Abstract
Phytomining is suggested as a technology to obtain platinum group metals (PGMs) nanoparticles from plants grown on the mineralized soils or tailings. Samples from North American Palladium (Canada) and gossans from Broken Hill (BH) (Australia) were studied to assess the possibility of using these PGM-rich samples as substrates for phytomining. The bioavailability of PGMs was indirectly assessed using geochemical procedures. The selective extractions showed that the highest available concentration of Pd is 5.38 ppm in BH gossan 1. The extraction of PGMs by ammonium acetate, fulvic acid or citrate-dithionite indicates natural availability to plants. The BH gossan 1 was the best of the five studied samples for phytomining of Pd due to available Pd concentration (> 2 mg/kg), low Electric Conductivity (< 2dS/m), high CEC (Cation Exchange Capacity) (38.8 meq/100g), and proper pH (6.5). Cu-tolerant plant species should be chosen to grow on BH gossan 1. A criterium for choosing substrates for phytomining of Pd was developed comprising various classical soil parameters plus selective extraction procedures.
Keywords:
phytomining; platinum group metals; bioavailability; plant growth parameters
1. Introduction
The concept of phytomining is an adaptation of the use of an old concept of using plants for prospecting metals (Chaney et al., 2018CHANEY R. L.; BAKER A. J. M.; MOREL J. L. The long road to developing agromining/phytomining. In: VAN DER ENT, A.; ECHEVARRIA, G.; BAKER, A.; MOREL, J. (ed.). Agromining: farming for metals. Springer, Cham, 2018. p.1-17. (Mineral Resource Reviews).). The phytomining requires selection of hyperaccumulating plants, as well understanding which metals species are bioavailable in the substrate and how toxic for the plants they are (Kramer, 2019KRAMER, U. The plants that suck up metal. German Research, v. 40, n. 3, p. 19-23, 2018.). Plant species and methods for phytomining for platinum group metals (PGMs) involves growing plants on PGM-rich substrates that are capable of selectively incorporating these metals into their cellular structures (Sheoran et al., 2009SHEORAN, V.; SHEORAN, A. S.; POONIA, P. Phytomining of gold: a review. J. Geochemical Exploration, v. 128, p. 42-50, 2009.). Theoretically, the plants can then be harvested and subjected to controlled pyrolysis in order to yield a material with stabilized PGM nanoparticles that can later be used in catalytic reactions (Siddiq, & Husen, 2016, Dodson et al., 2015DODSON, J. R.; PARKER, H. L.; MUÑOZ-GARCÍA, A.; HICKEN, A.; ASEMAVE, K.; FARMER, T. J.; HE, H.; CLARK, J. H.; HUNT, A. J. Bio-derived materials as a green route for precious & critical metal recovery and re-use. Green Chemistry, v. 17, n. 4, p.1951-1965, 2015.). Parker et al. (2014)PARKER, H. L.; RYLOTT, E. L.; HUNT, A. J.; DODSON, J. R.; TAYLOR, A. F.; BRUCE, N. C.; CLARK, J.H. Supported palladium nanoparticles synthesized by living plants as a catalyst for Suzuki-Miyaura reactions. PloS One, v. 9, n. 1, 2014. DOI:10.1371/journal.pone.0087192.
https://doi.org/10.1371/journal.pone.008...
reported the first use of living plants to recover palladium and the production of catalytically active palladium nanoparticles with excellent catalytic activity across a range of coupling reactions that produced higher yields than commercial Pd catalyst. This process can reduce the number of production steps compared to traditional catalyst production methods. In automotive industries, for example, platinum (Pt), along with palladium (Pd), and rhodium (Rh) are coated onto a substrate housed in the exhaust system and act as catalysts to convert toxic vehicle emissions, such as carbon monoxide (CO), hydrocarbons (HC) and oxides of nitrogen (NOx), to less harmful substances (Saguru et al., 2018SAGURU, C.; NDLOVU, S.; MOROPENG, D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy, v. 182, p. 44-56, 2018.). In the soil, a small portion of metals is available to plants as a large portion is bound to different minerals that are more difficult to be incorporated by plants but this can change gradually by chemical weathering and biological process (Dairy Soils and Fertiliser Manual, 2013DAIRY soils and fertiliser manual: australian nutrient management guidelines. Melbourne, Victoria, Australia: Department of Primary Industries, 2013. ). The primary determinant of metal uptake by a plant is the bioavailability of the metal in the soil-plant system, i.e. how easily plants can access the metal in a form they require. The type and nature of the substrate constituents, pH, organic matter, cation exchange capacity and competing ions have all been shown to influence PGM bioavailability (Ko et al., 2008KO, B. G.; ANDERSON, C. W.; BOLAN, N. S.; HUH, K. Y.; VOGELER. I. Potential for the phytoremediation of arsenic-contaminated mine tailings in Fiji. Soil Research, v. 46, n. 7, p. 493-501, 2008.; Wilson-Corral et al., 2012WILSON-CORRAL, V.; ANDERSON, C. W.; RODRIGUEZ-LOPEZ, M. Gold phytomining. A review of the relevance of this technology to mineral extraction in the 21st century. Journal of Environmental Management, v. 111, p. 249-257, 2012.). As such, the primary interest of this study was to use geochemical methods, such as sequential-selective extractions, to assess the bioavailability of PGMs from high grade PGM samples.
2. Materials and methods
Samples from two sites were collected: North American Palladium (NAP), Canada and from Broken Hill (BH), Australia.
2.1 North American Palladium (NAP)
North American Palladium is a platinum group metals producer that has operated Lac des Iles (LDI) Mine in northern Ontario, Canada since 1993. The mine lies in the southern end of the Lac des Iles, 106 km northwest of Thunder Bay, Ontario, Canada, in an elliptical mafic-ultramafic intrusive complex (NAP, 2018NORTH AMERICAN PALLADIUM. Feasibility study for Lac des Iles Mine incorporating underground mining of the Roby Zone. NAP, 2008. Avaiable in: https://s21.q4cdn.com/326998455/files/doc_downloads/2018/10/NAP-43-101-Technical-Report-October-2-2018.pdf.
https://s21.q4cdn.com/326998455/files/do...
). A majority of platinum-group minerals occur either interstitially to sulfides in association with gangue minerals such as plagioclase, amphibole, chlorite, orthopyroxene, and talc, or at sulfide-silicate boundaries. The relative abundance of PGM-bearing minerals in the mill feed and concentrates from recently mined zones on the property are: palladium tellurides > palladium antimonides > palladium sulfides > sperrylite (platinum arsenide) > gold-silver alloys. PGM grades show varying degrees of correlation with nickel and copper concentrations (Yu et al., 2010YU, S.; OLIVEIRA, J.; SALEMIN, R.; CHISHOLM, K. Lac des Iles Roby and offset zone ore characterization report. Ontario, Canada: Xstrata Process Support (XPS) : Lac des Iles Mines Ltd., 2010. 111 p. ). Feed, concentrate and tailings samples were collected from the mineral processing plant.
2.2 Broken Hill (BH)
The Broken Hill deposit in western New South Wales, Australia has one of the richest reserves of lead, zinc and silver in the world. The Broken Hill ore body consists of a series of closely-spaced sulfides-rich deposits separated by quartz- and garnet-rich host rocks. In the upper parts of the mine, many of the sulfide minerals were converted by weathering into a large suite of oxide minerals, some of which were first recognized as new minerals from their formation in the Broken Hill District. Two types of gossans were selected to be studied (gossan 1 and 2) with no visible lithological differences except for the fact that gossan 2 seems denser than gossan 1.
2.3 Analytical procedures
Inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses for whole-rock chemistry, and Instrumental Neutron Activation Analysis (INAA) for PGMs (Ir, Os, Pd, Pt, Rh, Ru) and Au were conducted by Acme Analytical Labs Ltd. in Vancouver. The mineralogy was examined through quantitative X-ray powder diffractometry (XRD) at the department of Earth, Ocean & Atmospheric Sciences at the University of British Columbia.
Due to the high grade of PGMs in the BH gossan samples only these samples were analyzed by a Philips XL-30 scanning electron microscope (SEM) equipped with a Bruker Quantax 200 energy-dispersion X-ray (EDX) microanalysis system.
The mineral association and bioavailability of PGMs and Au in all samples were investigated by selective extraction methods of samples ground below 0.074 mm. Cu and Ni were also analyzed due to their phytotoxicity that may limit plant growth. Ammonium acetate (NH4C2H3O2) was used to extract readily exchangeable metal species (Ferreira and Veiga, 1995FERREIRA, N.; VEIGA,M.M. Control of mercury bioavailability by sediment adsorption. In: ECO URBS 95, 1995, Rio de Janeiro. Proceedings[...]. Rio de Janeiro, Jun. 19-23, 1995. p. 53-55.). It is believed that exchangeable metals in soil are the most available to plants (Castilho and Rix, 1993CASTILHO, P. D.; RIX, I. Ammonium acetate extraction for soil heavy metal speciation; model aided soil test interpretation. International Journal of Environmental Analytical Chemistry, v. 51, n. 1-4, p. 59-64, 1993.). Citrate-dithionite was used by Gray et al. (1996)GRAY, D. J.; SCHORIN, K.; BUTT, C. Mineral associations of platinum and palladium in lateritic regolith, Ora Banda sill, Western Australia. Journal of Geochemical Exploration, v.57, n. 1, p. 245-255, 1996. to selectively dissolve amorphous and crystalline Hydrous Ferric Oxides (HFO) such as limonite and goethite as well as crystalline iron oxide like hematite. This work may be the first trial to use fulvic acid in selective extraction of PGMs and Au. It was expected that this humic substance could provide additional information about the fraction of the metals that would be bioavailable and possibly incorporated by plants. Reverse aqua regia was used to dissolve sulfides. This is a mixture of nitric acid and hydrochloric acid in a molar ratio of 3:1. Higher concentration of nitric acid increases dissolution of sulfides (Tessier et al., 1979TESSIER, A.; CAMPBELL, P.;G.; BISSON, M. Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, v. 5, n. 7, p. 844-851. 1979.). When sulfides are dissolved, hydrochloric acid provides complexing ligands for Pt and Pd that are associated with sulfides (Colombo et al., 2008COLOMBO, C.; OATES, C.; MONHEMIUS, A.; PLANT, J. Complexation of platinum, palladium and rhodium with inorganic ligands in the environment. Geochemistry: Exploration, Environment, Analysis, v. 8, n. 1. p. 91-101, 2008.).
2.4 First selective extraction scheme
Two sequences of selective extractions were employed. The first extraction consisted of the following steps:
-
PGM associated with exchangeable cations: 3 g of sample were agitated for 1 h with 50 mL 1 M NH4C2H3O2.
-
PGM associated with extractable cations extracted by humic substances: the residue from the previous extraction was washed and leached twice in a shaker with 50 mL of lab-grade fulvic acid for 5 h, at 500C.
-
Dissolution of amorphous and crystalline HFO and hematite: The residue from the previous step was washed and leached twice with 50 mL of 0.3 M ammonium citrate and 1 g sodium dithionite for 16 hours, heated at 500C.
-
Dissolution of sulfides: the residue was washed and leached twice for 16 h, heated at 500C with 30 ml 65% HNO3, 10 mL 30% HCl (inverse aqua-regia) and 10 mL H2O2.
-
Residual PGM associated with other minerals: the final solid residues were melted with borate and dissolved with aqua regia.
All solutions from each extraction step were analyzed by ICP-MS.
2.5 Second selective extraction scheme
The objective of the second extraction sequence was to remove more ferric oxides and associated metals from the gossan samples since the NAP samples had low ferric oxides. The extraction used a citrate-dithionite leaching process as described by Veiga et al., (1991)VEIGA, M. M.; SCHORSCHER, H. D.; FYFE, W. S. Relationship of copper with hydrous ferric oxides: Salobo, Carajás, Pará, Brazil. Ore Geology Reviews, v. 6, n. 2, p. 245-255, 1991.. The method leached 3 g of sample ground below 0.074 in a shaker at 50 oC with a solution of citrate-dithionite as described above (step 3). After 4 hours, the pulp was filtered and the solution analyzed for Pt,Pd and Au. The procedure was repeated 3 more times to guarantee that all ferric oxides were dissolved.
3. Results and discussion
3.1 Total concentration of PGMs and Au
The total concentration of PGMs and Au determined by INAA are shown in Table 1. Pt and Au concentrations in NAP concentrate (con) are the highest of all samples. All six PGM grades in BH gossan 1 and gossan 2 are very high.
3.2 XRD analysis
X-ray diffraction analysis (XRD, CuKa) using Rietveld refinement process for mineral quantification showed that NAP feed and tailings contained mainly silicate minerals: actinolite (Ca2(Mg,Fe)5Si8O22(OH)2), andesine ((Na,Ca)(Si,Al)4O8) and clinochlore ((Mg,Fe2+)5Al(Si3Al)O10(OH)8). These three minerals accounted for 75.8% and 76.4% by mass of the feed and tailings, respectively. Other minerals comprise quartz, talc, biotite, dolomite and traces of sulfides. The XRD of NAP concentrate enhanced the presence of the sulfide minerals: pyrite (FeS2), chalcopyrite (CuFeS2) and pentlandite ((Fe,Ni)9S8). These three minerals accounted for 71.3% of the mass of the sample. The BH gossan samples consisted mainly of Fe-oxide minerals: goethite (a−FeOOH) and hematite (Fe2O3), entirely accounting for 90.6% and 90.5% of gossan 1 and gossan 2 by mass, respectively.
3.3 SEM analysis
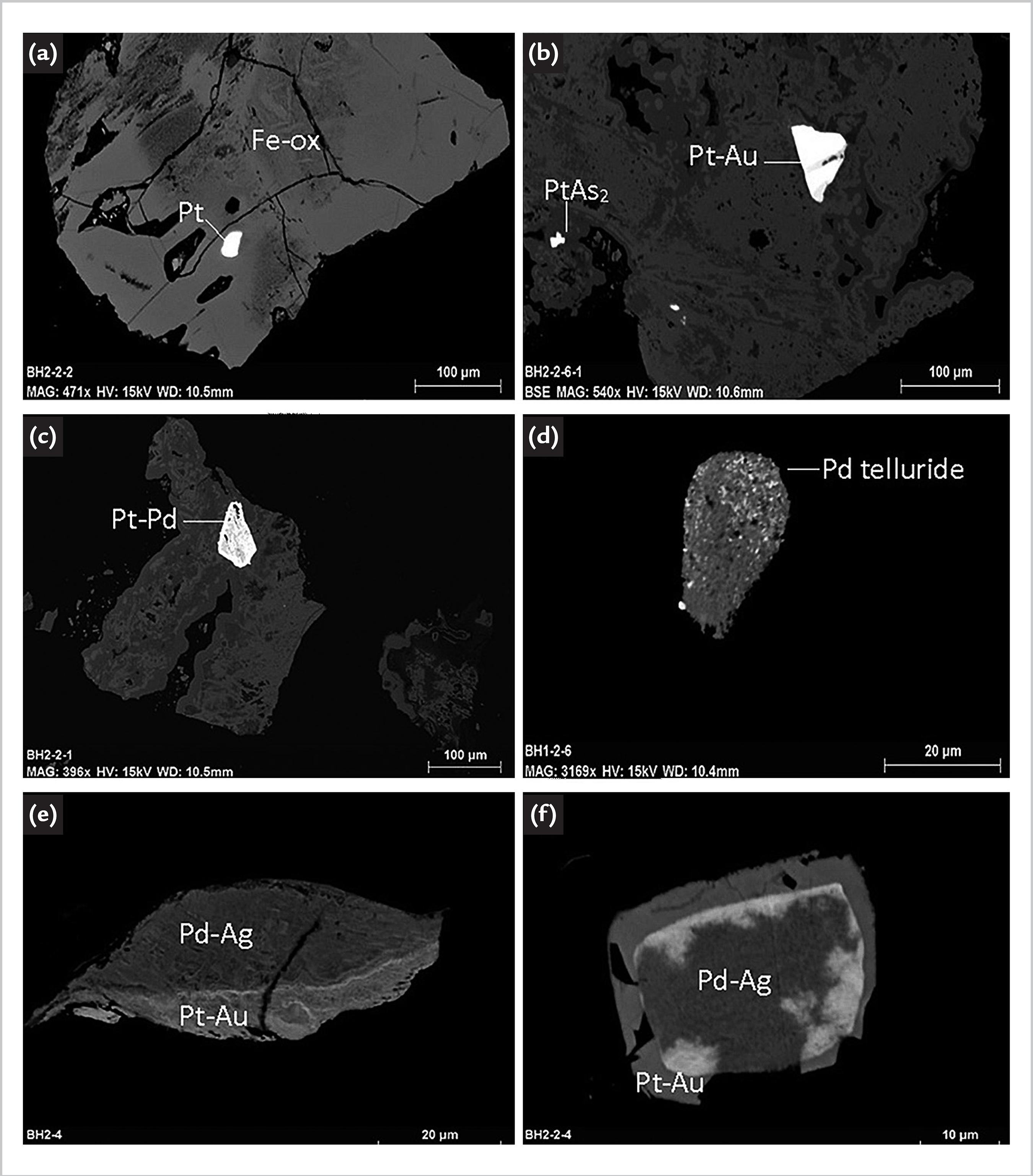
In the SEM study of BH gossan 1 and gossan 2, many barite grains were found in both gossan samples, and they were surrounded by iron oxides. Some grains of iodargyrite (AgI) were either liberated as independent grains or associated with a Fe-Cr-Ni mineral. Traces of galena (PbS) and chalcopyrite (CuFeS2) were also identified in both samples. Certain amounts of Cu and Ni were found associated with PGM. According to Elvy (1998)ELVY, S. B. Geochemical studies of base and noble metal compounds. PhD Thesis (Doctor of Philosophy) - School of Science, University of Western Sydney, Nepean, 1998., the observed PGM in the BH gossan include froodite (PdBi2), palladium-copper alloys, palladium-tellurium-mercury alloy, paolovite (Pd2Sn), platinum alloys, sperrylite (PtAs2) and unnamed palladium tellurides. Among them, palladium-copper alloys platinum-palladium alloy, sperrylite (PtAs2) and unnamed palladium tellurides were identified in BH samples (Fig. 1).
Micrography of Back-scattered Electrons showing: Pt , Pt-Au alloy, sperrylite and Pd-Pt alloy inclusions within Fe oxides (a,b,c); Pd telluride grain (d); Pd-Ag alloy is attached to Pt-Au alloy (e,f).
3.4 Sequential-selective extractions
The results of the sequential-selective extractions are shown in Tables 2, 3 and 4 with some comments provided as follows.
3.4.1 Exchangeable cations
The extraction of the PGMs weakly adsorbed onto the minerals, which can be exchanged by ammonium acetate, shows that from 0.1% to 2% of Pd in NAP samples and BH samples were dissolved by ammonium acetate. Gold had a better response to this extraction with recoveries from 0.2 to 12%. Almost no Pt in all samples was dissolved by ammonium acetate.
3.4.2 Extractable PGMs with Fulvic Acid
Fulvic acid was used to extract metallic PGMs that could be complexed with humic substances (Wood, 1996WOOD, S. A. The role of humic substances in the transport and fixation of metals of economic interest (Au, Pt, Pd, U, V). Ore Geology Reviews, v. 11, n. 1-3, p. 1-31, 1996.). Small proportions of Pd in NAP samples and BH samples were dissolved by fulvic acid (<1.1%). Again Au had better recoveries with fulvic acid showing up to 8.2% recovery in the NAP tailings. Almost no Pt in NAP samples and BH samples was dissolved by fulvic acid.
3.4.3 Extractable PGMs from Ferric Oxides
Citrate-dithionite can dissolve amorphous and crystalline HFO and Fe oxides. Up to 12.3% of Pd and to 24% of Pt in BH samples were dissolved by citrate-dithionite in two leaching batches of 16 h each (Table 3), indicating certain association of these metals with the Fe oxides. The results of the 2nd test with citrate-dithionite (Table 4) confirmed extractions up to 12.6% Pd and close to 32% of Pt from the gossans. The proportions of Pt dissolved by citrate-dithionite in both BH samples were similar, due to Pt being associated with Fe oxides in both BH samples. The different recoveries of Pt and Pd in the BH samples may suggest that the proportions of Pt and Pd associated with Fe oxides in the two samples are different.
3.4.4 Extractable PGMs with Reverse Aqua Regia
Reverse aqua regia is supposed to extract sulfides from NAP samples. As for BH samples, the major minerals are goethite and hematite, which can also be dissolved by reverse aqua regia. Ambiguities remained with respect to the proportions dissolved by reverse aqua regia in BH samples. Large amounts of Pd and Au in NAP samples were dissolved by reverse aqua regia. This suggests Pd and Au associations with sulfides. In contrast, the Pt solubility in reverse aqua regia was found to be considerably lower in the NAP samples. This can either indicate that most of the Pt is not in sulfides or the chloride concentration was not high enough to keep Pt in the solution. The proportions of Au and Cu dissolved in NAP feed, concentrate, and tailings are similar, probably due to Au being associated with Cu sulfides.
3.5 Soil Associated Factors
3.5.1 Nutrients, pH, EC and CEC
According to the nutrient analysis, the levels of N, K, P and Mg in the studied samples are very low: Nitrogen ranges from 9 to 24 ppm in NAP samples and around 34 ppm in the gossans; phosphorous in all samples was less than 3 ppm and potassium ranged from 4 to 44 in NAP samples and 155 to 660 in the gossan samples. Therefore, an adequate fertilization program must be implemented before cultivation of the substrate. The pH of NAP concentrate was 3.6 and as such, according to Dairy Soils and Fertiliser Manual (2013)DAIRY soils and fertiliser manual: australian nutrient management guidelines. Melbourne, Victoria, Australia: Department of Primary Industries, 2013. , the availability of macronutrients (nitrogen, phosphorus, potassium, sulfur, calcium and magnesium) as well as molybdenum in the soil is curtailed at pH of 3.6. Thus, the soil was too acidic for the survival of most plants. This acidity may be due to the presence of acid-generating sulfides in this substrate. Both the NAP feed and NAP tailings were slightly alkaline with pHs around 8. BH gossan 1, with pH 6.5 is acceptable for most plants, whereas BH gossan 2, with pH 8.5 is too alkaline for most plants. The electrical conductivity (EC) of all samples except NAP con were lower than 2 dS/m suggesting they may not have problems of salinity. The EC of NAP concentrate reached as high as 9 dS/m. According to Manitoba Agriculture, Food and Rural Initiatives Soil Management Guide (2008), this is moderately saline, meaning that plant growth can be limited by holding water more tightly than the plants can extract it.
The cation exchange capacity (CEC) of the BH gossan samples was the highest, 38.8 meq/100g, while those of NAP feed, concerntrate and tailings were below 10 meq/100. According to Spectrum Analytic Inc. (2015), soils with a CEC of 11-50 meq/100g have a greater capacity to hold nutrients and water than those with CEC of 1-10 meq/100g. Low CEC soils are more likely to develop potassium and magnesium (and other cations) deficiencies while high CEC soils are less susceptible to the leaching losses of these cations (Rayment and Higginson, 2012RAYMENT, G.; HIGGINSON, F. R. New, comprehensive soil chemical methods book for Australasia. Journal Communications in Soil Science and Plant Analysis, v. 43, n. 1-2, p. 412-418, 2012.). This indicates that an adequate fertilization program should be implemented when cultivating on NAP samples. Although normally, CEC is a function of clay and organic matter in the soil, in this particular situation, it may be a function of the weathered Fe-minerals.
3.5.2 Heavy metals
The total concentrations of heavy metals (As, Ba, Cd, Cr, Co, Cu, Hg, Pb, Mo, Ni and Zn) in all samples were investigated and compared with the Dutch standards (Ministry of Housing - Netherlands, 1994MINISTRY OF HOUSING OF NETHERLANDS. Dutch intervention values of heavy metals and organic pollutants in soils, sediments, and ground water. Physical Planning and Environmental Conservation Report HSE 94.021, 994.) for assessing soil contamination on the basis of the total concentration of heavy metals in the soil. None of the heavy metals concentrations of NAP tailings exceeded the intervention values of the Dutch standards. Cu and Ni contents in the other four samples went beyond the intervention values of the Dutch standards. This indicates that plants may have Cu and Ni toxicity problems when they grow on these substrates. Ba and Co concentrations of BH gossan 1 and gossan 2 were also very high. The phytotoxicity of Cu has been proposed as an important variable that will affect the yield of PGMs during phytomining, since toxicity due to Cu uptake will influence the rate of transpiration and thus the degree of PGM uptake (Wilson-Corral et al., 2012; Aquan, 2015AQUAN, H. M. Phytoextraction of palladium and gold from broken hill gossan. 2015, 126f. Thesis (Master of Environmental Management) - Massey University, North Palmerston, New Zealand, 2015.). The likely bioavailable exchangeable and extractable Cu in all samples exceed 190 mg/kg, which can be a problem for plants.
4. Conclusion
This study was conducted to predict bioavailability of Platinum Group Metals (PGM to plants), using selective chemical extractions and other soil and mineralogical techniques. Minerals of interest such as native Pt, Pt-Au alloy and Pd telluride were identified in SEM in both BH gossan samples. Sperrylite and Pd-Cu oxide were also found in sample BH gossan 2.
Regarding the selective extraction methods, the dissolution of ferric oxides by citrate-dithionite extracted as much as 12% of Pd and 24% of Pt from the BH gossan samples. This might suggest some association of these metals with the ferric oxides. Large amounts of Cu were found associated with exchangeable and extractable fractions in NAP samples and BH samples, which can be a problem to the Cu-sensitive plants development. The PGM that can be extracted by ammonium acetate are those weakly adsorbed on the minerals and seem to be a good indicator of their availability of these metals to plants. However, just low amounts of Pd and Pt were found associated with exchangeable fractions in all samples. Those PGM that can be extracted by fulvic acid and citrate-dithionite provide indication that they might be soluble in soils. The NAP samples responded very weakly to all selective extractive methods providing very little amounts of Pd and Pt in solution.
The NAP conc. sample has low pH and high salinity, which makes it unsuitable as a direct growth media for plants. The available Pd concentration of all NAP feed, concentrate, and tailing sanples are lower than 2 mg/kg which was considered by Anderson et al. (2005)ANDERSON, C.; MORENO, F.; MEECH, J.A. A field demonstration of gold phytoextraction technology. Minerals Engineering, v. 18, n. 4, p. 385-392, 2005. as the minimum concentration of precious metals in a substrate to yield significant concentration in a plant during the phytomining process. The NAP samples does not seem suitable for phytomining of PGM n. In addition, most Pd was found associated with sulfides in the NAP samples, but not enough data was obtained to suggest that Pt is also associated with sulfides.
The Pd concentration in BH gossan 1 is higher than 2 mg/kg and its low EC, high CEC, and proper pH make it a suitable substrate for plant growth. It is the best "one" of the five samples. Cu-tolerant plant species should be chosen to grow on both BH gossans. The available Pd concentration of BH gossan 2 is also higher than 2 mg/kg, however its pH (8.5) is slightly alkaline. This means amendments should be applied to adjust its pH before planting plants. The selective extractions show that the soluble Pd concentrations of BH gossan 2 are also higher than 2 mg/kg. However, the pH (8.5) is slightly alkaline. This means amendments should be applied to adjust its pH before cultivation.
Acknowledgment
This article is in memoriam of Dr John Meech who started this investigation and unfortunately could not see the results. The NSERC is acknowledged for the funds for this research. This project was part of the PHYTOCAT supported by the G8 Research Councils Initiative on Multilateral Research Funding. The team was led by the University of York in the UK with support from Yale University, the University of British Columbia and Massey University in New Zealand.
References
- ANDERSON, C.; MORENO, F.; MEECH, J.A. A field demonstration of gold phytoextraction technology. Minerals Engineering, v. 18, n. 4, p. 385-392, 2005.
- AQUAN, H. M. Phytoextraction of palladium and gold from broken hill gossan 2015, 126f. Thesis (Master of Environmental Management) - Massey University, North Palmerston, New Zealand, 2015.
- CASTILHO, P. D.; RIX, I. Ammonium acetate extraction for soil heavy metal speciation; model aided soil test interpretation. International Journal of Environmental Analytical Chemistry, v. 51, n. 1-4, p. 59-64, 1993.
- CHANEY R. L.; BAKER A. J. M.; MOREL J. L. The long road to developing agromining/phytomining. In: VAN DER ENT, A.; ECHEVARRIA, G.; BAKER, A.; MOREL, J. (ed.). Agromining: farming for metals. Springer, Cham, 2018. p.1-17. (Mineral Resource Reviews).
- COLOMBO, C.; OATES, C.; MONHEMIUS, A.; PLANT, J. Complexation of platinum, palladium and rhodium with inorganic ligands in the environment. Geochemistry: Exploration, Environment, Analysis, v. 8, n. 1. p. 91-101, 2008.
- DAIRY soils and fertiliser manual: australian nutrient management guidelines. Melbourne, Victoria, Australia: Department of Primary Industries, 2013.
- DODSON, J. R.; PARKER, H. L.; MUÑOZ-GARCÍA, A.; HICKEN, A.; ASEMAVE, K.; FARMER, T. J.; HE, H.; CLARK, J. H.; HUNT, A. J. Bio-derived materials as a green route for precious & critical metal recovery and re-use. Green Chemistry, v. 17, n. 4, p.1951-1965, 2015.
- ELVY, S. B. Geochemical studies of base and noble metal compounds PhD Thesis (Doctor of Philosophy) - School of Science, University of Western Sydney, Nepean, 1998.
- FERREIRA, N.; VEIGA,M.M. Control of mercury bioavailability by sediment adsorption. In: ECO URBS 95, 1995, Rio de Janeiro. Proceedings[...] Rio de Janeiro, Jun. 19-23, 1995. p. 53-55.
- GRAY, D. J.; SCHORIN, K.; BUTT, C. Mineral associations of platinum and palladium in lateritic regolith, Ora Banda sill, Western Australia. Journal of Geochemical Exploration, v.57, n. 1, p. 245-255, 1996.
- KO, B. G.; ANDERSON, C. W.; BOLAN, N. S.; HUH, K. Y.; VOGELER. I. Potential for the phytoremediation of arsenic-contaminated mine tailings in Fiji. Soil Research, v. 46, n. 7, p. 493-501, 2008.
- KRAMER, U. The plants that suck up metal. German Research, v. 40, n. 3, p. 19-23, 2018.
- MINISTRY OF HOUSING OF NETHERLANDS. Dutch intervention values of heavy metals and organic pollutants in soils, sediments, and ground water Physical Planning and Environmental Conservation Report HSE 94.021, 994.
- NORTH AMERICAN PALLADIUM. Feasibility study for Lac des Iles Mine incorporating underground mining of the Roby Zone NAP, 2008. Avaiable in: https://s21.q4cdn.com/326998455/files/doc_downloads/2018/10/NAP-43-101-Technical-Report-October-2-2018.pdf
» https://s21.q4cdn.com/326998455/files/doc_downloads/2018/10/NAP-43-101-Technical-Report-October-2-2018.pdf - PARKER, H. L.; RYLOTT, E. L.; HUNT, A. J.; DODSON, J. R.; TAYLOR, A. F.; BRUCE, N. C.; CLARK, J.H. Supported palladium nanoparticles synthesized by living plants as a catalyst for Suzuki-Miyaura reactions. PloS One, v. 9, n. 1, 2014. DOI:10.1371/journal.pone.0087192.
» https://doi.org/10.1371/journal.pone.0087192 - RAYMENT, G.; HIGGINSON, F. R. New, comprehensive soil chemical methods book for Australasia. Journal Communications in Soil Science and Plant Analysis, v. 43, n. 1-2, p. 412-418, 2012.
- SHEORAN, V.; SHEORAN, A. S.; POONIA, P. Phytomining of gold: a review. J. Geochemical Exploration, v. 128, p. 42-50, 2009.
- SAGURU, C.; NDLOVU, S.; MOROPENG, D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy, v. 182, p. 44-56, 2018.
- SIDDIQI, K. S.; HUSEN, A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Research Letters, V. 11, n. 482, 2016. DOI: https://doi.org/10.1186/s11671-016-1695-z
» https://doi.org/10.1186/s11671-016-1695-z - TESSIER, A.; CAMPBELL, P.;G.; BISSON, M. Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, v. 5, n. 7, p. 844-851. 1979.
- VEIGA, M. M.; SCHORSCHER, H. D.; FYFE, W. S. Relationship of copper with hydrous ferric oxides: Salobo, Carajás, Pará, Brazil. Ore Geology Reviews, v. 6, n. 2, p. 245-255, 1991.
- WILSON-CORRAL, V.; ANDERSON, C. W.; RODRIGUEZ-LOPEZ, M. Gold phytomining. A review of the relevance of this technology to mineral extraction in the 21st century. Journal of Environmental Management, v. 111, p. 249-257, 2012.
- WOOD, S. A. The role of humic substances in the transport and fixation of metals of economic interest (Au, Pt, Pd, U, V). Ore Geology Reviews, v. 11, n. 1-3, p. 1-31, 1996.
- YU, S.; OLIVEIRA, J.; SALEMIN, R.; CHISHOLM, K. Lac des Iles Roby and offset zone ore characterization report Ontario, Canada: Xstrata Process Support (XPS) : Lac des Iles Mines Ltd., 2010. 111 p.
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
Jan-Mar 2020
History
-
Received
05 Apr 2019 -
Accepted
27 Sept 2019