Abstracts
OBJECTIVE: To evaluate whether differences are present in microvascular response to the schemia induced by dynamic videocapillaroscopy (VCD), through analysis of the measured capillar transverse segment area (CTSA) in patients with type 1 diabetes mellitus (T1DM). METHODS: The vascular reactivity of the CTSA was studied by VCD, using a reactive hyperemia test in 61 volunteers, being 31 healthy controls without diabetes family history (Group 1) and 30 patients with T1DM without complications (Group 2). The images were captured every two seconds, during reperfusion after one minute induced ischaemia, and they were analyzed by the program Studio Version 8 and Motic Image Plus. The pre-ischemia capillary transverse segment (basal area, BA), the maximum strain post-ischemia (maximum area, MA), and time to achieve it (MAt) were measured during reperfusion, and the increased area percentage (Ap) was estimated. RESULTS: The mean differences between groups were evaluated by the t-test. The median comparisons between the groups were studied by the Mann-Whitney test. There was no difference in BA between the groups. The Ap was significantly lower among the diabetic patients, and there was a significant increase in the Mat among the patients of Group 2 when compared to Group 1. CONCLUSIONS: These data suggest that type 1 diabetes provokes earlier endothelial dysfunction, before the onset of clinically detectable degenerative complications. The outcomes from these alterations need further studies.
Diabetes mellitus; microcirculation; angioscopy microscopy; endothelium-abnormalities
OBJETIVO: Avaliar se há diferença de resposta microcirculatória à isquemia induzida pela videocapilaroscopia dinâmica (VCD), por meio da análise de medida da área do segmento transverso capilar (ASTC) em pacientes com diabetes melito tipo 1 (DMT1). MÉTODOS: A reatividade vascular do ASTC foi estudada pela VCD usando o teste de hiperemia reativa em 61 voluntários, sendo 31 controles sadios sem história familiar de diabetes (Grupo 1) e 30 pacientes com DMT1, sem complicações (Grupo 2). As imagens foram capturadas a cada dois segundos, durante a reperfusão após um minuto de isquemia induzida, e analisadas pelo programa Studio Version 8 e Motic Image Plus. O segmento transverso pré-isquemia (área basal, AB), a área máxima pós-isquemia (área máxima, AM) e o tempo para alcançá-la foram medidos durante a reperfusão, e o percentual de incremento foi estimado. RESULTADOS: As principais diferenças entre os grupos foram avaliadas pelo teste t. As médias comparativas entre os grupos foram avaliadas pelo teste Mann-Whitney. Não houve diferença na área basal entre os dois grupos. O percentual de incremento foi significativamente menor entre os pacientes diabéticos e houve um aumento significativo no ASTC entre os pacientes do Grupo 2 quando comparados com o Grupo 1. CONCLUSÕES: Os dados sugerem que o diabetes tipo 1 provoca disfunção endotelial precoce, antes mesmo de complicações degenerativas serem detectadas clinicamente. Os fatores que levam a essas alterações necessitam de estudos adicionais.
Diabetes melito; microcirculação; angioscopia microscópica; endotélio-anormalidades
ORIGINAL ARTICLE
Microvascular reactivity in type 1 diabetics
Reatividade microvascular em diabéticos tipo 1
Tereza Cristina Abi-ChahinI; Moema de Alencar HausenI; Claudia Moraes Mansano-MarquesI; Vera Lucia Rabello de Castro HalfounII
IDepartamento de Ciências Morfológicas, Faculdade de Medicina Souza Marques, Rio de Janeiro, RJ, Brasil
IIDepartamento de Clínica Médica, Faculdade de Medicina, Universidade Federal do Rio de Janeiro, UFRJ, Rio de Janeiro, RJ, Brasil
Correspondence to Correspondence to: Vera Lucia Rabello de Castro Halfoun Departamento de Clínica Médica, Faculdade de Medicina, UFRJ Nutrologia, Hospital Universitário Clementino Fraga Filho, 9º andar Av. Brigadeiro Trompowsky, s/nº 21941-590 - Rio de Janeiro, RJ, Brasil halfounvl@gmail.com
ABSTRACT
OBJECTIVE: To evaluate whether differences are present in microvascular response to the schemia induced by dynamic videocapillaroscopy (VCD), through analysis of the measured capillar transverse segment area (CTSA) in patients with type 1 diabetes mellitus (T1DM).
METHODS: The vascular reactivity of the CTSA was studied by VCD, using a reactive hyperemia test in 61 volunteers, being 31 healthy controls without diabetes family history (Group 1) and 30 patients with T1DM without complications (Group 2). The images were captured every two seconds, during reperfusion after one minute induced ischaemia, and they were analyzed by the program Studio Version 8 and Motic Image Plus. The pre-ischemia capillary transverse segment (basal area, BA), the maximum strain post-ischemia (maximum area, MA), and time to achieve it (MAt) were measured during reperfusion, and the increased area percentage (Ap) was estimated.
RESULTS: The mean differences between groups were evaluated by the t-test. The median comparisons between the groups were studied by the Mann-Whitney test. There was no difference in BA between the groups. The Ap was significantly lower among the diabetic patients, and there was a significant increase in the Mat among the patients of Group 2 when compared to Group 1.
CONCLUSIONS: These data suggest that type 1 diabetes provokes earlier endothelial dysfunction, before the onset of clinically detectable degenerative complications. The outcomes from these alterations need further studies.
Keywords:Diabetes mellitus; microcirculation; angioscopy microscopy; endothelium-abnormalities
RESUMO
OBJETIVO: Avaliar se há diferença de resposta microcirculatória à isquemia induzida pela videocapilaroscopia dinâmica (VCD), por meio da análise de medida da área do segmento transverso capilar (ASTC) em pacientes com diabetes melito tipo 1 (DMT1).
MÉTODOS: A reatividade vascular do ASTC foi estudada pela VCD usando o teste de hiperemia reativa em 61 voluntários, sendo 31 controles sadios sem história familiar de diabetes (Grupo 1) e 30 pacientes com DMT1, sem complicações (Grupo 2). As imagens foram capturadas a cada dois segundos, durante a reperfusão após um minuto de isquemia induzida, e analisadas pelo programa Studio Version 8 e Motic Image Plus. O segmento transverso pré-isquemia (área basal, AB), a área máxima pós-isquemia (área máxima, AM) e o tempo para alcançá-la foram medidos durante a reperfusão, e o percentual de incremento foi estimado.
RESULTADOS: As principais diferenças entre os grupos foram avaliadas pelo teste t. As médias comparativas entre os grupos foram avaliadas pelo teste Mann-Whitney. Não houve diferença na área basal entre os dois grupos. O percentual de incremento foi significativamente menor entre os pacientes diabéticos e houve um aumento significativo no ASTC entre os pacientes do Grupo 2 quando comparados com o Grupo 1.
CONCLUSÕES: Os dados sugerem que o diabetes tipo 1 provoca disfunção endotelial precoce, antes mesmo de complicações degenerativas serem detectadas clinicamente. Os fatores que levam a essas alterações necessitam de estudos adicionais.
Descritores: Diabetes melito; microcirculação; angioscopia microscópica; endotélio-anormalidades
INTRODUCTION
Diabetes mellitus (DM) can be defined as a heterogeneous group of metabolic disturbances that has hyperglycemia as a common finding. Type 1 diabetes mellitus (T1DM) occurs in 5% to 10% of the cases, resulting in a total destruction of β-pancreatic cells and, according to the Counsel of the Brazilian Society of Diabetics-2006 (3), it could be caused by autoimmune or idiopathic conditions.
With the advent of insulinotherapy, there was an increase in life expectancy of these patients. As a result, it was observed a substancial decrease in acute complications, which was continuously substituted for chronic complications, such as micro and macrovascular. In fact, coronary arterial disease mortality is three to ten times more frequent in patients with T1DM (1).
Microcirculatory net is a suitable place for the study of some systemic diseases that can appear in this territory, owing to alterations in the tissue blood flow (2,5).
After following healthy patients during annual capillaroscopy and others laboratory exams, Coget, observed the presence of microvascular complications before diabetes onset (5). The same was observed by another group (6), in diabetic when compared to healthy relatives controls.
In DM, the microangiopathy emerges as one of the most prevalent clinical complications which can be detected by periungueal videocapillaroscopy, as emphasized by many researchers (7-10). The procedure involving nail fold to observe the microcirculation is considered innocuous and non-invasive. In basal conditions, several authors (9-15) noticed hard visibility pattern because of the presence of edema, distension of capillary transversal segment, tortuosity of efferent branch, microectasy and shortening of capillary loops in diabetics, when compared to healthy subjects (12-16). However, there are few available studies using this method in T1DM.
Most studies suggest that diabetic patients have endothelial dysfunction because there is a deficit in vasodilatation relaxing factors. The nitric oxid (NO) is the primary mediator of endothelium dependent vasodilatation and it is known as endothelium-derived relaxing factor. This mediator is severely diminished among those patients and seems to be the main factor to reduce endothelium dependent vasodilatation.
This study aimed to evaluate the response of microcirculation to isquemia induced by dynamic videocapillaroscopy (VCD) through the measure's analysis of the area of capillary transverse segment in type 1 diabetic patients. These findings were also studied concerning to age, gender, family history of diabetes, capillary glycemia (CG), glicated hemoglobin (GH), time of disease, body mass index (BMI) and fundoscopy.
METHODS
A transversal, descriptive study was carried out from February 2007 until June 2008, at the Hospital Universitário Clementino Fraga Filho (HUCFF) of Universidade Federal do Rio de Janeiro (UFRJ). Sixty-one subjects, men and women, from all ethnic groups and with age ranging from 18 to 36 years old, took part in the study after signing a consent form.
Volunteers were divided into two groups: Group 1 (G1, Control Group), with 31 subjects without family history of diabetes and with normal oral glucose tolerance test (OGTT), and Group 2 (G2), with 30 type 1 diabetic individuals, without complications or family history of diabetes in next relatives. The following criteria were used for exclusion of patients: use of oral hypoglycemiant and/or vasoactive substances; clinical evidence of hepatopathies; BMI > 25 kg/m2; creatinine > 1.5 mg/dL; peripheral arterial insufficiency; dermatological lesions or inflammatory process in the hands; gestation; triglycerides (TCG) > 150 mg/dL; fasting glycemia (FG) > 100 mg/dL (Control Group); glycemia after glucose overload > 140 mg/dL (Control Group). Additionally, the arterial hypertension was included as exclusion criteria, according to the Brazilian Counsel of Arterial Hypertension-2006 (4).
In the day of the videocapillaroscopic exam, the patients should not smoke or use other type of drug or caffeine. The levels of CG should not be superior to 250 mg/dL. The images were captured by a CCD camera, Kocon, model KCC-31 OND (Sony, Japan) connected to a Sony 21" TV and to a computer with image software for capture (Studio Version 8) and analysis (Motic Image Plus). Also, an image analysis digital cuff (Hokanson®) was connected to a manometer of mercury to perform functional test (reactive hyperemia test) and to a thermometer (IncoTerm®) to register environmental temperature.
Reactive hyperemia test (RTH), was performed under room temperature up to 25ÚC, with blood pressure gauges adjusted preferably in the forth finger of the dominant hand. The best visualized distal capillary was selected for the RHT. The manometer was inflated 20 mmHg above the maximum arterial pressure of the patient for one minute. The images were captured during one minute pre-ischemia, with a magnification of 100X. Afterwards, the cuff was uninflated and images were taken every two seconds, during a time of 40 seconds, for posterior analysis. The transverse capillary segment area was delimitated by a perpendicular line to the largest axis of the selected capillary, tangent to internal arch limit and, in the sequence, by passing the external perimeter. Pre-ischemia basal area (BA) and maximum post-ischemia area (MA) were measured using the same capillary. BA was represented by three consecutively measurements of the same vessel. During post-ischemia, the maximum increase area, expressed in percentage (Ap), as well as the time spent to reach it (MAt) were registered. BA, MA, Ap and MAt were compared. Differences between the means from the two groups were compared by t-Student's test when distribution curves were considered normal. Differences between medians were tested by Mann-Whitney test.
RESULTS
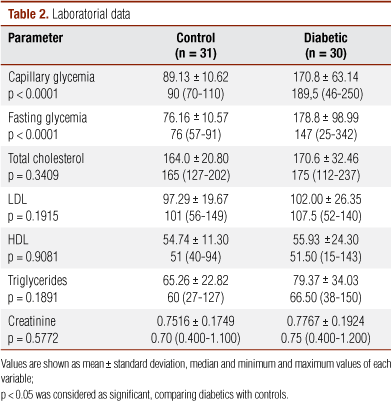
The detailed information of clinical and laboratorial parameters are presented in Tables 1 and 2. In G1, 67.74% were women, while in G2, they were 70%. Average age was 23.39 ± 5.38 versus 23.67 ± 4.31 in G1 and G2, respectively.
As for ethnic group, the percentage of white subjects, in G1, was 74.2% and, in G2, 43.3%. Among non-white individuals, the percentages were 25.8% and 56.7% in Group 1 and 2, respectively. In G1, there were only nonsmokers, and G2 had only one smoker. Disease duration ranged from 8 to 336 months (median = 132; average = 155.46 ± 80.17). Fifty percent of the subjects in G2 had duration of diabetes between 101 and 200 months.
FG (p < 0.0001) and CG (p < 0.0001) were significantly different between the groups. Conversely, HDL, LDL, total cholesterol (TC) and TCG levels were very similar between both groups, without significant differences. There was no statistical difference concerning other laboratorial parameters between the groups (p > 0.05, Table 2).
There was no difference between BA (pre-ischemia) and MA between the groups. The MAt, however, was significantly greater in diabetics (p < 0.0001, Table 3). Otherwise, Ap was significantly reduced among diabetic patients when compared to controls.
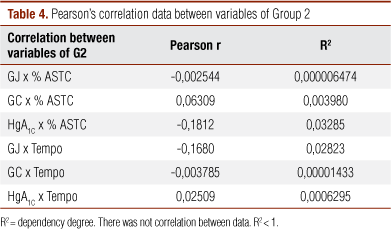
There was no significant correlation between FG, CG, GH and data from increase and time to reach maximum dilatation in G2 (Table 4).
Furthermore, there was not significant correlation between environmental and digital temperature, when analyzed in each group, separately or together (p-value > 0.05, Table 5).
DISCUSSION
In this study, variations in the measurement of post-ischemia transverse capillary segment area was used as vascular reactivity indicative on the basis that it is a validated, cheap, innocuous and easily reproducible method (15). By this method, there were well documented evidences of endothelial dysfunction in these patients. Concerning T1DM, rare studies are available in literature and none of them used the present method. In general, studies use indirect methods for evaluating microcirculation based on measurement of blood flow in large arteries in association with Doppler laser and pletismography (14,17-22). VCD is a dynamic technique, once it provides a direct evaluation of microcirculation and possible alterations in microvascular bed.
It's noteworthy to mention that this method offers some limitations, since it depends on personal experience and interpretation of the observer. In the present study, only one observer did the measurements, after training under supervision and agreement with instructor's observations. The measurement was limited to the lumen of the capillaries, thus measuring only the column of red cells inside the transverse segment. In fact, considering that this characteristic is present in every measurement, it minimized the mistake.
The variations in environmental temperature, which influence arteriolar vasodilatation, can be mentioned as other limitation, once the study was performed with variations between 24 and 34ºC. However, there was no correlation between environmental and digital temperatures when analyzed within each group or taking the two groups together. Moreover, averages and medians were similar between the two groups. In this way, it is hard to believe that this variable could have influenced significantly the capillary distensibility. No exam was performed with environmental temperatures under 24ºC to avoid vasoconstriction.
As far as sample selection is concerned, according to previous data (23), there was a reduction in vasodilatation capacity with ageing beginning after 30 years old, fact that was not proved by other work (24) which described this finding only after 60 years old. In the present study, 15 controls were in their thirties, varying from 31 to 38 years old, and two diabetic patients had ages in their thirties (31 and 35 years old). This fact could reduce the differences in MAt between the groups because of the slower maximum dilatation in Control Group. However, the delay in dilatation was more important in Group 2. Thus, these patients were not excluded from the sample.
Ethnic differences were not considered, since the Brazilian population is highly multiracial, so that it is very difficult to identify the racial origin based only on the skin color.
The presence of microangiopathy was evaluated by fundoscopy, to exclude subjects with microvascular lesions, provided that the objective of the study was to evaluate the influence of metabolic alterations in an early stage of the disease, without the well-known impact of diabetes upon microcirculation. Patients with signs of arterial or neurological alterations that could justify changes in vascular reactivity were excluded.
Furthermore, individuals with diastolic pressure > 90 mmHg, systolic pressure > 140 mmHg and BMI > 25 kg/m2 were also excluded. Obesity has been linked to hypertension through increase in vessel tonus by the reduction in NO biodisponibility, oxidative stress and increase in sympathetic tonus (25). These facts highlight the assertion that diabetes is often accompanied by arterial hypertension, obesity, hyperlipidemia, which characterize metabolic syndrome and, in isolated form, could justify endothelial dysfunction - with or without diabetes. Hyperlipidemia in male young adults alters vascular reactivity when measured by braquial artery ultrasound (26). In this study, the greatest values were found in the diabetic group, without significant statistical differences between the groups.
Subjects that used vasoactive substances, hypolipemiants and oral hypoglycemiant were not included to avoid possible bias. Oral hypoglycemiants are described in the literature as substances that interfere with endothelial dysfunction in DM (27-31). None of the subjects in G2 took these sorts of medicines.
One smoker was not excluded from G2; however, he was advised to stop smoking 24 hours before the exam, to normalize endothelial function and avoid vasoconstriction, as it was shown by others authors (32,33) as an adequate interval time. Besides that, their vasoactive response was similar to the other patients of the same group. In the day of the exam, all patients were advised to avoid substances similar to caffeine, tea, mate and soda to prevent other alterations in vasomotility. All subjects were clinically followed and had a laboratorial routine to evaluate renal and hepatic functions, which were normal to both groups. The course time of diabetes ranged from 8 to 336 months (8 months to 28 years), being 50% of the individuals between 101 to 200 months of disease (approximately 9 to 15 years), with median of 120 months (8 to 336).
In this study, the capillary distensibility was reduced among diabetic patients and took more time to reach MA after induced ischemia-MAt. These alterations are probably due to the decreased release of NO and other factors linked to arteriolar vasodilatation, as many works in the literature describe (12,14,27,34-36). Furthermore, these alterations precede the detectable retinopathy by fundoscopy, which was not found in the subjects of the study. This fact put forward that the mechanisms of circulating vasodilators inhibition can be related to oxidative stress which is usually present in these patients. Our patients had, in average, hemoglobin of 8.36%, and, therefore, they could be under that condition mentioned before. However, more studies are required to support this interpretation.
The results of this study suggest that patients with type 1 diabetes present endothelial dysfunction, which has early onset regarding the evolution of the disease, preceding clinically detectable microvascular alterations. Further studies can elucidate the metabolic control role in the evolution of these alterations and in the prognosis of that disease.
Acknowledgements: The authors wish to thank HUCFF for the equipment support.
Disclosure: no potential conflict of interest relevant to this article was reported.
Received in Mar/19/2009
Accepted in Apr/23/2009
- 1. Bozza ACT. Macroangiopatias. In: Oliveira JE, Milech A, editores. Diabetes mellitus: clínica, diagnóstico, tratamento multidisciplinar. 1Ş ed. Rio de Janeiro: Atheneu; 2004. p. 125-41.
- 2. Bollinger A, Butti P, Barras JP, Trachsler H, Siegenthaler W. Red blood cell velocity in nailfold capillaries of man measured by a television microscopy technique. Microvasc Res. 1974;7(1):61-72.
-
3Counsel of the Brazilian Society of Diabetics. 2006.
-
4Brazilian Counsel of Arterial Hypertension. 2006.
- 5. Coget JM, Dupuis-Uvny C, Merlen JF. The value of studying the ocular conjunctiva in case-finding in prediabetic states. J Mal Vasc. 1989;14(1):68-70.
- 6. Danilova AI. State of the microcirculation in the relatives of diabetes mellitus patients. Probl Endokrinol (Mosk). 1979;25(2):20-4.
- 7. Danilishina VS, Kushnir VL. Functional and biochemical characteristics of 2 types of diabetes mellitus. Ter Arkh 1984;56(10):86-8.
- 8. Gasser P, Berger W. Nailfold videomicroscopy and local cold test in type I diabetics. Angiology. 1992;43(5):395-400.
- 9. Jörneskog G, Brismar K, Fagrell B. Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia. 1995;38(4):474-80.
- 10. Jörneskog G, Fagrell B. Discrepancy in skin capillary circulation between fingers and toes in patients with type 1 diabetes. Int J Microcirc Clin Exp. 1996;16(6):313-9.
- 11. Volgin EG, Stroev Iu I, Yonskaia LI, Pechenkin ELU. Television capillaroscopy in the diagnosis of diabetic microangiopathies. Probl Endokrinol (Mosk). 1986;32(3):27-30.
- 12. Fernandes TJ, Bernardini EMT, Morais IC, Castilho C, Halfoun VLRC. Capilaroscopia em crianças com diabetes mellitus tipo 1. Arq Bras Endocrinol Metab. 2001;45(5):441-6.
- 13. Meyer MF, Pfohl M, Schatz H. Assessment of diabetic alterations of microcirculation by means of capillaroscopy and laser-Doppler anemometry. Med Klin (Munich). 2001;96(2):71-7.
- 14. Halfoun VL, Pires ML, Fernandes TJ, Victer F, Rodrigues KK, Tavares R. Videocapillaroscopy and diabetes mellitus: area of transverse segment in nailfold capillar loops reflects vascular reactivity. Diabetes Res Clin Pract. 2003;61(3):155-60.
- 15. Halfoun VLRC, Fernandes TJ, Pires MLE, Braun E. Estudos morfológicos e funcionais da microcirculação da pele no diabetes mellitus. Arq Bras Endocrinol Metab. 2003;47:271-9.
- 16. Morais IC, Bernardini EMT, Halfoun VLRC. Capilaroscopia em pacientes portadores de diabetes tipo II. Rev Angiol Cir Vasc. 1993;2(4):186-94.
- 17. Fagrell B. Advances in microcirculation network evaluation: an update. Int J Microcirc Clin Exp. 1995;15 Suppl 1:34-40.
- 18. Vayssairat M, Carpentier P. Microcirculation clinique. Paris: Masson; 1996.
- 19. Abink EJ, Wollersheim, Netten PM, Smits P. Reproducibility of skin microcirculatory measurements in humans, with special emphasis on capillaroscopy. Vasc Med. 2001;6(4):203-10.
- 20. Carpentier PH. Current techniques for the clinical evaluation of the microcirculation. J Mal Vasc. 2001; 26(2):142-7.
- 21. Tooke JE, Lins PE, Ostergreen J, Fagrell B. Skin microvascular autoregulatory responses in type 1 diabetes: the influence of duration and control. Int J Microcirc Clin Exp. 1985;4(3):249-56.
- 22. Rooijens PP, Burgmans JP, Yo TI, Hop WC, de Smet AA, van den Dorpel HA, et al. Autogenous radial-cephalic or prosthetic brachial-antecubital forearm loop AVF in patients with compromised vessels? A randomized, multicenter study of the patency of primary hemodialysis access. J Vasc Surg. 2005;42(3):481-6; discussions 487.
- 23. Jin SM, Nch CI, Yang SLU, Bae EJ, Shin CH, Chung HR, et al. Endothelial dysfunction and microvascular complications in type 1 diabetes mellitus. J Korean Med Sci. 2008;23(1):77-82.
- 24. Gerhard MM, Roddy MA, Creager SJ. Alterações endoteliais no envelhecimento. Endotélio e doenças cardiovasculares. 1Ş ed. Rio de Janeiro: Atheneu; 1996. p. 369-79.
- 25. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001:38(2): 274-9.
- 26. de Jongh RT, Serné EH, Jzerdan RG, de Vries G, Stehouwer CD, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004:109(21):2529-35.
- 27. Lundman P, Eriksson MJ, Stühlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol. 2001;38(1):111-6.
- 28. McVeigh GE, Brennan GE, Johnston GD, Mc Derrmott BJ, MC Grath LT, Henry WR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(8):771-6.
- 29. Fava D, Cassone-Faldetta M, Laurenti O, De Luca O, Ghiselli A, De Mattia G. Gliclazide improves anti-oxidant status and nitric oxide-mediated vasodilation in type 2 diabetes. Diabet Med. 2002;19(9):752-7.
- 30. De Mattia G, Laurenti O, Fava D. Diabetic endothelial dysfunction: effect of free radical scavenging in Type 2 diabetic patients. J Diabetes Complications. 2003;17(2 Suppl):30-5.
- 31. Grant PJ. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes Metab. 2003;29(4 Pt 2):6S44-52.
- 32. Wiernsperger NF, Bouskela E. Microcirculation in insulin resistance and diabetes: more than just a complication. Diabetes Metab. 2003;29(4 Pt 2):6S77-87.
- 33. Pazos-Moura CC, Moura EG, Bouskela E, Torres Filho IP, Breitenbach MM. Nailfold capillaroscopy in non-insulin dependent diabetes mellitus: blood flow velocity during rest and post-occlusive reactive hyperaemia. Clin Physiol. 1990:10(5):451-61.
- 34. Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31(5):1047-60.
- 35. Halfoun VLRC, Fernandes TJ, Pires MLE, Victer F, Tavares R, Cardozo M, et al. Videocapilaroscopia e diabetes mellitus: projeção da área do segmento transverso em processamento de imagem. Arq Bras Endocrinol Metab. 2001;45(5):S584.
- 36. Consentino F, Hishikawa K, Katusik ZS, Lüscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96(1):25-8.
- 37. Perfett F, Tarquini R, Tapparini L, Tarquini B. Influence of non-insulin-dependent diabetes mellitus on plasma endothelin-1 levels in patients with advanced atherosclerosis. J Diabet Complications. 1998;12(4):187-92.
Publication Dates
-
Publication in this collection
28 Oct 2009 -
Date of issue
Aug 2009
History
-
Received
19 Mar 2009 -
Accepted
23 Apr 2009