Abstract
Ocotea notata (Lauraceae) is popularly known as white-cinnamon. Ocotea species have several medicinal uses, especially for treating chest pain, rheumatism and wounds. The present study aimed to analyze the chemical composition of O. notata n-hexane fraction, in addition to its anti-mycobacterial and immunomodulatory activities. The n-hexane fraction was analyzed by GC-MS and was chromatographed to afford 15 subfractions (SF1-15), where SF5 was identified, by GC-MS and NMR, as the sesquiterpene spathulenol. The n-hexane fraction was the most potent in inhibiting nitric oxide (NO) and tumor necrosis factor-alpha (TNF-α) production on LPS-stimulated macrophages (IC50 8.3 ± 0.9 and 5.9 ±1.0 μg/mL, respectively). SF4, a major subfraction, that presents a spathulenol analogous as a constituent, also inhibited NO and TNF-α production. Spathulenol only modulated NO production (IC50 45.6 ± 1.4 μg/mL). The n-hexane fraction, SF4, and spathulenol revealed antimycobacterial activity against Mycobacterium bovis BCG, M. tuberculosis H37Rv, and M299 strains. Spathulenol inhibited the growth of Mtb H37Rv with MIC50 36.9 ± 1.5 μg/mL (167.5 ± 6.8 μM), and Mtb M299 with MIC5042.1 ± 0.5 μg/mL (191.0 ± 2.2 μM). This is the first report describing the isolation of spathulenol from O. notata leaves and its anti-mycobacterial activity.
Key words:
antiinflammatory; anti-mycobacterial;
Ocotea notata
; Sesquiterpenes
Resumo
Ocotea notata (Lauraceae) é popularmente conhecida como canela-branca. As espécies do gênero Ocotea têm vários usos medicinais, especialmente no tratamento de dores no peito, reumatismo e feridas. O presente estudo teve como objetivo analisar a composição química da fração n-hexano de O. notata, além de suas atividades antimicobacteriana e imunomoduladora. A fraçao n-hexano foi analisada por GC-MS e fracionada fornecendo 15 subfrações (SF1-15), onde SF5 foi identificado, por GC-MS e RMN, como o sesquiterpeno espatulenol. A fração n-hexano foi a mais potente na inibição da produção de óxido nítrico (NO) e do fator de necrose tumoral-alfa (TNF-α) em macrófagos estimulados por LPS (IC50 8,3 ± 0,9 e 5,9 ±1,0 μg/mL, respectivamente). SF4, urna subfração majoritária, que apresenta um análogo do espatulenol como constituinte, também inibiu a produção de NO e TNF-α. O espatulenol modulou apenas a produção de NO (IC50 45,6 ±1,4 μg/mL). A fração n-hexano, SF4 e espatulenol apresentaram atividade antimicobacteriana contra as cepas de Mycobacterium bovis BCG, M. tuberculosis (Mtb) H37Rv e M299. O espatulenol inibiu o crescimento de Mtb H37Rv com MIC50 36,9 ± 1,5 μg/mL (167,5 ± 6,8 μM) e Mtb M299 com MIC50 42,1 ± 0,5 μg/mL (191,0 ± 2,2 μM). Este é o primeiro relato que descreve o isolamento do espatulenol das folhas de O. notata e sua atividade antimicobacteriana.
Palavras-chave:
anti-inflamatório; antimicobacteriano;
Ocotea notata
; Sesquiterpenes
Introduction
Tuberculosis (TB) is a major cause of death worldwide aggravated by the emergence of Mycobacterium tuberculosis (Mtb) multidrug-resistant (MDR-TB) strains. The World Health Organization reported 10 million new TB cases in 2017, with a high mortality rate in developing countries (WHO 2018WHO - World Health Organization (2018) Global tuberculosis report 2018. World Health Organization, Geneva. Available at <http://www.who.int/tb/publications/global_report/en/>. Access on May 20, 2019.
<http://www.who.int/tb/publications/glob...
). The long treatment duration of at least 6 months and complex regimens which involve expensive and toxic drugs hinder improvement in therapy outcomes (Dartois 2014Dartois V (2014) The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nature Review Microbiology 12: 159-167.).
Uncomplicated drug-sensitive pulmonary TB treatment is based on the use of multiple antibiotics. However, severe destructive and disseminated forms of TB such as meningitis and tuberculosis pericarditis require anti-inflammatory adjunct therapy to prevent excessive inflammation (Zumla et al. 2014Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, Mchugh TD, Schito M, Maeurer M & Nunn AJ (2014) New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. The Lancet Infectious Diseases 14: 327-340.).
The search for new molecules with dual anti-inflammatory and anti-mycobacterial activity has been encouraged for severe pulmonary TB therapy and 70% of the antibiotics in use are natural products or are derived from them (Singh & Macdonald 2010Singh B & Macdonald CA (2010) Drug discovery from uncultivable microorganisms. Drug Discovery Today 15: 17-18.).
Ocotea notata (Nees & Mart.) Mez is a medium-sized tree belonging to the Lauraceae family, popularly known as white-cinnamon. Within the family, Ocotea genus presents the highest number of medicinal species, being used to treat chest pain, rheumatism, and cutaneous wounds, among others (Marques 2001Marques CA (2001) Importância econômica da familia Lauraceae Lindl. Floresta e Meio ambiente 8: 195.; Neto & Morais 2003Neto GG & Morais GR (2003) Recursos medicinais de especies do cerrado de Mato Grosso: um estudo bibliográfico. Acta Botanica Brasilica 17: 561-584.).
Species of the Ocotea genus have several activities reported in the literature such as antiinflammatory (Zschocke et al. 2000Zschocke S, Drewes SE, Paulus K, Bauer R & Van Staden J (2000) Analytical and pharmacological investigation of Ocotea bullata (black stinkwood) bark and leaves. Journal of Ethnopharmacology 71: 219-230.; Madubanya et al. 2005Madubanya LA, Jäger AK, Makunga NP, Geldenhuys CJ & Van Staden J (2005) DNA fingerprinting and anti-inflammatory activity of Ocotea bullata bark from different locations. South African Journal of Botany 71: 38-44.), antioxidant (Bruni et al. 2004Bruni R, Medici A & Andreotti E (2004) Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chemistry 85: 415-421.; Guerrini et al. 2006Guerrini A, Sacchetti G & Muzzoli M (2006) Composition of the volatile fraction of Ocotea bofo Kunth (Lauraceae) calyces by GC-MS and NMR fingerprinting and its antimicrobial and antioxidant activity. Journal of Agricultural and Food Chemistry 54: 7778-7788.), antiprotozoal (Fournet et al. 2007Fournet A, Ferreira ME, Rojas de Arias A, Guy I, Guinaudeau H & Heinzen H (2007) Phytochemical and antiprotozoal activity of Ocotea lancifolia. Fitoterapia 78: 382-384.), anti-allergic (Serra et al. 1997Serra MF, Diaz BL & Barreto EO (1997) Anti-allergic properties of the natural PAF antagonist yangambin. Planta Medica 63: 207-212.), central nervous system depressant (Pachú et al. 1993Pachú CO, Almeida RN & Barbosa-Filho JM (1993). Atividade depressora do sistema nervoso central pela Iangambina. Ciencias Cultura Saúde 12: 14-16.), antimicrobial (Bruni et al. 2004Bruni R, Medici A & Andreotti E (2004) Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chemistry 85: 415-421.; Souza et al. 2004Souza GC, Haas APS, Von Poser GL, Schapoval EES & Elisabetsky E (2004) Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. Journal of Ethnopharmacology 90: 135-143.) and anti-herpetic (Dias et al. 2003Dias CS, Silva IG, Cunha EVL, Silva MS, Braz- Filho R & Barbosa-Filho JM (2003) Isolamento e identificação de novos alcaloides de Ocotea duckei Vattimo (Lauraceae). Revista Brasileira de Farmacognosia 13:62-63.), and have been studied for their diversity in secondary metabolites such as alkaloids, neolignans, lignans, terpenes, and flavonoids (Dias et al. 2003Dias CS, Silva IG, Cunha EVL, Silva MS, Braz- Filho R & Barbosa-Filho JM (2003) Isolamento e identificação de novos alcaloides de Ocotea duckei Vattimo (Lauraceae). Revista Brasileira de Farmacognosia 13:62-63.; Zanin & Lordello 2007Zanin SM W & Lordello ALL (2007) Aporphine alkaloids in Ocotea species (Lauraceae). Química Nova 30: 92-98.; Barbosa-Filho et al. 2008Barbosa-Filho JM, Cunha RM & Dias CSI (2008) GC-MS analysis and cardiovascular activity of the essential oil of Ocotea duckei. Revista Brasileira de Farmacognosia 18: 37-41.; Funasaki et al. 2009Funasaki M, Lordello ALL & Viana AM (2009) Neolignans and sesquiterpenes from leaves and embryogenic cultures of Ocotea catharinensis (Lauraceae). Journal of the Brazilian Chemical Society 20: 853-859.; Cuca et al. 2009Cuca LE, Leon P & Coy ED (2009) A bicyclo[3.2.1] octanoid neolignan and toxicity of the ethanol extract from the fruit of Ocotea heterochroma. Chemistry of Natural Compounds 45: 179-181.; Garrett et al. 2012Garrett R, Romanos MTV, Borges RM, Santos MG, Rocha L, Silva AJR (2012) Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Revista Brasileira de Farmacognosia 22: 306-313.).
We had previously shown that the crude ethanol extract of Ocotea notata leaves presented anti-mycobacterial activity and NO production inhibitory effect on LPS-stimulated RAW 264.7
macrophages. Moreover, two isolated flavonoids from ethyl acetate fraction, kaempferol-3-O-β-L-ramnopyranoside (afzelin), and quercetin-3-O-β-D-glucopyranoside (isoquercitrin) were shown to contribute to suppressing NO production, although they did not inhibit mycobacterial growth (Costa et al. 2015Costa IFJB, Calixto SD, Araujo MH, Konno TUP, Wanderley LT, Guimarães DO, Lasunskaia EB, Leal IRC & Muzitano MF (2015) Antimycobacterial and nitric oxide production inhibitory activities of Ocotea notata from Brazilian Restinga. The Scientific World Journal 2015: 1-9.).
Thus, the aim of the present study was to continue our efforts in the study of Ocotea notata, collected in the Restinga of Jurubatiba (Rio de Janeiro state, Brazil). Specifically, to investigate the chemical composition of its n-hexane fraction, associated with anti-mycobacterial and immunomodulatory activities. The n-hexane fraction stood out as an anti-mycobacterial promising fraction and was not studied before.
Materials and Methods
Plant material
Leaves of Ocotea notata were collected in the early morning, on a sunny day, at Parque Nacional da Restinga de Jurubatiba, Quissamã, Rio de Janeiro, Brazil, under legal authorization (SISBIO 39673-2, SisGenAAA989F) at March 2011. A voucher specimen (RFA38751) was deposited at the herbarium of the Universidad Federal do Rio de Janeiro (Brazil).
Extraction and isolation
Fresh O. notata leaves (1.3 kg) were triturated and extracted exhaustedly with ethanol ACS at room temperature (crude extract). An aliquot (60.0 g) of the dried extract (78.1 g) was resuspended with methanol and partitioned with n-hexane to obtain the n-hexane fraction (26.7 g). An aliquot of n-hexane fraction (1.5 g) was chromatographed and submitted to column chromatography on a Si gel 60 (40-63 microns) (SILICYCLE®) (60 cm in height and 2.7 cm in diameter) and then eluted with an increasing gradient varying in between n-hexane/AcOEt and MeOH (0–100%) in steps of 10% (40 mL each), and separated into 15 subfractions on the basis of TLC analyses revealed with vanillin followed by sulfuric acid. The evaluation of the biological properties was restricted to n-hexane fraction, and subfractions SF2, SF4, SF5, SF7, and SF15 due to their amount, solubility and toxicity.
Subfraction 5, (SF5, 11 mg, eluted with EtOAc/Hex, 9:1) was identified as a pure compound by TLC and was submitted to NMR and GC-MS analyses.
Analysis by gas chromatography coupled to mass spectrometry
Fraction, subfraction, and isolated compound were submitted to GC-MS performed on Shimadzu GC-MS 2010 equipment using capillary columns of fused silica RTX-5MS (30 m × 0.25 urn) (Restek Corporation Pennsylvania, USA). The column temperature was kept at 60 °C for 1 min and was subsequently increased to 280 °C at a rate of 15 °C/min and held for 10 min. Helium was used as carrier gas with a flow rate of 1.1 mL/min. One milligram of the SF5 pure fraction was dissolved in n-hexane and 1 μL was injected with the aid of an autosampler. Chromatogram peaks were tentatively identified by comparing their mass spectra with those of the National Institute of Standards and Technology (NIST) library and those reported in the literature (Adams 2007Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Allured Pub. Corp, Carol Stream. 804p.). All hits that presented similarity ≥ 90% were considered and checked manually. In addition, the identity of spathulenol was confirmed by comparison of its retention time and full scan mass spectrum with the isolated sample. Furthermore, sub-fractions were compared to n-hexane fraction due to their chromatograms, considering peaks retention time and full scan mass spectrum.
Nuclear magnetic resonance
The isolated spathulenol compound was analyzed by mono and two-dimensional 1H and 13C NMR spectra and recorded on a Bruker-DRX-400 NMR spectrometer (1H: 400 MHz; 13C: 100 MHz) of the Instituto de Pesquisas de Produtos Naturais Walter Mors (IPPN/UFRJ).
Mycobacterial culture and antimycobacterial activity
Three mycobacterial strains differing in virulence were used in this study: an avirulent Mycobacterium bovis Bacillus Calmette-Guérin (BCG), Moreau vaccine strain, and two Mycobacterium tuberculosis strains (low virulent laboratory strain H37Rv, ATCC 27294, and highly virulent Mtb Beijing strain M299, isolated from a TB patient in Mozambique) evaluated for virulence in a previous study (Ribeiro et al. 2014Ribeiro SCM, Gomes LL, Amaral EP, Andrade MRM, Almeida FM, Rezende AL, Lanes VR, Carvalho ECQ, Suffys PN, Mokrousov I & Lasunskaia EB (2014) Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. Journal Clinical Microbiology 52: 2615-2624.). Mycobacterial strains were grown in suspension in 7H9 Middlebrook broth containing 10% albumin dextrose complex (ADC) and 0.05% Tween-80 at 37 °C under Biosecurity level 3 containment conditions. The hexane fraction, SF2, SF4, SF7, SF15, and spathulenol were evaluated for their
antimycobacterial activity using an MTT assay (tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazole, Sigma Aldrich®) in a 96-well plate. During the logarithmic growth phase, 50 uL of bacterial suspensions were plated at 1 × 106 CFU/well and incubated with 50 uL of samples at concentrations of 4 to 500 μg/mL. The sealed plate was incubated at 37 °C and 5% CO2 for 7 days for Mbv BCG and 5 days for the other strains. After this period, the bacterial culture was then incubated for 3 h with 10 uL of tetrazolium salt (5 mg/mL in sterile phosphate-buffered saline [PBS]) and then 100 uL of lyses buffer (20% w/v SDS/50% DMF - dimethylformamide in distilled water - pH 4.7) were added (Gomez-Flores et al. 1995Gomez-Flores R, Gupta S, Tamez-Guerra R & Mehta RT (1995) Determination of MICs for Mycobacterium avium - M. intracellulare complex in liquid medium by a colorimetric method. Journal of Clinical Microbiology 33: 1842-1846.). The plate was incubated overnight, and the reading was made using a spectrophotometer at 570 nm. A bacterial culture treated with antibiotic rifampin (Sigma Aldrich®, 95% purity) was used as a positive control, while an untreated bacterial suspension was used as a negative control.
Cell culture, treatments, and quantification of Nitric Oxide and TNF-α production
RAW 264.7 cells obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) were cultured in Dulbecco Modified Eagle medium (DMEM-F12) with 10% fetal bovine serum and gentamicin (50 μg/mL) in the presence of 5% CO2 at 37 °C. The cells were seeded in 96--well plates (1 × 105 cells/well) in the presence or absence of samples (4 to 500 μg/mL) and/or lipopolysaccharide (LPS-Escherichia coli 055:B5; SigmaAldrich). A NO inhibitor (NG-methyl-L-arginine acetate salt - L-NMMA, Sigma Aldrich®, 98% purity) was used as a positive control of NO inhibition at 20 μg/mL (32.7 ± 0.9 μM/reducing NO production by 52.5 ± 1.3%) in the experiments. Culture supernatants were collected for NO and TNF-α assays after 24 h. The nitrite concentration, a stable NO metabolite, was determined using the Griess method (Park et al. 2009Park PH, Kim HS, Jin XY, Jin F, Hur J, Ko G & Sohn DH (2009) KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. European Journal of Pharmacology 606: 215-224.). TNF-α a was measured by an L929 fibroblast bioassay, based on the sensitivity of L929 cells to the cytotoxic effect of TNF-α. To do so, the L929 cells were seeded in 96-well plates (2 × 105 cells/well). The resulting cell monolayers were treated with the macrophage culture supernatants in the presence of actinomycin D (2 μg/mL) after 24 h of incubation. Then, after 24 h of additional incubation, the viability of the L929 cells was assayed by the MTT assay (Mosmann 1983Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65: 55-63.). The cytokine concentration was determined using a recombinant mouse cytokine to obtain a standard curve to correlate cellular viability and TNF-α concentration.
Macrophage cytotoxicity assay
Cytotoxic effects of samples on RAW 264.7 cell viability in cultures stimulated with LPS were determined using the MTT assay (Mosmann 1983Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65: 55-63.). A cell culture was obtained as previously described and evaluated using tetrazolium salt MTT. The optical density was measured at 570 nm employing a microplate reader after incubation for 2 h with MTT solution (5 mg/ml in sterile PBS). Cytotoxicity was calculated by subtracting the ratio of the mean absorbance value for treated cells from the mean absorbance value for non-treated cells. The cytotoxicity percentage was calculated in relation to the negative control (untreated macrophages) and stimulated, and to the positive control (stimulated macrophages) culture treated with 1% (v/v) Triton X-100. Final concentrations of dimethyl sulfoxide (DMSO) were used as the solvent of the samples and were tested in parallel as a control.
Statistical analysis
The tests were performed in triplicate and values were reported as the mean ± standard error of the mean (SEM). Statistical analyses were performed by one-way ANOVA, followed by Tukey post-test, employing GraphPad Prism 4 software. The outcomes were considered significant for p < 0.05. The IC50 and MIC50 values were calculated by non-linear regression.
Results and Discussion
The phytochemical profile of the n-hexane fraction from O. notata extract and its subfractions were investigated in the present study. Furthermore, their anti-mycobacterial and immunomodulatory potential and cytotoxicity were evaluated, as well as for the isolated sesquiterpene spathulenol.
The permeability of mycobacteria to compounds is controlled by an outer lipid barrier based on a monolayer of characteristic mycolic acids and a capsule-like coat of polysaccharide and protein. The mycolate layer prevents entry of small hydrophilic molecules, which obtain access to the cell by way of pore-forming proteins resembling porins of Gram-negative bacteria. More lipophilic molecules can diffuse through the lipid layer (Draper 1998Draper P (1998) The outer parts of the Mycobacterial envelope as permeability barriers. Frontiers in Bioscience 3: 1253-1261.). The mycobacterial cell wall has
a high permeability to hydrophobic compounds (Lee et al. 2013Lee SH, Choi M, Kim P & Myung PK (2013) 3D-QSAR and Cell Wall Permeability of Antitubercular nitroimidazoles against Mycobacterium tuberculosis. Molecules 18: 13870-13885.), which encourages investigation of the n-hexane fraction, a non-polar, commonly composed by terpenes, sterols and fatty acids (Cantrell et al. 2011Cantrell CL, Franzblau SG & Fischer NH (2001) Antimycobacterial plant terpenoids. Planta Medica 67: 685-694.).
GC-MS analyses were conducted in order to identify putative active compounds present within the O. notata n-hexane fraction. Then, 11 derivatives were identified in comparison to NIST library and to literature data (Adams 2007Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Allured Pub. Corp, Carol Stream. 804p.). The major components identified in the n-hexane fraction of O. notata leaves were the terpenes: (Z)-epi-β-santalol (17.98%), (Z)-α-santalol (10.27%), caryophyllene oxide (8.19%) and spathulenol (10.79%), with a similarity of 94, 90, 92 and 93%, respectively, to the NIST library; and in accordance to the literature data (Adams 2007Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Allured Pub. Corp, Carol Stream. 804p.). Table 1 presents the proposed identity for n-hexane fraction compounds.
Chemical composition of n-hexane fraction from Ocotea notata by gas chromatography coupled with mass spectrometer GC-MS.
The essential oil of O. notata leaves was analyzed in previous studies by GC-MS and 12 compounds were identified, accounting for 83.3% of the total components present in the essential oil. Germacrene A (22.7%) and β-caryophyllene (22.9%) sesquiterpenes and α-pinene, β-pinene, and terpinolene (8.7, 6.9 and 5.5%, respectively) monoterpenes were the main metabolites identified. The brine shrimp (Artemia salina) lethality test was performed and showed high toxicity profile for this oil with an LC50 value of 2.4 μg/ml (Garret et al. 2010Garret R, Cruz RAS, Rocha L, Santos MG & Silva AJR (2010) Chemical Composition and Toxicity of Ocotea notata (Nees) Mez Essential Oil. Journal of Essential Oil-Bearing Plants 13: 455-459.).
The chemical composition of extracts, fractions, and essential oil could vary among species, in the same species and in different plant parts. Several factors influence the production of secondary metabolites, such as salinity, temperature, harvest season, soil quality, and other factors (Gobbo-Neto & Lopes 2007Gobbo-Neto L & Lopes NP (2007) Plantas medicinais: fatores de influencia no conteúdo de metabólites secundários. Química Nova 30: 374-381.).
Then, SF2, SF4, and SF5, which are subfractions obtained from n-hexane fraction, were analyzed by GC-MS because they were the most active samples, considering anti-mycobacterial and immunomodulatory assays, as could be seen below. For subfractions, in addition to NIST information, GC-MS data were also analyzed considering the comparison of retention time and mass spectrum with those obtained for the n-hexane fraction. The major identified constituents in these subfractions were SF2: (Z)-α-santalol (11.74%) and spathulenol (12.81%), in Table 2; SF4: aromadendrane-type sesquiterpenoid (37%), in Table 3. SF5 presented the highest degree of purity. It was analyzed by GC-MS and sesquiterpene spathulenol (tR 10.115 min), previously assigned in the chromatogram of the n-hexane fraction, was identified as the major volatilized component of this subfraction (99% of the relative area in GC-MS chromatogram and 90% of similarity) (Fig. 1).
a. Chromatogram of the SF5 obtained by gas chromatography coupled with mass spectrometer (GC-MS). b. Mass spectrum of the major peak at tR 10.115 min, spathulenol.
Chemical composition of SF2 fraction from Ocotea notata by gas chromatography coupled with mass spectrometer GC-MS.
Chemical composition of SF4 fraction from Ocotea notata by gas chromatography coupled with mass spectrometer GC-MS.
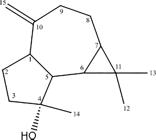
The sample was submitted to 1H and 13C NMR spectroscopy analysis to support structural elucidation. 13C NMR spectrum was decisive to the characterization of spathulenol (1) (Fig. 2), that was completed by comparison to the literature data (Inagaki & Abe 1985Inagaki F & Abe A (1985) Analysis of 1H and 13C nuclear magnetic resonance spectra of spathulenol by two-dimensional methods. Journal of the Chemical Society (Perkin Transactions 2) 11: 1773-1778.; Farias et al. 2019Farias ALF, Rodrigues ABL, Martins RL, Rabelo ÉM, Farias CWF & Almeida SSMS (2019) Chemical characterization, antioxidant, cytotoxic and microbiological activities of the essential oil of leaf of Tithonia diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals (Basel) 12: 34.). The 13C NMR spectrum showed fifteen carbon signals characteristic of an aromadendrane type sesquiterpene, as listed below. It is important to highlight the two carbons signals of the exocyclic double-bond, C-10 (δ 153.3 ppm) and C-15 (δ 106.3 ppm); and the one signal characteristic of a sesquiterpene alcohol (C-4; δ 81.0 ppm).
Spathulenol (1): Colorless oil; purity 99% (GC/ MS); tR 10.115 min; NMR data: 13C NMR (100 MHz, DMSOd6): S ppm 53.5 (C-1), 26.7 (C-2), 41.8 (C-3), 81.0 (C-4), 54.4 (C-5), 29.9 (C-6), 27.5 (C-7), 24.8 (C-8), 38.9 (C-9), 153.3 (C-10), 20.3 (C-11), 28.7 (C-12), 16.3 (C-13), 26.1 (C-14), 106.3 (C-15).
The n-hexane fraction and its subfractions (SF2, SF4, SF5, SF7, and SF15) were initially evaluated for their immunomodulatory activity through evaluation of the inhibitory effect on the production of NO and TNF-α by LPS-stimulated RAW 264.7 macrophages. NO is an important chemical mediator that has several physiologic functions such as immunomodulatory action in response to many immune cells and microbicide activities during inflammatory responses. However,
the increased levels of NO have a deleterious role leading to tissue damage and must be tightly regulated (Guzik et al. 2003Guzik TJ, Korbut R & Adamek-Guzik T (2003) Nitric oxide and superoxide in inflammation and immune regulation. Journal of Physiology and Pharmacology 54: 469-487.; Garlanda et al. 2007Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C & Caccamo N (2007) Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1 -related receptor, a negative regulator of IL-1/ TLR signaling. The Journal of Immunology 179: 3119-3125.).
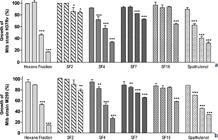
The n-hexane fraction from O. notata leaves was the most potent in inhibiting NO production on LPS-stimulated macrophages when compared to control groups. Its inhibitory capacity was of 72.2 ± 1.6% at 100 μg/mL, and at 20 μg/mL inhibited 55.9 ± 1.5% of the NO production, with IC50 of 8.3 ± 0.9 μg/mL (Fig. 3a; Tab. 4).
a-c. Effect of n-hexane fraction, subfractions and spathulenol from Ocotea notata on NO and TNF-α production by LPS-stimulated RAW 264.7 macrophages and evaluation of cytotoxicity by MTT test. RAW 264.7 macrophages were treated with LPS (1 μg/mL) in the presence of samples (4, 20, 100 and 500 μg/mL) – a. NO production as concentration of nitrite. Untreated cells were used as a negative control (C-) and cells treated with LPS only were used as a positive control of macrophage stimulation (50.4 ± 0.5 μg/mL). Treatment with L-NMMA was used as a positive control of NO inhibition, reducing NO production by 52.5 ± 1.3 at 20 μg/mL; b. TNF-α production was measured using a L929 fibroblast bioassay. As a negative control it was used macrophages not treated and not LPS stimulated and as positive control macrophages stimulated with 1 mg/mL LPS and not treated (1395.6 ±1.2 μg/ mL); c. The cytotoxicity was evaluated by mitochondrial reduction of MTT to formazan and toxicity percentage was calculated in relation to the negative control (untreated macrophages, 2.2 ± 0.4%; O. D. 1.596) and to the positive control (stimulated macrophages culture treated with 1% (v/v) Triton X-100 (100.1 ± 3.6%; O.D. 0.128). * =p < 0.05 , ** =p < 0.01; *** =p < 0.001 in relation to untreated group.
Minimum inhibitory concentration of n-hexane fraction, sub-fractions, and spathulenol from Ocotea notata on the production of NO and TNF-α by LPS-stimulated RAW 264.7 macrophages, and evaluation of cytotoxicity by MTT test.
Among the studied subfractions, the more pronounced inhibitory activity was presented by SF4 and followed by SF2. SF4 particularly demonstrated higher inhibitory capacity on NO production (IC50 15.6 ± 1.1 μg/mL) than SF2 (IC50 37.7 ±1.1 μg/mL), although the latter inhibited 51.7 ± 1.3% of the NO production at the 100 μg/mL. The SF15 and SF7 subfractions showed the worst profiles (higher IC50 values). Spathulenol (SF5) inhibited 50.9 ± 1.0% of NO production at 100 μg/mL, but the same was not observed in lower concentrations (20 and 4 μg/mL).
The spathulenol IC50 for NO inhibitory activity was calculated as 45.6 ± 1.4 μg/mL, equivalent to 206.0 ± 4.5 μM (Fig. 3a; Tab. 4).
When compared to L-NMMA, a known NO inhibitor used as a positive control (NO inhibition by 52.5 ± 1.3% at 20 μg/mL), the n-hexane fraction and SF4 presented higher inhibitory activity on NO production and the n-hexane fraction was two times more active. According to the chemical profile, the major identified constituent of SF4 is an aromadendrane-type sesquiterpenoid (a spathulenol analogous), and it probably contributes to SF4 activity. Twenty-seven (27) azulenes have been reported to inhibit NO production in LPS-stimulated macrophages RAW 264.7 (Hashiba et al. 2004Hashibal K, Yokoyama K, Wakabayashi H, Hashimoto K, Satoh K, Kurihara T, Motohashi N & Sakagami H (2004) Inhibition of LPS-stimulated NO Production in Mouse Macrophage-like Cells by Azulenes. Anticancer Research 24: 3939-3944.). The different compounds present in the SF2 and SF4 subfractions, with emphasis on the aromadendrane-type sesquiterpenoids as spathulenol, are associated with the inhibitory activity on NO production observed for the n-hexane fraction. Spathulenol showed antiinflammatory activity in mice ear edema produced by 12-O-tetradecanoylphorbol-13-acetate (TPA) (Aguilar-Guadarrama & Rios 2004Aguilar-Guadarrama AB & María Yolanda Rios (2004) Three New Sesquiterpenes from Croton arboreous. Journal of Natural Products 67: 914-917.)
Thus, combining the results obtained herein with our previously reported data, it is possible to suggest that the inhibitory activity observed for the crude extract of O. notata on NO production might be related to an additive or synergistic effect between compounds present in n-hexane fraction, such as spathulenol, with other compounds (isoquercitrin and afzelin) present in ethyl acetate fraction (Costa et al. 2015Costa IFJB, Calixto SD, Araujo MH, Konno TUP, Wanderley LT, Guimarães DO, Lasunskaia EB, Leal IRC & Muzitano MF (2015) Antimycobacterial and nitric oxide production inhibitory activities of Ocotea notata from Brazilian Restinga. The Scientific World Journal 2015: 1-9.).
Tumor necrosis factor-α (TNF-α) is a cytokine that exhibits potent pro-inflammatory capacity, mainly for its effects on diverse cells including immune cells which lead to the expression of a cascade of downstream chemical mediators. Although TNF-α is involved in
various protective physiological functions and antimicrobial immunity, excessive production of TNF-α is strongly associated with fever, wasting, and tissue injury (Sedger & Mc Dermott 2014Sedger LM & McDermott MF (2014) TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine & Growth Factor Reviews 25: 453-472.).
As seen in Fig. 3b, the n-hexane fraction strongly inhibited TNF-α production by LPS-stimulated macrophages RAW 264.7, 95.3 ± 0.5% at 100 μg/mL and 44.8 ± 1.7% at 20 μg/mL, with IC50 15.9 ± 1.0 μg/mL. A greater inhibitory effect on TNF-α production was only seen for subfraction SF4, IC50 82.1 ± 2.1 μg/mL. SF2, SF7 and SF15 subfractions presented higher IC50 values exhibiting a poor capacity of inhibiting TNF-α production, as also observed for spathulenol (Fig. 3b; Tab. 4). The obtained results showed that the samples were more potent inhibitors of NO than TNF-α and that the SF4 subfraction partially contributes to the inhibitory capacity of the n-hexane fraction.
Anti-inflammatory activity has been reported for some species of the Ocotea genus. Ocotea quixos essential oil and isolated trans-cinnamaldehyde were reported to reduce LPS-induced NO production and COX-2 expression on J774 macrophages and in vivo anti-inflammatory effects in a carrageenan-induced rat paw edema model (Ballabeni et al. 2010Ballabeni V, Tognolini M, Giorgio C, Bertoni S, Bruni R & Barocelli E (2010) Ocotea quixos Lam. essential oil: in vitro and in vivo investigation on its antiinflammatory properties. Fitoterapia 81: 289-295.). It was also reported anti-inflammatory activity for n-hexane extracts of O. bullata on COX expression and, it was showed that n-hexane extracts from O. bullata leaves and bark inhibit in vitro COX-1 and 5-lipoxygenase activity (Zschocke et al. 2000Zschocke S, Drewes SE, Paulus K, Bauer R & Van Staden J (2000) Analytical and pharmacological investigation of Ocotea bullata (black stinkwood) bark and leaves. Journal of Ethnopharmacology 71: 219-230.; Madubanya et al. 2005Madubanya LA, Jäger AK, Makunga NP, Geldenhuys CJ & Van Staden J (2005) DNA fingerprinting and anti-inflammatory activity of Ocotea bullata bark from different locations. South African Journal of Botany 71: 38-44.). Sesquiterpene spathulenol isolated from Salvia mirzayanii (Lamiaceae) methanol extract showed immunomodulatory capacity of inhibiting lymphocyte proliferation (Ziaei et al. 2011Ziaei A, Ramezani M, Wright L, Paetz C, Schneider B & Amirghofran Z (2011) Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects. Phytotherapy Research 25: 557-562.).
The viability of the samples treated by macrophages was measured by the ability of the macrophages to metabolize MTT to formazan in order to exclude the possibility that the inhibitory effects of the studied samples on macrophages were due to their cytotoxicity. The tested samples only showed cytotoxic effect at the highest concentration (500 μg/mL), with an exception for SF7 subfraction which exhibited elevated cytotoxicity levels at 100 μg/mL (IC50 16.9 ± 1.8 μg/mL). Therefore, inhibitory activities described for tested samples were generally not affected by their cytotoxicity to macrophages (Fig. 3c; Tab. 4).
Infectious diseases, including tuberculosis, are highly involved with the inflammatory response. The production of chemical mediators such as NO and TNF-α is generally essential for the immune response of mycobacteria-infected macrophages. Nonetheless, the exacerbated inflammatory response in severe forms of TB contributes to immunopathology, requiring the use of anti-inflammatory adjunct therapy combined with the use of anti-mycobacterial drugs to prevent injury tissue and mortality (Zumla et al. 2014Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, Mchugh TD, Schito M, Maeurer M & Nunn AJ (2014) New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. The Lancet Infectious Diseases 14: 327-340.).
As can be seen in Fig. 4, the n-hexane fraction was notably potent against Mbv BCG. Its ability to inhibit mycobacterial growth at 100 μg/mL was 83.6 ± 1.9%, and the growth inhibition even at 20 μg/mL was of 68.1 ±1.4 %, showing the lowest MIC50 values (MIC50 6.6 ± 0.8 μg/mL) (Tab. 5).
Effect of n-hexane fraction, subfractions and spathulenol from Ocotea notata on growth of Mycobacterium bovis BCG. M. bovis BCG suspensions were treated with samples at concentrations of 4, 20, 100 and 500 μg/mL or rifampicin. Culture medium without bacteria was used as a positive control and culture medium with bacteria and rifampin treated-bacterial culture at concentrations of 0.001-0.03 μg/mL as negative control for M bovis BCG (MIC50 0.01 ± 0.3 μg/mL). * =p < 0.05; ** =p < 0.01; *** =p < 0.001 in relation to untreated group.
Minimum inhibitory concentration of n-hexane fraction, sub-fractions, and spathulenol from Ocotea notata on the growth of Mycobaterium strains.
In comparison with our previous results (Costa et al. 2015Costa IFJB, Calixto SD, Araujo MH, Konno TUP, Wanderley LT, Guimarães DO, Lasunskaia EB, Leal IRC & Muzitano MF (2015) Antimycobacterial and nitric oxide production inhibitory activities of Ocotea notata from Brazilian Restinga. The Scientific World Journal 2015: 1-9.), the n-hexane fraction showed a similar inhibitory activity profile to that seen for the crude extract of O. notata, and more potent than the ethyl acetate fraction on Mbv BCG growth. The SF2 and SF4 subfractions were very active, inhibiting 76.0 ± 1.1% and 69.7 ± 1.7% of Mbv BCG growth at 100 μg/mL, and 54.9 ± 0.5% and 40.6 ± 0.3% at 20 μg/mL, respectively (MIC50 8.2 ± 1.0 μg/mL and MIC50 12.8 ±1.1 μg/ mL). The inhibitory potential of the SF7 and SF15 subfractions were low compared to others (higher IC50 values), while SF7 showed no selectivity for anti-mycobacterial activity, being cytotoxic to macrophages at 100 and 500 μg/mL. Spathulenol
inhibited 55.4 ± 0.4% at 100 μg/mL, although its inhibitory activity was not maintained in lower concentrations, MIC5025.2 ± 1.4 μg/mL (114.3 ± 6.3 μM) (Fig. 4; Tab. 5).
We additionally evaluated the effects of the samples against the laboratory Mtb strain H37Rv and the highly virulent Mtb clinical isolate, strain M299, belonging to the modern M tuberculosis Beijing sublineage. The Mtb strains were generally more resistant to anti-mycobacterial agents, as well as rifampicin, mainly against Mtb M299, since it required a ten-fold concentration of rifampicin compared to the H37Rv strain (Ventura et al. 2015Ventura, TLB, Calixto SD, Abrahim-Vieira BA, de Souza AMT, Mello MVP, Rodrigues, CR, Mariz e Miranda LS, Souza ROMA, Leal ICR, Lasusnkaia EB & Muzitano MF (2015) Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20: 8072-8093.).
The n-hexane fraction at 100 μg/mL substantially reduced mycobacterial growth, about 50–55% for both Mtb strains, with MIC50 30.6 ± 1.1 μg/mL (Mtb H37Rv) and 35.6 ± 0.7 μg/mL (Mtb M299) (Fig. 5; Tab. 5). Among subfractions, only the SF4 subfraction exhibited selective activity against Mycobaterium and reduced Mtb H37Rv and M299 growth by about 45-50% at 100 μg/ mL (MIC5044.7 ± 0.8 μg/mL). Spathulenol (SF5) showed a similar activity profile against both Mtb strains, inhibiting almost 60 % of Mtb H37Rv growth (MIC5036.9 ± 1.5 μg/mL; 167.5 ± 6.8 μM) and Mtb M299 (MIC5042.1 ± 0.5 μg/mL; 191.0 ± 2.2 μM) (Fig. 5; Tab. 5).
Effect of n-hexane fraction, subfractions and spathulenol from Ocotea notata on growth of Mycobacterial tuberculosis H37Rv and clinical isolate M299. Bacterial suspensions were treated with samples at concentrations of 4, 20,100 and 500 μg/mL for M. tuberculosis H37Rv (a.) and hypervirulent M tuberculosis M299 (b.) or rifampicin. Culture medium without bacteria was used as a positive control and culture medium with bacteria and rifampin treated-bacterial culture (at 0.00032 to 1 μg/mL for Mtb H37Rv and at 0.008 to 10 μg/mL for clinical Mtb isolate M299) as negative control. *=p < 0.05; ** =p < 0.01; ***= p < 0.001 in relation to untreated group.
This may suggest that the SF4 subfraction and the spathulenol are related to the n-hexane fraction inhibitory potential on Mycobacterium growth.
Previous reports have demonstrated the antimicrobial activity of the Ocotea species. The essential oil of Ocotea bofo obtained by steam distillation exhibited antimicrobial activity against yeasts. Antimicrobial activity has also been reported for Ocotea quixos essential oil, as methanol extract of Ocotea odorífera reported MIC of 180 μg/ ml for the organic extract of Ocotea sp. against Staphylococcus aureus and Enterococcus faecalis (Yamaguchi et al. 2011Yamaguchi MU, Garcia FP, Cortez DA, Ueda-Nakamura T, Filho BP & Nakamura CV ( 2011) Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorífera (Lauraceae). Antonie Van Leeuwenhoek 99: 507-14.; Suffredini et al. 2006Suffredini IB, Paciencia MLB, Varella AD & Younes RN (2006) Antibacterial activity of Brazilian Amazon plant extracts. Brazilian Journal of Infectious Diseases 10: 400-402.).
Previous studies have attributed the antimicrobial activity of spathulenol observed in Ocotea extracts and fractions. Spathulenol is one of the major constituents found in Ocotea nectandrqfilia, and partially inhibited Cladosporium sphaerospermum and C. cladosporioides growth, and presented moderate activity against Aspergillus niger and Candida albicans (Raggi 2008Raggi L (2008) Estudo da composição química e das atividades biológicas de óleos voláteis de especies de Lauraceae, em diferentes épocas do ano. Instituto de Botanica da Secretaria de Estado do Meio Ambiente, Sao Paulo. 67p.).
The abundant compounds found in Espeletia nana essential oil: a-pinene (38.1%), β-pinene (17.2%), myrcene (15.0%), spathulenol (4.2%), bicyclogermacrene (4.0%), α-zingiberene (4.0%) and himachalene (3.7%) presented antibacterial activity (MIC values were determined for S. aureus ATCC 25923 as 200 μg/mL and E. faecalis ATCC 29212 as 600 μg/mL) (Peña et al. 2012Peña A, Rojas L, Aparicio R, Alarcón L, Baptista JG, Velasco J, Carmona J & Usubillaga A (2012) Chemical composition and antibacterial activity of the essential oil of Espeletia nana. Natural Product Communications 7: 661-662.).
Previously, spathulenol was reported with moderate active against M tuberculosis H37Rv strain (MIC 231.9 μg/mL) (Nascimento et al. 2018Nascimento KF, Moreira FMF, Alencar Santos J, Kassuya CAL, Croda JHR, Cardoso CAL, Vieira MDC, Góis Ruiz ALT, Ann Foglio M, Carvalho JE & Formagio ASN (2018) Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. Journal of Ethnopharmacology 210: 351-358.). However, recently, spathulenol, isolated from Azorella compacta (Apiaceae), was evaluated due to its anti-mycobacterial activity, against M tuberculosis H37Rv strain and showed a MIC of 12.50 μg/mL (Dzul-Beh et al. 2019Dzul-Beh AJ, Sosa KG, Uc-Cachón AH, Bórquez J, Loyola LA, García HBB, Peña-Rodríguez LM & Molina-Salinas LMGM (2019) In vitro growth inhibition and bactericidal activity of spathulenol against drug-resistant clinical isolates of Mycobacterium tuberculosis. Revista Brasileira de Farmacognosia 29: 798-800.). It is important to mention that these two works used a different M tuberculosis inoculum, 1.5 × 107 (Nascimento et al. 2018Nascimento KF, Moreira FMF, Alencar Santos J, Kassuya CAL, Croda JHR, Cardoso CAL, Vieira MDC, Góis Ruiz ALT, Ann Foglio M, Carvalho JE & Formagio ASN (2018) Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. Journal of Ethnopharmacology 210: 351-358.) and 6 × 106 (Dzul-Beh et al. 2019Dzul-Beh AJ, Sosa KG, Uc-Cachón AH, Bórquez J, Loyola LA, García HBB, Peña-Rodríguez LM & Molina-Salinas LMGM (2019) In vitro growth inhibition and bactericidal activity of spathulenol against drug-resistant clinical isolates of Mycobacterium tuberculosis. Revista Brasileira de Farmacognosia 29: 798-800.) CFU/mL, and this fact was used in the last work as a possible justification for MIC difference. In the present work, spathulenol MIC50 was determined as 36.9 ± 1.5 μg/mL, and M tuberculosis inoculum used was 1 × 107 CFU/mL. Certainly, the M tuberculosis inoculum seems to be important when comparing MIC values between different experiments and methodologies. Although the MIC difference, spathulenol stood out as a promising anti-mycobacterial compound and as a candidate for further studies.
In addition, a spathulenol analogous is one of the major constituents of SF4, and the antibacterial potential of aromadendrane-type sesquiterpenes has been partially investigated in the literature. Aromadendrane-type sesquiterpenes showed antibacterial activity against Streptococcus pyogenes methicillin, as well as sensitive and resistant strains of Staphylococcus aureus (Naz et al. 2015Naz T, Packer J, Yin P, Brophy JJ, Wohlmuth H, Renshaw DE, Smith J, Elders YC, Vemulpad SR & Jamie JF (2015) Bioactivity and chemical characterisation of Lophostemon suaveolens - an endemic Australian Aboriginal traditional medicinal plant. Natural Product Research 06: 11.). Furthermore, viridiflorol, one aromadendrane-type sesquiterpene, has shown anti-mycobacterial activity against Mtb H37Rv with MIC 190 μg/mL (Trevizan et al. 2016Trevizan LNF, Nascimento KF, Santos JA, Kassuya CAL, Cardoso CAL, Vieira MC, Moreira FMF, Croda J & Formagio ASN (2016) Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St. -Hil, A. Juss. & Cambess.) Radlk. Journal of Ethnopharmacology 4: 510-515.).
In conclusion, the isolation and identification of the spathulenol sesquiterpene from O. notata leaves was reported for the first time in the present study, besides its anti-mycobacterial and immunomodulatory activities. It is interesting to highlight the capacity of spathulenol in inhibiting M tuberculosis M299 growth, a resistant strain belonging to the Beijing family. This sesquiterpene notably contributed to the hexane fraction activity and did not show cytotoxicity when assessed on RAW 264.7 macrophages. In sum, this in vitro study importantly contributes to identify O. notata aromadendrane-type sesquiterpenes as promising anti-TB agents, which present interesting anti-mycobacterial activity combined with an immunomodulatory profile. These results show their eligibility for further in vivo studies in murine models of severe pulmonary tuberculosis, as recently established by our group (Almeida et al. 2017Almeida FM, Ventura TLB, Amaral ER, Ribeiro SC, Calixto SD, Manhães MR, Rezende AL, Souza GS, Carvalho IS, Silva EC, Silva JA, Carvalho ECQ, Kritski AL & Lasunskaia EB (2017) Hypervirulent Mycobacterium strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57BL/6 mice. Plos One 12(3): e 0173715.).
Acknowledgements
This work was supported by FAPERJ and CNPq research grants.
References
- Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Allured Pub. Corp, Carol Stream. 804p.
- Aguilar-Guadarrama AB & María Yolanda Rios (2004) Three New Sesquiterpenes from Croton arboreous. Journal of Natural Products 67: 914-917.
- Almeida FM, Ventura TLB, Amaral ER, Ribeiro SC, Calixto SD, Manhães MR, Rezende AL, Souza GS, Carvalho IS, Silva EC, Silva JA, Carvalho ECQ, Kritski AL & Lasunskaia EB (2017) Hypervirulent Mycobacterium strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57BL/6 mice. Plos One 12(3): e 0173715.
- Ballabeni V, Tognolini M, Giorgio C, Bertoni S, Bruni R & Barocelli E (2010) Ocotea quixos Lam. essential oil: in vitro and in vivo investigation on its antiinflammatory properties. Fitoterapia 81: 289-295.
- Barbosa-Filho JM, Cunha RM & Dias CSI (2008) GC-MS analysis and cardiovascular activity of the essential oil of Ocotea duckei. Revista Brasileira de Farmacognosia 18: 37-41.
- Bruni R, Medici A & Andreotti E (2004) Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chemistry 85: 415-421.
- Cantrell CL, Franzblau SG & Fischer NH (2001) Antimycobacterial plant terpenoids. Planta Medica 67: 685-694.
- Costa IFJB, Calixto SD, Araujo MH, Konno TUP, Wanderley LT, Guimarães DO, Lasunskaia EB, Leal IRC & Muzitano MF (2015) Antimycobacterial and nitric oxide production inhibitory activities of Ocotea notata from Brazilian Restinga. The Scientific World Journal 2015: 1-9.
- Cuca LE, Leon P & Coy ED (2009) A bicyclo[3.2.1] octanoid neolignan and toxicity of the ethanol extract from the fruit of Ocotea heterochroma. Chemistry of Natural Compounds 45: 179-181.
- Dartois V (2014) The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nature Review Microbiology 12: 159-167.
- Dias CS, Silva IG, Cunha EVL, Silva MS, Braz- Filho R & Barbosa-Filho JM (2003) Isolamento e identificação de novos alcaloides de Ocotea duckei Vattimo (Lauraceae). Revista Brasileira de Farmacognosia 13:62-63.
- Draper P (1998) The outer parts of the Mycobacterial envelope as permeability barriers. Frontiers in Bioscience 3: 1253-1261.
- Dzul-Beh AJ, Sosa KG, Uc-Cachón AH, Bórquez J, Loyola LA, García HBB, Peña-Rodríguez LM & Molina-Salinas LMGM (2019) In vitro growth inhibition and bactericidal activity of spathulenol against drug-resistant clinical isolates of Mycobacterium tuberculosis. Revista Brasileira de Farmacognosia 29: 798-800.
- Farias ALF, Rodrigues ABL, Martins RL, Rabelo ÉM, Farias CWF & Almeida SSMS (2019) Chemical characterization, antioxidant, cytotoxic and microbiological activities of the essential oil of leaf of Tithonia diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals (Basel) 12: 34.
- Fournet A, Ferreira ME, Rojas de Arias A, Guy I, Guinaudeau H & Heinzen H (2007) Phytochemical and antiprotozoal activity of Ocotea lancifolia. Fitoterapia 78: 382-384.
- Funasaki M, Lordello ALL & Viana AM (2009) Neolignans and sesquiterpenes from leaves and embryogenic cultures of Ocotea catharinensis (Lauraceae). Journal of the Brazilian Chemical Society 20: 853-859.
- Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C & Caccamo N (2007) Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1 -related receptor, a negative regulator of IL-1/ TLR signaling. The Journal of Immunology 179: 3119-3125.
- Garret R, Cruz RAS, Rocha L, Santos MG & Silva AJR (2010) Chemical Composition and Toxicity of Ocotea notata (Nees) Mez Essential Oil. Journal of Essential Oil-Bearing Plants 13: 455-459.
- Garrett R, Romanos MTV, Borges RM, Santos MG, Rocha L, Silva AJR (2012) Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Revista Brasileira de Farmacognosia 22: 306-313.
- Gobbo-Neto L & Lopes NP (2007) Plantas medicinais: fatores de influencia no conteúdo de metabólites secundários. Química Nova 30: 374-381.
- Gomez-Flores R, Gupta S, Tamez-Guerra R & Mehta RT (1995) Determination of MICs for Mycobacterium avium - M. intracellulare complex in liquid medium by a colorimetric method. Journal of Clinical Microbiology 33: 1842-1846.
- Guerrini A, Sacchetti G & Muzzoli M (2006) Composition of the volatile fraction of Ocotea bofo Kunth (Lauraceae) calyces by GC-MS and NMR fingerprinting and its antimicrobial and antioxidant activity. Journal of Agricultural and Food Chemistry 54: 7778-7788.
- Guzik TJ, Korbut R & Adamek-Guzik T (2003) Nitric oxide and superoxide in inflammation and immune regulation. Journal of Physiology and Pharmacology 54: 469-487.
- Hashibal K, Yokoyama K, Wakabayashi H, Hashimoto K, Satoh K, Kurihara T, Motohashi N & Sakagami H (2004) Inhibition of LPS-stimulated NO Production in Mouse Macrophage-like Cells by Azulenes. Anticancer Research 24: 3939-3944.
- Inagaki F & Abe A (1985) Analysis of 1H and 13C nuclear magnetic resonance spectra of spathulenol by two-dimensional methods. Journal of the Chemical Society (Perkin Transactions 2) 11: 1773-1778.
- Lee SH, Choi M, Kim P & Myung PK (2013) 3D-QSAR and Cell Wall Permeability of Antitubercular nitroimidazoles against Mycobacterium tuberculosis. Molecules 18: 13870-13885.
- Madubanya LA, Jäger AK, Makunga NP, Geldenhuys CJ & Van Staden J (2005) DNA fingerprinting and anti-inflammatory activity of Ocotea bullata bark from different locations. South African Journal of Botany 71: 38-44.
- Marques CA (2001) Importância econômica da familia Lauraceae Lindl. Floresta e Meio ambiente 8: 195.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65: 55-63.
- Nascimento KF, Moreira FMF, Alencar Santos J, Kassuya CAL, Croda JHR, Cardoso CAL, Vieira MDC, Góis Ruiz ALT, Ann Foglio M, Carvalho JE & Formagio ASN (2018) Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. Journal of Ethnopharmacology 210: 351-358.
- Naz T, Packer J, Yin P, Brophy JJ, Wohlmuth H, Renshaw DE, Smith J, Elders YC, Vemulpad SR & Jamie JF (2015) Bioactivity and chemical characterisation of Lophostemon suaveolens - an endemic Australian Aboriginal traditional medicinal plant. Natural Product Research 06: 11.
- Neto GG & Morais GR (2003) Recursos medicinais de especies do cerrado de Mato Grosso: um estudo bibliográfico. Acta Botanica Brasilica 17: 561-584.
- Pachú CO, Almeida RN & Barbosa-Filho JM (1993). Atividade depressora do sistema nervoso central pela Iangambina. Ciencias Cultura Saúde 12: 14-16.
- Park PH, Kim HS, Jin XY, Jin F, Hur J, Ko G & Sohn DH (2009) KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. European Journal of Pharmacology 606: 215-224.
- Peña A, Rojas L, Aparicio R, Alarcón L, Baptista JG, Velasco J, Carmona J & Usubillaga A (2012) Chemical composition and antibacterial activity of the essential oil of Espeletia nana. Natural Product Communications 7: 661-662.
- Raggi L (2008) Estudo da composição química e das atividades biológicas de óleos voláteis de especies de Lauraceae, em diferentes épocas do ano. Instituto de Botanica da Secretaria de Estado do Meio Ambiente, Sao Paulo. 67p.
- Ribeiro SCM, Gomes LL, Amaral EP, Andrade MRM, Almeida FM, Rezende AL, Lanes VR, Carvalho ECQ, Suffys PN, Mokrousov I & Lasunskaia EB (2014) Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. Journal Clinical Microbiology 52: 2615-2624.
- Sedger LM & McDermott MF (2014) TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine & Growth Factor Reviews 25: 453-472.
- Serra MF, Diaz BL & Barreto EO (1997) Anti-allergic properties of the natural PAF antagonist yangambin. Planta Medica 63: 207-212.
- Singh B & Macdonald CA (2010) Drug discovery from uncultivable microorganisms. Drug Discovery Today 15: 17-18.
- Souza GC, Haas APS, Von Poser GL, Schapoval EES & Elisabetsky E (2004) Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. Journal of Ethnopharmacology 90: 135-143.
- Suffredini IB, Paciencia MLB, Varella AD & Younes RN (2006) Antibacterial activity of Brazilian Amazon plant extracts. Brazilian Journal of Infectious Diseases 10: 400-402.
- Trevizan LNF, Nascimento KF, Santos JA, Kassuya CAL, Cardoso CAL, Vieira MC, Moreira FMF, Croda J & Formagio ASN (2016) Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St. -Hil, A. Juss. & Cambess.) Radlk. Journal of Ethnopharmacology 4: 510-515.
- Ventura, TLB, Calixto SD, Abrahim-Vieira BA, de Souza AMT, Mello MVP, Rodrigues, CR, Mariz e Miranda LS, Souza ROMA, Leal ICR, Lasusnkaia EB & Muzitano MF (2015) Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20: 8072-8093.
- WHO - World Health Organization (2018) Global tuberculosis report 2018. World Health Organization, Geneva. Available at <http://www.who.int/tb/publications/global_report/en/> Access on May 20, 2019.
» <http://www.who.int/tb/publications/global_report/en/> - Yamaguchi MU, Garcia FP, Cortez DA, Ueda-Nakamura T, Filho BP & Nakamura CV ( 2011) Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorífera (Lauraceae). Antonie Van Leeuwenhoek 99: 507-14.
- Zanin SM W & Lordello ALL (2007) Aporphine alkaloids in Ocotea species (Lauraceae). Química Nova 30: 92-98.
- Ziaei A, Ramezani M, Wright L, Paetz C, Schneider B & Amirghofran Z (2011) Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects. Phytotherapy Research 25: 557-562.
- Zschocke S, Drewes SE, Paulus K, Bauer R & Van Staden J (2000) Analytical and pharmacological investigation of Ocotea bullata (black stinkwood) bark and leaves. Journal of Ethnopharmacology 71: 219-230.
- Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, Mchugh TD, Schito M, Maeurer M & Nunn AJ (2014) New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. The Lancet Infectious Diseases 14: 327-340.
Edited by
Publication Dates
-
Publication in this collection
11 June 2021 -
Date of issue
2021
History
-
Received
13 Dec 2019 -
Accepted
07 May 2020