Abstracts
The cellular and humoral immune responses of mice inoculated with rabies virus and treated with the Bacillus of Calmette-Guérin, Avridine and Propionibacterium acnes were evaluated in this paper. There was a higher percentage of surviving mice in groups submitted to P. acnes treatment. Lower levels of interferon-<FONT FACE="Symbol">g</font> (IFN-<FONT FACE="Symbol">g</font>) were found in infected mice. The intra-pad inoculation test (IPI) was not effective to detect cellular immune response, contrary to the results found in MIF reaction. The survival of mice did not present correlation with the levels of antirabies serum neutralizing (SN) antibodies titers, IFN-<FONT FACE="Symbol">g</font> concentration and MIF response.
Rabies; Immunomodulators; Cellular immunity; Humoral immunity; <FONT FACE=Symbol>g</font>-interferon; Mice
Avaliou-se a resposta imune celular e humoral de camundongos inoculados com vírus rábico de rua e submetidos aos imunomoduladores Onco-BCG, avridina e Propionibacterium acnes. Os animais submetidos ao tratamento com P.acnes apresentaram um maior percentual de sobrevivência quando comparados aos dos demais tratamentos. Foram observados menores níveis de IFN-<FONT FACE="Symbol">g</font> nos animais infectados, sugerindo imunossupressão viral. O teste do Coxim Plantar não foi eficaz para a detecção da resposta de hipersensibilidade retardada na metodologia utilizada, contrariamente ao MIF. A sobrevivência dos animais não apresentou correlação com os níveis de anticorpos soroneutralizantes, concentração de IFN-<FONT FACE="Symbol">g</font> e resposta ao MIF.
EFFECT OF BACILLUS OF CALMETTE-GUÉRIN, AVRIDINE AND Propionibacterium acnes AS IMMUNOMODULATORS ON RABIES IN MICE
J.MEGID(1 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. ), M.T.S. PERAÇOLI(2 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. ), P.R. CURI(3 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. ), C.R.ZANETTI(4 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. ), W.H. CABRERA(5 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. ), R.VASSAO(5 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. ) & F.H.ITO(6 (1 Abstract presented at the Pan American Veterinary Congress, October 1996, Campo Grande-MS, Brazil. Research conducted with the financial support from FUNDUNESP. ) Assistant Professor-PhD, Department of Veterinary Hygiene and Public Health, FMVZ-UNESP, Botucatu, SP, Brazil. (2 ) Assistant Professor-PhD, Department of Immunology and Microbiology, Biosciences Institute, UNESP, Botucatu, SP, Brazil. (3 ) Professor of Statistics, FMVZ-UNESP, Botucatu, SP, Brazil. (4 ) Researcher of Pasteur Institute, São Paulo, SP, Brazil. (5 ) Researcher of Butantan Institute, São Paulo, SP, Brazil. (6 ) Associate Professor of Department of Preventive Veterinary Medicine and Animal Health, FMVZ-USP, São Paulo,SP, Brazil. )
SUMMARY
The cellular and humoral immune responses of mice inoculated with rabies virus and treated with the Bacillus of Calmette-Guérin, Avridine and Propionibacterium acnes were evaluated in this paper. There was a higher percentage of surviving mice in groups submitted to P. acnes treatment. Lower levels of interferon-g (IFN-g) were found in infected mice. The intra-pad inoculation test (IPI) was not effective to detect cellular immune response, contrary to the results found in MIF reaction. The survival of mice did not present correlation with the levels of antirabies serum neutralizing (SN) antibodies titers, IFN-g concentration and MIF response.
KEYWORDS: Rabies; Immunomodulators; Cellular immunity; Humoral immunity; g-interferon; Mice.
INTRODUCTION
The immune response in infection with rabies virus has been studied and reported by several authors6,8,27,31,33 characterizing the importance of cellular and humoral immune response and induction of interferon in the survival of animals, as well as the immunosupression induced by rabies virus25,34.
Evidence has shown that immunomodulators are capable of enhancing the immune response induced by vaccines, restore the immunocompetence of immunocompromised animals, and strengthen inespecifically the resistance to infections and increase the efficacy of conventional therapy12.
Among the immunomodulating substances more widely used, the bacillus of Calmette-Guérin (BCG) can be mentioned as one of the most well known immunomodulators. Research using BCG was reported by BARAKAT et al.4 in sheep, and later challenged with Rift Valley Fever virus; by SPENCER et al.28 with the influenza virus in mice and by GLASGOW et al.15 with Corynebacterium acnes, Corynebacterium parvum and BCG in mice.
P. acnes has been used in different studies evaluating its mechanism of action1,10; compared to several immunomodulators22; used in human carriers of infectious diseases21; treatment of malignant neoplasias26 and in animals with infectious diseases32.
HOFFMAN et al.17, while searching for an atoxic inducer of IFN found the Avridine (CP-20. 961); tested against the DNA and RNA viruses in mice they reported its antiviral activity. ANDERSON & REYNOLDS2 demonstrated that Avridine induced to an increase of lymphocytes through local lymph nodes, in which it was observed the appearance of increasing number of macrophages around germinal center, considered as an excellent antigen presenting cells for clones of T and B lymphocytes.
Other investigations were conducted using Avridine as the adjuvant of rabies vaccines9,14.
Therapeutic trials in rabies were initiated in 1889 by BABES & LEPP (apud BAER3) with application of serum, and followed by other works using vaccines alone, serum combined with vaccines, interferons and interferon inducers.
This work was designed to evaluate the cellular and humoral immune response of animals infected with rabies virus and treated with the bacillus of Calmette-Guérin (BCG), Propionibacterium acnes (Corynebacterium parvum) and Avridine.
MATERIAL AND METHODS
ANIMALS: swiss albino mice, outbred, females, aging approximately four weeks, were provided by the Central Animal Facility of UNESP-Botucatu.
RABIES VIRUS: the virus isolate used was from dog brain, which have been maintained with one intracerebral and one intramuscular passage in mice.
DILUENT FOR THE IPI AND MIF TESTS: a 2% six to seven day old mice nervous tissue suspension without rabies virus inoculation, homogeneized in buffered diluent was used as diluent. The suspension was preserved with 0.1% phenol and 0.01% timerosal. The final product constituted the negative control antigen for the IPI and MIF tests.
IMMUNOMODULATORS: ONCO-BCG: produced by Butantan Institute, administering dose of 200 µg per mouse, according to SPENCER28; P. acnes: produced by Laboratório Farmacêutico do Estado de Pernambuco (LAFEPE), and this product was used at dose of 0.2 ml per animal; Avridine: kindly provided by PFIZER S.A., using dose of 0.04 mg per animal, according to NIBLACK et al.23.
ANIMAL INOCULATION: performed through intramuscular route, with small modification of the methodology described by KOPROWSKY19.
DIRECT IMMUNOFLUORESCENCE: the test was run according to the methodology of GOLDWASSER & KISSLING16.
MIF TECHNIQUE: the technique used was the one described by DEFAVERI et al. 11; the migration chamber was filled with: a) 10% of horse serum added in Eagle medium (control chamber); b) Eagle medium containing 10% horse serum and antigen added in, here represented by an inactivated rabies vaccine (Fuenzalida-Palacios type) at 10-2 dilution; c) 10% horse serum added in Eagle medium and with an inactivated rabies vaccine at 10-2 dilution.
IPI TEST: the technique used was according to that described by TSIANG & LAGRANGE31.
The pad of the right hind limb was injected through intradermic route with 40 µl antigen constituted of rabies vaccine diluted at 10-3 and similarly the pad of the left hind limb was injected with 40 µl of negative control antigen, constituted of the diluent of the vaccine at 10-3 dilution. The increase of the pad thickness was measured using a caliper (Mitutoyo, precision of 0.01 mm), calculating the difference in the mean of 3 measurements made immediately before and 24 hs after the injection of antigen.
DETERMINATION OF IFN-g: performed according to the methodology of CHERWINSKI et al.7, by using the ELISA technique.
SERUM NEUTRALIZATION (SN): the technique adopted was described by FAVORETTO et al.13, using the PV strain and BHK21 cell culture; the sera were first diluted at 1:5 and thereafter used the two-fold serial dilution until the dilution of 1:40 and the SN titers were expressed in IU/ ml.
EXPERIMENTAL PROCEDURES
This experiment was conducted in 2 phases:
Phase I: In this phase 220 healthy mice were injected intramuscularly with 0.03 ml of normal mouse brain suspension, and after being divided into 4 groups of 55 animals each and 24 hs after the injection, they were treated with immunomodulators, as follows:
Group 1 = without immunomodulator;
Group 2 = 0.02 ml of BCG through IM route, into the right hind pad;
Group 3 = 0.2 ml of P. acnes through intraperitonial route;
Group 4 = 0.1 ml of Avridine through intraperitonial route.
Phase II: Through intramuscular route, 220 mice have been inoculated with 0.03 ml of rabid mouse brain suspension. Twenty-four hours later, the animals were separated into 4 groups of 55 animals each and treated with immunomodulators as follows:
Group 5 = without immunomodulator;
Group 6 = 0.02 ml of BCG through IM route, into the right hind pad;
Group 7 = 0.2 ml of P. acnes through intraperitonial route;
Group 8 = 0.1 ml of Avridine through intraperitonial route.
After treating the animals with the immunomodulators, 5 mice from each group were bled through cardiac punction and sacrificed, at 3, 6, 12 and 24 hs after administering the immunomodulators. On the 5th day after the application of the immunomodulators, 2 mice from each group were identified and submitted to IPI test. Twenty-four hours later the thickness of the pads were measured, and soon after the mice were bled and sacrificed and the spleen collected, kept in Eagle's medium until its use for MIF test. At the 6th day three animals were selected from each group and submitted to the same procedures. At the 12 and 13th day after the treatment with immunomodulators, 5 mice from each group were submitted for the same procedures described above. Sera taken 24 hs after the administration of immunomodulators were pooled accordingly to the groups and after this period, all sera were kept frozen individually, until the SN titers and IFN-g determination. The animals were daily observed for six months and then, the survival rate was calculated. The results of MIF and IPI tests were analyzed considering the differences obtained between the responses of the groups of animals to vaccine or to the negative control antigen. The results of different tests were submitted to completely randomized factor analysis (nonparametric) according to ZAR35.
RESULTS
The results of IPI, MIF, IFN-g and SN tests are presented in the following Tables and Figures:
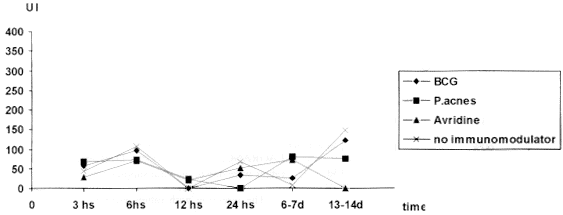
Fig. 1 - IPI values expressed in millimeter of thickness, in mice belonging to different groups at the 6-7 and 13-14th day, 1996
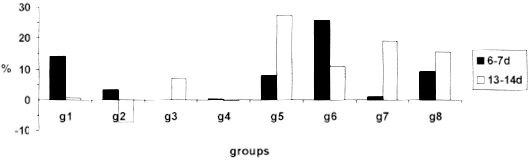
Fig. 2 - MIF values expressed in percentage of migration inhibition,in mice belonging to different groups at the 6-7 and 13-14th day, 1996.
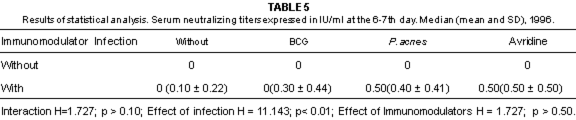
Fig. 3 - Serum neutralizing titers expressed in IU/ml, in mice belonging to different groups, at the 6-7 and 13-14th day, 1996.
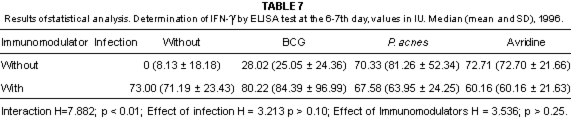
Fig 4 - IFN-g determination by the ELISA test in pooled sera taken from non infected mice belonging to different groups. Values expressed in IU, 1996.
Fig. 5 - IFN-g determination by the ELISA test in pooled sera taken from infected mice belonging to different groups. Values expressed in IU, 1996.
DISCUSSION
In this experiment, contrary to what was observed by TSIANG & LAGRANGE31 the IPI test was not effective on the detection of antirabies cellular immunity characterized by delayed type hypersensibility. The results were extremely variable. This lack of response to the test in infected animals could be explained by the necessity of previous application of an adjuvant of T cells, like BCG, in order to obtain higher levels and more constant DTH response20, which is in accordance with BLANCOU et al.5 who found more intense reaction in vaccinated mice treated with BCG. It was observed the decrease in the thickness of the pads in almost all infected groups at the 13-14th day, with exception of mice treated with P.acnes. This result could be explained by consequence of the weight losses observed in those animals at the peak of the disease. Moreover, TSIANG & LAGRANGE31 considered that DTH reactions of low intensity in rabies were due to the viral suppression.
The infection with rabies virus induced a cellular response, manifested by MIF test at the 6-7th day, reaching the maximum inhibition intensity at the 13-14th day. Evidence of the cellular immune response after the infection with rabies virus was reported by several authors25,33, who detected the maximum peak at the first nine days after the infection, with gradual decrease thereafter; PERRIN et al.25 reported reduction in the production of IL-2 after infection of mice with rabies virus; KAWANO et al.18 described cytotoxic activity of splenic cells, contrasting to the results found in this experiment in which the peak of MIF reaction was observed at the 13-14th day.
The levels of serum neutralizing antibodies were not associated with the survival rate, in line with the report of NUNBERG et al.24.
In this experiment, infected mice were found with lower production of IFN-g at the 13-14th day, coinciding with the peak of the symptoms of the disease. Immunosuppression due to viral infection was reported by PERRIN et al.25, through the decrease in IL-2 production.
The interferon inducing capacity of Avridine was characterized at the 13-14th day in infected mice which presented higher IFN-g titers than the other groups, according to the report of NIBLACK et al.23.
In spite of the higher number of surviving mice observed in the groups treated with P.acnes, no statistically significant differences were found between the period of incubation, this fact agrees with the work of SMITH et al.27 who found, in immunosuppressed and immunocompetent animals, the same mean period of mortality although there was difference in the survival rate.
The results found with P. acnes could be explained by its activity at the macrophage level, which release IL-1, TNF-a and IL-6 few hours after the administration of P. acnes 29. P. acnes is considered as a stimulator of NK cells activity through the liberation of IFN and TNF30.
According to the methodology and biological model used, P. acnes has been characterized as the best immunomodulator in this experiment.
The survival rate, even after the inoculation of rabies virus was not correlated with the titers of serum neutralizing antibodies, IFN-g concentration and intensity of MIF response.
RESUMO
Efeito do bacilo de Calmette-Guérin, Avridina e Propionibacterium acnes como imunomoduladores na raiva em camundongos
Avaliou-se a resposta imune celular e humoral de camundongos inoculados com vírus rábico de rua e submetidos aos imunomoduladores Onco-BCG, avridina e Propionibacterium acnes. Os animais submetidos ao tratamento com P.acnes apresentaram um maior percentual de sobrevivência quando comparados aos dos demais tratamentos. Foram observados menores níveis de IFN-g nos animais infectados, sugerindo imunossupressão viral. O teste do Coxim Plantar não foi eficaz para a detecção da resposta de hipersensibilidade retardada na metodologia utilizada, contrariamente ao MIF. A sobrevivência dos animais não apresentou correlação com os níveis de anticorpos soroneutralizantes, concentração de IFN-g e resposta ao MIF.
Correspondence to: Jane Megid,Departamento de Higiene Veterinária e Saúde Pública, FMVZ-UNESP. P.O. Box 560, 18618-000 Botucatu, SP, Brazil. Phone (014)821-2121 R:2270/2191 Fax (014) 8212121 R:2075 Email: JANE@FMVZ.UNESP.BR
Received: 01 October 1998
Accepted: 22 February 1999
- 1. AL-IZZI, S.A & MAXIE, M.G. - Effect of Corynebacterium parvum on bone marrow macrophage colony production, peripheral blood leucocytes, and histologic changes of tissues in calves. Amer. J. vet. Res., 43: 2244-2247, 1982.
- 2. ANDERSON, A.O. & REYNOLDS, J.A. - Adjuvant effects of the lipid amine CP-20,961. J. reticuloendoth. Soc., 26:667-680, 1979.
- 3. BAER, G.M. - Animal models in the pathogenesis and treatment of rabies. Rev. infect. Dis., 10 (suppl.4): 739-750, 1988.
- 4. BARAKAT, A.A.; SABER, M.S.; EMAD, N. et al. - Preliminary studies on the use of BCG as an immunopotentiating agent against Rift Valley Fever among sheep in Egypt. Bull. Off. int. Epizoot., 93: 1387-1393, 1981.
- 5. BLANCOU, J.; ANDRAL, L.; LAGRANGE, P.H. & TSIANG, H. - Kinetics of different specific immunological parameters after rabies vaccination in mice. Infect. Immun., 24: 600-605, 1979.
- 6. CELIS, E.; MILLER, R.W.; WIKTOR, T.J.; DIETZSCHOLD, B. & KOPROWSKI, H. - Isolation and characterization of human T cell lines and clones reactive to rabies virus: antigen specificity and production of interferon-g. J. Immunol., 136: 692-697, 1986.
- 7. CHERWINSKI, H.; SCHUMACHER, J.; BROWM, K. & MOSMANN, F. - Two types of mouse helper cell clone. 3. Further differences in limphokynes synthesis between tH1 and tH2 clones revelated by DNA hybridization functionally monospecific bioassay and monoclonal antibody. J. exp. Med., 166: 1229-1244, 1987.
- 8. CHO, S.; NARAHARA, H.; MIFUNE, K. & KAWAI, A. - Murine T cell clones directed to rabies virus: isolation and some of their properties. J. gen. Virol., 68: 1115-1123, 1987.
- 9. CÔRTES, J.A.; RWEYEMAMU, M.M.; ITO, F.H. et al. - Immune response of cattle induced by inactivated rabies vaccine adjuvanted with either aluminium hidroxide alone or additionally with avridine. Rev. Sci. Téch.,12: 941-955, 1993.
- 10. COX, W.I. - Examining the immunologic and hematopoietic properties of an immunostimulant. Vet. Med., 83: 424-428, 1988.
- 11. DEFAVERI, J.; REZKALLAH-IWASSO, M.T. & FRANCO, M.F. - Experimental pulmonary paracoccidioidomycosis in mice: morphology and correlations of lesions with humoral and cellular immune responses. Mycopathologia (Den Haag), 77: 3-11, 1982.
- 12. DESIDERIO, J.D & RANKIN, B.M. - Imunomoduladores. In: KIRK,R.W. Atualizaçăo Terapęutica Veterinária Săo Paulo, Manole, 1988. v.1, p.1379-1385.
- 13. FAVORETTO, S.R.; CARRIERI, M.L.; TINO, M.S.; ZANETTI, C.R. & PEREIRA, O.A.C. - Simplified fluorescence inhibition microtest for the titration of rabies neutralizing antibodies. Rev. Inst. Med.trop. S. Paulo, 35: 171-175, 1993.
- 14. GERMANO, P.M.L.; SILVA, E.V.; SILVA, E.V.; CORDEIRO, C.F. & PRETO, A.A. - Vacina anti-rábica PV/BHK com avridine como adjuvante. Avaliaçăo da eficácia em camundongos. Arq. Biol.Tecnol, 33: 865-878, 1990.
- 15. GLASGOW, L.A.; FISCHBACH, J.; BRYANT, S.M. & KERN, E.R. - Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille Calmett-Guérin. J. infect. Dis., 135: 763-770, 1977.
- 16. GOLDWASSER, R.A. & KISSLING, R.E. - Fluorescent antibody staining of street and fixed rabies virus antigens. Proc. Soc. exp. Biol. (N. Y.), 98: 219-223, 1958.
- 17. HOFFMAN, W.W.; KORST, J.J.; NIBLACK, J.F. & CRONIN. T.H. - N, N-dioctadecyl-N', N'-bis (2-hydroxyethyl) propanediamine: antiviral activity and interferon stimulation in mice. Antimicrob. Agents Chemother., 3: 498-502, 1973.
- 18. KAWANO, H.; MIFUNE, K.; OHUCHI, M. et al. - A. protection against rabies in mice by a cytotoxic T cell clone recognizing the glycoprotein of rabies virus. J gen. Virol., 71: 281-287, 1990.
- 19. KOPROWSKI, H. - Prueba de inoculacion al raton. In: KAPLAN, M.M. & KOPROWSKI, H. La rabia, tecnicas de laboratorio 3.ed. Ginebra, Organizacion Mundial de la Salud, 1976. p.88-97.
- 20. LAGRANGE, P.H.; TSIANG, H.; HURTREL, B. & RAVISSE, P. - Delayed-type hypersensitivity to rabies virus in mice: assay of active or passive sensitization by the footpad test. Infect. Immun., 21: 931-939, 1978.
- 21. LOPES, C.A.C.& SANTANA, C.F. - Novas observaçőes sobre o emprego do Corynebacterium parvum em pacientes portadores de doenças infecciosas. Pernambuco, Ministério da Educaçăo e Cultura, Universidade Federal de Pernambuco, 1984.
- 22. MÜLLER-BRUNECKER, G.; ERFLE, V. & MAYR, A. - Comparison of the effect of viral paramunity inducers PIND-AVI and PIND-ORF with that of BCG, Corynebacterium parvum and Levamisole on the growth of radiation-induced murine osteosarcoma. Zbl. Vet. Med. (B), 33: 188-195, 1986.
- 23. NIBLACK, J.F.; OTTERNESS, I.G.; HEMSWORTH, G.R. et al. - CP-20,961: a structurally novel, synthetic adjuvant. J. reticuloendoth. Soc., 26: 655-666, 1979.
- 24. NUNBERG, J.H.; DOYLE, M.V.; YORK, S.M. & YORK, C.J. - Interleukin 2 acts as an adjuvant to increase the potency of inactivated rabies virus vaccine. Proc. nat. Acad. Sci. (Wash), 86: 4240-4243, 1989.
- 25. PERRIN, P.; JOFFRET, M.L.; LECLERC, C. et al. - Interleukin 2 increases protection against experimental rabies. Immunobiology, 177: 199-209, 1988.
- 26. SANTANA, C.F.; ASFORA, J.J.; LINS, L.P.; LOPES, C.A.C. & SANTOS, E.R. - Efeitos imunoestimulantes do Corynebacterium parvum em pacientes portadores de neoplasias malígnas. Rev. Inst. Antibiót. (Recife), 19: 137, 1979.
- 27. SMITH, J.S.; McCLELLAND, C.L.; REID, F.L. & BAER, G.M. - Dual role of the immune response in street rabiesvirus infection of mice. Infect. Immun., 35: 213-221, 1982.
- 28. SPENCER, J.C.; GANGULY, R. & WALDMAN, R.H. - Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacillus Calmette-Guérin. J. infect. Dis., 136: 171-175, 1977.
- 29. TIZARD, I. - Use of immunomodulators as an aid to clinical management of feline leukemia virus-infected cats. J. Amer. vet. Med. Ass., 199: 1482-1485, 1991.
- 30. TIZARD, I. - Round-table discussion. EqstimŽ immunostimulant. 37 Annual AAEP Meeting, 1992. J. Equine vet. Sci., 12: 209-214, 1992.
- 31. TSIANG, H. & LAGRANGE, P.H. - In vivo detection of specific cell-mediated immunity in street rabies virus infection in mice. J. gen. Virol., 47: 183-191, 1980.
- 32. VAIL, C.D.; NESTVED, A.J.; ROLLINS. B.J et al. - Adjunct treatment of equine respiratory disease complex (ERDC) with the Propionibacterium acnes, immunostimulant, EqStimŽ J. Equine vet. Sci., 10: 399-403, 1990.
- 33. WIKTOR, T.J.; DOHERTY, P.C.& KOPROWSKI, H. - In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc. nat. Acad. Sci. (Wash),74: 334-338, 1977b.
- 34. WIKTOR, T.J.; DOHERTY, P.C. & KOPROWSKI, H. - Suppression of cell-mediated immunity by street rabies virus. J. exp. Med., 145: 1617-1622, 1977a.
- 35. ZAR, H. - Biostatistical analysis 2.ed. Englewood Cliffs, Prentice-Hall, 1984.
Publication Dates
-
Publication in this collection
02 July 1999 -
Date of issue
Mar 1999
History
-
Accepted
22 Feb 1999 -
Received
01 Oct 1998