, p. 37-41, 2001.). The stability and recalcitrance of the BC, once incorporated in the soil, can contribute over the long term to retention of water and ions in the soil.
Extraction of metals from biochars in acid medium
Extraction of metals in acid medium evaluates the degree of availability of these elements in acid conditions and also the recalcitrance of different BCs, contributing to evaluation of retention of C and of elements that may contribute to soil fertility and environmental aspects, such as uptake of phytotoxic elements.
Acid extraction of chemical elements with HNO3 in increasing concentrations occurred in a different manner in the BCs produced at 500 °C. In the extraction curve of the macronutrients (Ca, Mg, P, K, S), it was found that BC-Sex hibited greater release of these elements compared to the other BCs. The BCs of eucalyptus and sugarcane bagasse exhibited low release for these macronutrients, below 10% of the total content at the different concentrations of HNO3 (Figure 2).
Extraction of the chemical elements Ca, Mg, P, K, and S from the samples of biochars (BC) from sugarcane bagasse, eucalyptus, and sewage sludge produced at 500 °C
In BC-S, the release of Ca, Mg, P, K, and S was gradual as of the concentration of 0.01 mol L-1 up to the maximum concentration of HNO3 (2 mol L-1). Mg and K exhibited release of 50% of the total content at the concentration of 0.5 mol L-1 of HNO3, reaching 80 and 100%, respectively, as the concentration of the acid increased. At this same concentration, the S and P exhibited a lower rate of release, around 20% at the concentration of 2 mol L-1 of HNO3. Ca had a similar response, but with higher releases at lower concentrations, arriving at 50% in 2 mol L-1 of HNO3.
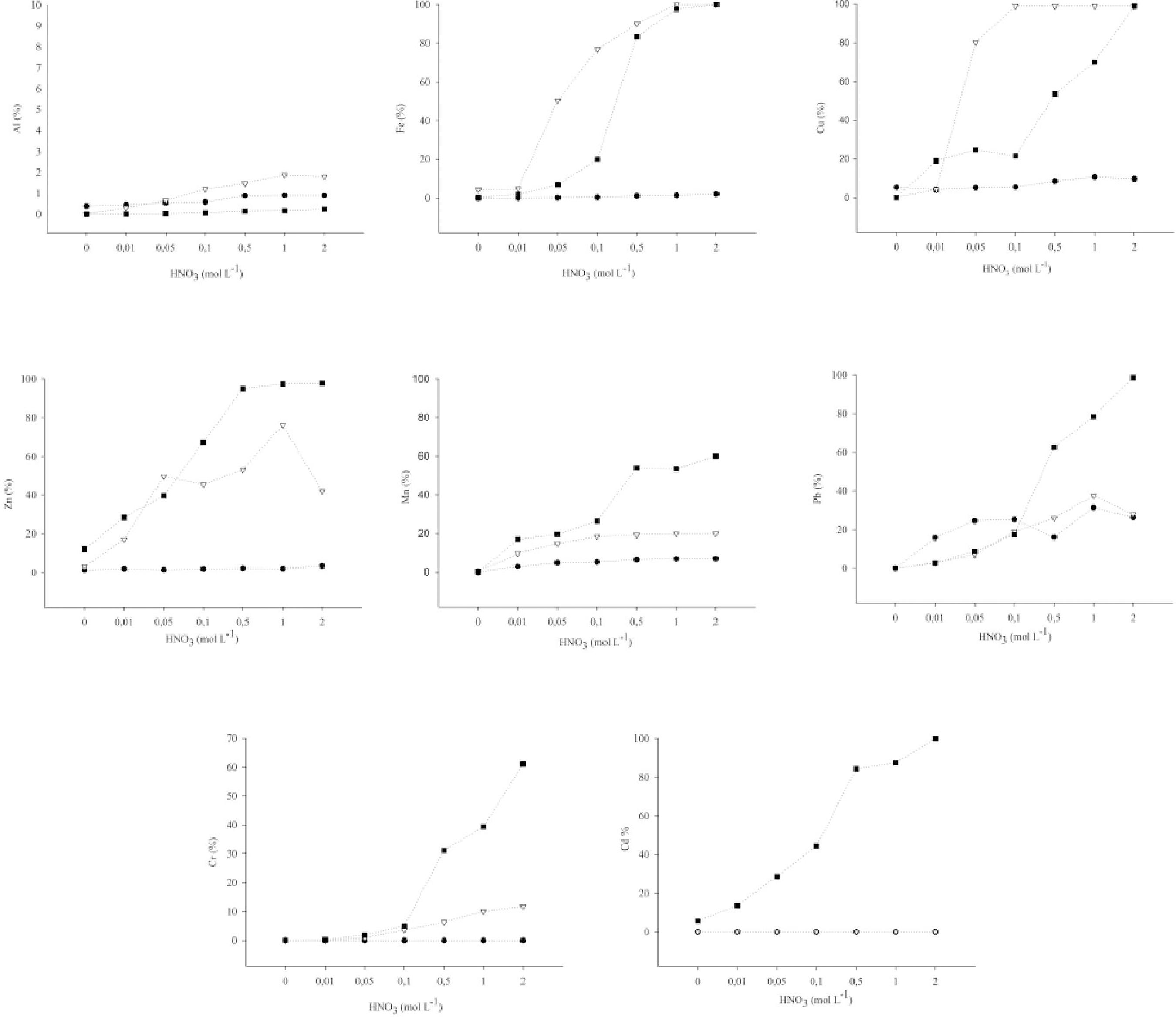
In relation to micronutrients, the BCs of sugarcane bagasse and sewage sludge exhibited growing releases of the elements analyzed as of 0.01 mol L-1. In BC-C, Fe and Cu were totally extracted at concentrations of 1 and 0.1 mol L-1 of HNO3, respectively. Zn was released to the solution at the level of 15% of the total content at the concentration of 0.01 mol L-1of HNO3, and under the condition of greater concentration of the acid (1 mol L-1), it arrived at 76% of the total (133 mg kg-1). Al and Mn were hardly released under acid conditions (Figure 3).
Extraction of the elements Al, Fe, Zn, Cu, Mn, Cd, Cr, and Pb in the samples of biochars (BC) of sugarcane bagasse, eucalyptus, and sewage sludge produced at 500 °C
In BC-S, Fe, Zn,and Cu exhibited total solubilization, 26,113, 480,and 375 mg kg-1, respectively, as of the concentration of 0.5 mol L-1 of HNO3. The release rate of Al, as in BC-C, was low, and of Mn, near 20% at 0.01 mol L-1 and 50% as of the concentration of 0.5 mol L-1 of HNO3. In BC-E, itexhibited low solubility also for the micronutrients, not releasing Cu to the solution, and for Zn there was a small release, near 10% of the total content (363 mg kg-1).
In regard to phytotoxic elements, release of these elements to the solution increased as the concentration of HNO3 increased. Pb and Cr were nearly totally extracted with 2 mol L-1 HNO3. Pb, highly polluting and toxic, was released at 50% in BC-E and 39.5% in BC-C, being made available in reaction with 0.01 mol L-1of HNO3. In BC-S, Cd was totally released to the solution and the Pb and Cr neared total release with HNO3 (2 mol L-1) (Figure 3). Thus, in acid situations, these metals are extractable, which makes it necessary to control application of these materials in the environment to avoid contamination of soil and water resources.
The biochar of sewage sludge has highly differentiated characteristics in regard to the greatest concentrations of the elements because its mineral part is composed of kaolinite, goethite, and calcite, high CEC, a lower pH, and a higher O/C ratio, which indicates more functional groups in relation to biochars from biomasses. These factors influenced the surface charge generated by compounds among the organic and the mineral functional groups, releasing more elements under these acid conditions.
The higher concentration of the chemical elements in BC-C compared to BC-E, the minerals present (kaolinite and hematite), high CEC, and more alkaline pH affected the release rates of nutrients and contaminants of these materials in acid medium. Release of chemical elements in BC-E was very low, even raising the concentrations of HNO3to 2 mol L-1. This low extraction represents the force for retaining chemical elements in BC-E, which may be due to the low concentrations of nutrients in this material, the amorphous mineralogy, and the more recalcitrant structure. Another important factor is the presence of aliphatic groups (carboxyls and ketones), which can interact with the cations and H+of the acid solution.
In general, in acid medium, the chemical elements were released to the solution, and this method of extraction is important for knowing the capacity for retaining elements in acidic situations since biochars can be used for purposes of soil management or as sorbents of contaminants.
CONCLUSIONS
-
The biochar of sewage sludge exhibited a diversity of nutrients that can be bioavailable and contribute to improvement in soil fertility;
-
Trace elements were totally extractable in acid conditions in all the biochars, especially for the biochar from sewage sludge, and may be a source of pollution if they are not applied under controlled conditions;
-
The eucalyptus biochar has greater chemical stability, making its capacity for release of chemical elements low, which may be highly beneficial in the environment upon retaining ions, even in acid conditions.
-
1
Parte da tese financiada pelo CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico
AKNOWLEDGMENTS
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico for financial support and for granting a scholarship.
REFERENCES
- AMARAL SOBRINHO, N.M.B.et al. Metais pesados em alguns fertilizantes e corretivos. Revista Brasileira de Ciência do Solo, v. 16, n. 2, p. 271-276, 1992.
- BOEHM, H.-P. Surface chemical characterization of carbons from adsorption studies. In: BOTTANI, E.J.; TASCÓN, J.M.D. (Ed.). Adsorption by carbons. Amsterdam: Elsevier, 2008. p. 301-328.
- CASE, S.D.C.et al Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biology and Biochemistry, v. 81, p. 178-185, 2015.
- CHEN, D. et al Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresource Technology, v. 218, p. 1303-1306, 2016.
- DEMIRBAS, A. Determination of calorific values of biochars and pyrolysis from pyrolysis of beech trunkbarks. Journal of Analytical and Applied Pyrolysis, v. 72, n. 2, p. 215-219, 2004.
- GARCIA-PEREZ, M. et al Fast pyrolysis of oil mallee woody biomass:effect of temperature on the yield and quality of pyrolysis products. Industrial & Engineering Chemistry Research, v. 47, n. 6, p. 1846-1854, 2008.
- GASKIN J.W. et al Effect of low temperature pyrolysis conditions on biochars for agricultural use. Transactions of the Asabe, v. 51, n. 6, p. 2061-2069, 2008.
- GLASER, B. et al The Terra Preta phenomenon - a model for sustainable agriculture in the humid tropics. Naturwissens chaften v. 88, p. 37-41, 2001.
- GRAY, M. et al Water uptake in biochars: the roles of porosity and hydrophobicity. Biomass and Bioenergy, v. 61, p. 196-205, 2014.
- HOSSAIN, M.K. et al Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, v. 92, n. 1, p. 223-228, 2011.
- HUANLIANG, L. et al. Characterization of sewage sludge-derived biochar from different feedstocks and pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 102, p. 137-143, 2013.
- HWANG, I.H. et al Characteristics of leachate from pyrolysis residue of sewage sludge. Chemosphere, v. 68, n. 10, p. 1913-1919, 2007.
- KEILUWEIT, M.et al. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environmental Science & Technology, v. 44,n. 4, p. 1247-1253, 2010.
- KOOKANA, R.S. et al Biochar application to soil:agronomic and environmental benefits and unintended consequences. Advances in Agronomy,, v. 112, p. 103-143, 2011.
- LEHMANN, J. et al Biochar effects on soil biota:a review. Soil Biology and Biochemistry, v. 43, n. 9, p. 1812-1836, 2011.
- MENDONÇA, E.S; MATOS, E.S. Matéria orgânica do solo: métodos de análise. Viçosa, MG: UFV, 2005. 107 p.
- MITCHELL, P.J. et al. Shifts in microbial communityand water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biology and Biochemistry, v. 81, p. 244-254, 2015.
- NGUYEN, B.T. et al Temperature sensitivity of black carbon decomposition and oxidation. Environmental Science & Technology, v. 44, n. 9, p. 3324-3331, 2010.
- OLIVEIRA, C. et al. Solubilidade de metais pesados em solos tratados com lodo de esgoto enriquecido. Revista Brasileira da Ciência do Solo, v. 27, n. 1, p. 171-181, 2003.
- REEVES, J.B. et al Near infrared spectroscopic examination of charred pine wood, bark, cellulose and lignin: implications for the quantitative determination of charcoal in soils. Journal Near Infrared Spectroscopy,v. 15, p. 307-315, 2007.
- SONG, W.; GUO, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, v. 94, p. 138-145, 2012.
- UCHIMIYA, M.; CHANG, S.C. Screening biochars for heavy metal retention in soil: role of oxygen functional groups. Journal of Hazardous Materials,v. 190, n. 1/3, p. 432-441, 2011.
- ZHANG, L., CHUNBAO, C., XU, P.C. Overview of recent advances in thermo-chemical conversion of biomass. Energy Conversion and Management, v.51, n. 5, p. 969-982, 2010.
Publication Dates
-
Publication in this collection
Jul-Sep 2017
History
-
Received
08 May 2016 -
Accepted
28 Aug 2016