ABSTRACT
The purpose of this case series is to report eight patients with giant prolactinomas emphasizing presentations and a treatment complication. The study group included six men and two women. The median age was 29 years (18–54 years); median serum prolactin level was 4,562 ng/ml (1,543–18,690 ng/ml); three patients (37.5%) had panhypopituitarism; median tumor diameter was 50 mm (41–60 mm). Five patients (62.5%) had visual field defects and three had improvement during treatment; six patients (75%) reached prolactin normalization, with a median time of 10.5 months (7–84 months) and median dose of 2.0 mg/week (1.0 to 3.0 mg/week). One patient presented as a true incidentaloma. One patient presented a cerebrospinal fluid leakage during medical treatment and refused surgery, however this resolved with conservative measures. This case series illustrate a rare subtype of macroprolactinomas, the importance of considering unusual presentations at the diagnosis, the effectiveness of pharmacological treatment and its possible complications.
prolactinoma; dopamine agonists; hyperprolactinemia
RESUMO
O objetivo desta série de casos é relatar oito pacientes com prolactinomas gigantes enfatizando as formas de apresentação e uma complicação do tratamento. O estudo incluiu seis homens e duas mulheres. A mediana de idade foi 29 anos (18–54); e dos níveis de prolactina foi 4.562 ng/ml (1.543–18.690); três pacientes (37,5%) apresentaram pan-hipopituitarismo; a mediana do máximo diâmetro tumoral foi 50 mm (41–60 mm). Cinco pacientes (62,5%) apresentaram alterações no campo visual e três tiveram melhora durante o tratamento; seis pacientes (75%) alcançaram normalização da prolactina em 10,5 meses (7–84) com dose mediana de cabergolina de 2,0 mg / semana (1,0 a 3,0). Um paciente se apresentou como um verdadeiro incidentaloma. Um paciente apresentou uma fistula liquórica durante o tratamento medicamentoso e recusou correção cirúrgica. No entanto a fistula foi resolvida com medidas conservadoras. Esta série de casos ilustra um subtipo raro de macroprolactinomas, a importância de considerar apresentações incomuns no diagnóstico, a eficácia do tratamento farmacológico e suas possíveis complicações.
prolactinoma; agonistas de dopamina; hiperprolactinemia
Giant prolactinomas correspond to 0.5–4.4% of all pituitary tumors, based on retrospective analysis11. Molitch ME, Thorner MO, Wilson C. Management of prolactinomas. J Clin Endocrinol Metab 1997;82(4):996-1000. doi:10.1210/jcem.82.4.3845
https://doi.org/10.1210/jcem.82.4.3845...
,22. Shrivastava RK, Arginteanu MS, King WA, Post KD. Giant prolactinomas: clinical management and long-term follow up. J Neurosurg. 2002;97(2):299-306. doi:10.3171/jns.2002.97.2.0299
https://doi.org/10.3171/jns.2002.97.2.02...
,33. Corsello SM, Ubertini G, Altomare M, Lovicu RM, Migneco MG, Rota CA et al. Giant prolactinomas in men: efficacy of cabergoline treatment. Clin Endocrinol (Oxf). 2003;58(5):662-70. doi:10.1046/j.1365-2265.2003.01770.x
https://doi.org/10.1046/j.1365-2265.2003...
,44. Colao A, Sarno AD, Cappabianca P, Briganti F, Pivonello R, Somma CD et al. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocrinol. 2003;148(3):325-31. doi:10.1530/eje.0.1480325
https://doi.org/10.1530/eje.0.1480325...
,55. Schaller B. Gender-related differences in prolactinomas: a clinicopathological study. Neuro Endocrinol Lett. 2005;26(2):152-9.. They represent 2–3% of all prolactin-secreting tumors and are more prevalent in men with a male to female ratio of 9:1 and a mean age at diagnosis of 40 years. The linear relationship between prolactin levels and tumor size can be lost in this group of patients, either because of the hook effect or due to the presence of necrotic or hemorrhagic components in the tumor66. Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170(6):R213-27. doi:10.1530/EJE-14-0013
https://doi.org/10.1530/EJE-14-0013...
. Hook effect is caused by an excess of soluble antigen binding separately to both the solid-phase and the soluble antibody in one-step immunometric assays. The ‘sandwich’ in which the antigen molecule binds simultaneously to both antibodies is not formed and during the washout phase these antigen-soluble antibody complexes are removed therefore resulting in a lower than expected reading. The hook effect can be eliminated by using a two-step process or more usually by performing analysis at two sample dilutions77. Haller BL, Fuller KA, Brown WS, Koenig JW, Eveland BJ, Scott MG. Two automated prolactin immunoassays evaluated with demonstration of a high-dose “hook effect” in one. Clin Chem. 1992;38(3):437-8.. Criteria for the definition of a giant prolactinoma vary among authors. Recently Maiter and Delgrange66. Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170(6):R213-27. doi:10.1530/EJE-14-0013
https://doi.org/10.1530/EJE-14-0013...
suggested that these tumors should be defined on the basis of the following: 1) Size – largest diameter of 4 cm or more in any direction with massive extrasellar extension; 2) prolactin (PRL) levels at diagnosis usually above or equal to 1,000 µg/L using a modern and well-standardized assay; 3) Exclusion of concomitant GH and ACTH secretion. The therapeutic goals are the same as for the non-giant prolactinomas, however rapid relief of neurologic symptoms is usually critical. There are no specific evidence-based recommendations for the management of these tumors and most of the literature consists of case reports or small case series.
The purpose of this case series is to report eight patients with giant prolactinomas followed at the Endocrinology Clinics of the Hospital Universitário Antônio Pedro, adding information of such a rare condition to the literature.
METHODS
A review of the medical records of patients with prolactinomas from the endocrinology outpatient clinics was performed with special interest to the patients with tumors with maximum diameter of more than 4 cm. The study was approved by the local Ethics Committee.
Eight patients (six men; male/female ratio 3:1) were included in the analysis.
At diagnosis median age was 29 years (18–54) and median PRL concentration was 4,562 ng/mL (1,543–18,690 ng/mL; Reference values: male 2.5–17; female 1.9–25). All patients had hypogonadotropic hypogonadism and additional pituitary hormone deficiencies were observed in three patients (#4, #7, and #8), the latter two being affected in all adenohypophyseal axis.
RESULTS
Median maximum tumor diameter was 50 mm (41–60). Five patients (62.5%) had visual field defects (detailed in Table 1). Regarding the presenting features, the two female patients (#7 and #8) had the classic amenorrhea/galactorrhea syndrome and in one patient (#8), additionally, significant visual symptoms were also noted. One patient (#3) sought medical care in the context of an erectile dysfunction and laboratory tests confirmed hyperprolactinemia. Three patients (#2, #4 and #5) had symptoms predominantly related to the tumor mass effect with headache and visual field defects (suprasellar extension). Patient #2 also manifested left oculomotor nerve paresis (cavernous sinus extension). One patient (#6) had a very rare presentation due to large anterior tumoral extension. Giant prolactinoma was discovered in the context of the investigation of chronic nasal obstruction unresponsive to usual treatment (“incidental” finding of paranasal sinus computed tomography). Finally, one patient (#1) presented as an incidentaloma in imaging studies ordered in the context of an acute ischemic stroke.
Data are summarized in Table 1.
Treatment and follow up
All patients but one (#4) were initially treated with cabergoline (CAB). Patient #4 underwent transfrontal surgery at diagnosis, in 1998, in another center, for unclear reasons.
During follow up, six patients treated with CAB achieved normal PRL levels after a median time of 10.5 months (7–84), with a median dose of 2.0 mg/week (1.0–3.0 mg/week). Patients #3 and #4 only reached normalization of PRL levels after 49 and 84 months, respectively, due to noncompliance with treatment. Two patients did not normalize PRL levels (#2 and #5). Nevertheless patient #2 had 93% reduction in serum PRL levels, with the last imaging study showing a partial empty sella. Patient #5 developed a cerebrospinal fluid (CSF) leak diagnosed on the basis of a clear rhinorrhea which started two months after CAB was initiated (1.5 mg/week). As he refused surgical correction of the CSF leak, CAB dose was reduced (0.5 mg/week) to allow tumor re-growth as an attempt to stop CSF leak, however it was unsuccessful and after two months CAB dose was progressively increased (3.0 mg/week) to attain PRL level reduction/normalization and tumor shrinkage. Patient was constantly told to keep the head of his bed elevated, among other conservative measures, as well as repeatedly informed of the risks and gold standard surgical treatment for his condition. During follow up, CSF leak resolved spontaneously after seven months of continued CAB treatment with no intercurrent infection.
Of the five patients who had visual field defects, three showed improvement during treatment. Only one patient (#3) recovered gonadal function. No other patients with hypopituitarism at diagnosis experienced recovery of the pituitary function. At the final evaluation, median maximum tumor diameter was 22 mm (patients #2 and #4 showed partial empty sella and empty sella, respectively). All patients showed significant tumor shrinkage (reduction ≥ 25% in tumor volume and/or ≥ 30% in tumor diameter) (Figures 1 and 2).
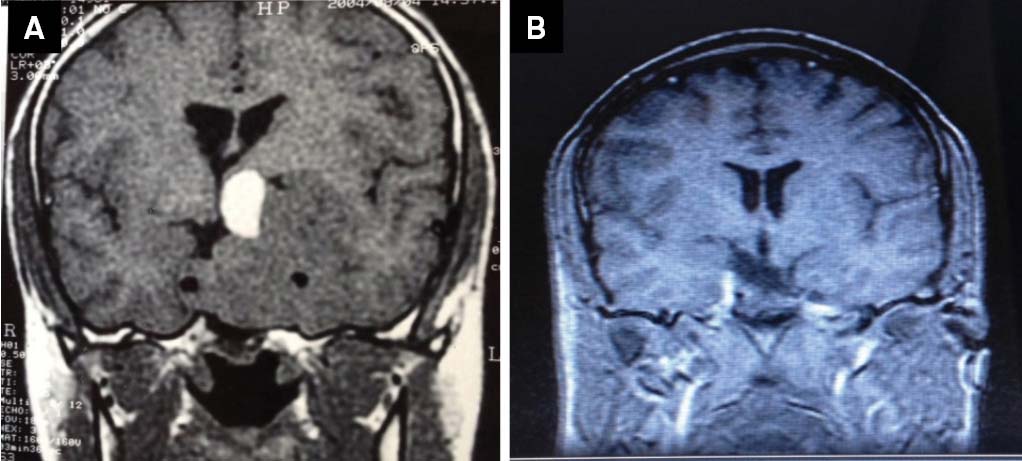
(A) Patient #1. Sella turcica MRI coronal view T1WI, showing a large tumor extending from the sella to supra, infra and parasellar regions, with heterogeneous contrast enhancement, measuring 4.8 x 4.0 x 5.5 cm. (B) After two years of treatment with cabergoline 3.0 mg/week, residual lesion, with cystic areas, irregular enhancement of contrast, measuring 1.0 x 2.2 x 2.4 cm.
(A) Patient #2. Sella turcica MRI coronal view T1WI, showing an expansive sellar tumor measuring 6.0 x 5.2 x 5.3 cm, extending to the suprasellar region and to the left temporal lobe and periventricular region, with massive left cavernous sinus invasion. In the superior portion a hyperintensity area suggests acute bleeding inside the tumor. (B) Partial empty sella (after seven years of treatment with cabergoline 3.5 mg/week).
Data are summarized in Table 2.
DISCUSSION
Giant prolactinomas show presenting features different from non-giant tumors, generally reflecting massive tumor extension into surrounding structures66. Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170(6):R213-27. doi:10.1530/EJE-14-0013
https://doi.org/10.1530/EJE-14-0013...
.
In the present case series, two patients (#1 and #6) had presentations that are worthy to note. In one patient, giant prolactinoma was an incidental finding of a head MRI ordered in the context of an acute ischemic stroke (#1). In the other (#6), giant prolactinoma was discovered during the investigation of chronic nasal obstruction which was due to large anterior tumor extension into the nasopharynx.
Patient (#1) is a 50-year-old man who was admitted to the emergency department with a right hemiplegia. In addition to an acute ischemic stroke in topography in the semiovale centrum, corona radiata, caudate nucleus, internal capsule and insula on the left, head MRI showed an expansive sellar lesion with upper extension to the suprasellar cistern and below to the sphenoid sinus as well as a lateral extension, especially to the right, involving the cavernous portion of the right carotid artery, measuring 4.8 x 4.0 x 5.5 cm. Patient reached normalization of PRL levels and significant reduction of tumor diameter with CAB (data are summarized in Tables 1 and 2). A few cases of stroke related to giant pituitary tumours have been described in the literature. However, they are often associated with pituitary apoplexy (PA)88. Navarro-Bonnet J, Martínez-Anda JJ, Balderrama-Soto A, Pérez-Reyes SP, Pérez-Neri I, Portocarrero-Ortiz L. Stroke associated with pituitary apoplexy in a giant prolactinoma: a case report. Clin Neurol Neurosurg. 2014;116:101-3. doi:10.1016/j.clineuro.2013.09.039
https://doi.org/10.1016/j.clineuro.2013....
. Multiple hypotheses have been proposed to explain a stroke in PA, but the most accepted is the extraluminal compression of the main brain vessels caused by the acute growth of the tumor. This seems not to be the case of the present patient since no clinical or radiological evidence of PA were present at the time of acute ischemic stroke.
In patient #6, as previously stated, giant prolactinoma was discovered during the investigation of chronic nasal obstruction which was due to large anterior tumor extension into the nasopharynx. There are few cases in the literature reporting this type of presentation. In one of them99. Sunil B, Reddy A, Bryant N, Young DW, Ashraf AP. Invasive giant prolactinoma presenting as a nasal polyp. J Pediatr. 2013;162(2):435-435.e1. doi:10.1016/j.jpeds.2012.08.029
https://doi.org/10.1016/j.jpeds.2012.08....
a 14-year-old boy presented with progressive weight gain, nasal obstruction, snoring, and a polypoid mass in his left nostril. Imaging studies revealed a homogenously enhancing mass extending from the floor of sella to the nasopharynx, measuring in the maximum diameter 9.9 cm. Only after biopsy and laboratory analysis the diagnosis of giant prolactinoma was done and successful treatment with CAB was instituted. In another case1010. Care RH, Sunkaraneni VS, Theaker J, Harries PG. Rapidly progressing giant invasive prolactinoma. J Laryngol Otol. 2012;126(8):840-3. doi:10.1017/S0022215112001296
https://doi.org/10.1017/S002221511200129...
a 30-year-old man presented with recurrent sinusitis not relieved by several courses of antibiotics. Imaging studies revealed an extensive skull base mass with a nasopharyngeal component which suggested a nasopharyngeal carcinoma. Patient was submitted to a biopsy and the histological pattern suggested a low grade neuroendocrine tumour. Only after laboratory tests and specific staining techniques, the diagnosis of a giant invasive prolactinoma was made. As previously seen, sometimes the diagnosis of prolactinoma may be made through the biopsy of a nasal polypoid mass. These cases should remind the clinician that giant pituitary tumors, and notably giant prolactinomas, may extend far from the sella and cause symptoms different from cranial nerve compression66. Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170(6):R213-27. doi:10.1530/EJE-14-0013
https://doi.org/10.1530/EJE-14-0013...
.
Regarding the follow up, it is important to mention that patient #5 developed a CSF leak, a possible complication of dopamine agonist (DA) treatment, after two months of starting CAB (1.5 mg/week). There have been several reports of development of CSF rhinorrhea following the initiation of medical treatment for pituitary adenomas. The classical description of this phenomenon has been in the setting of DA therapy for prolactinomas1111. Afshar F, Thomas A. Bromocriptine induced cerebrospinal fluid rhinorrhoea. Surg Neurol. 2004;18(1):61-3. doi:10.1016/0090-3019(82)90020-9
https://doi.org/10.1016/0090-3019(82)900...
. Lam et al.1212. Lam G, Mehta V, Zada G. Spontaneus and medically induced cerebrospinal fluid leakage in the setting of pituitary adenomas: review of the literature. Neurosurg Focus. 2012;32(6):E2. doi:10.3171/2012.4.FOCUS1268
https://doi.org/10.3171/2012.4.FOCUS1268...
reviewed the literature between the years 1980 and 2011, describing 52 patients with spontaneous or medically induced CSF leaks in the setting of pituitary adenomas. In 38 patients (73%), CSF rhinorrhea developed following initiation of medical therapy. Forty-two patients (81%) had prolactinomas and the medical agents associated with CSF leakage were DA (97%) and somatostatin analogs (3%; one patient). The average time from initiation of medical treatment to onset of rhinorrhea was 3.3 months (range 3 days–17 months).
Another complication from medical treatment of prolactinomas is empty sella which occurs quite frequently and may cause or aggravate pre-existent visual disturbances. In the present series, patients #2 and #4 had respectively partial and total empty sella, both asymptomatic and not associated with worsened visual field defects. Secondary empty sella usually develops following radiation therapy or surgery of the sellar region or during dopamine agonist treatment for prolactinomas1313. Gallardo E, Schächter D, Cáceres E, Becker P, Colin E, Martínez C et al. The empty sella: results of treatment in 76 successive cases and high frequency of endocrine and neurological disturbances. Clin Endocrinol (Oxf). 1992;37(6):529-33. doi:10.1111/j.1365-2265.1992.tb01484.x
https://doi.org/10.1111/j.1365-2265.1992...
,1414. Hamlyn PJ, Baer R, Afshar F. Transsphenoidal chiasmopexy for long standing visual failure in the secondary empty sella syndrome. Br J Neurosurg. 1988;2(2):277-9. doi:10.3109/02688698808992681
https://doi.org/10.3109/0268869880899268...
,1515. Jones SE, James RA, Hall K, Kendall-Taylor P. Optic chiasmal herniation: an under recognized complication of dopamine agonist therapy for macroprolactinoma. Clin Endocrinol (Oxf). 2000;53(4):529-34. doi:10.1046/j.1365-2265.2000.01039.x
https://doi.org/10.1046/j.1365-2265.2000...
. In that scenario, visual disturbance is caused by traction of the chiasm or the optic nerves toward the sella floor by the adhesion scar or pituitary stalk1616. Czech T, Wolfsberger S, Reitner A, Görzer H. Delayed visual deterioration after surgery for pituitary adenoma. Acta Neurochir (Wien). 1999;141(1):45-51. doi:10.1007/s007010050265
https://doi.org/10.1007/s007010050265...
,1717. Guinto G, Valle R, Nishimura E, Mercado M, Nettel B, Salazar F. Primary empty sella syndrome: the role of visual system herniation. Surg Neurol. 2002;58(1):42-7. doi:10.1016/S0090-3019(02)00766-8
https://doi.org/10.1016/S0090-3019(02)00...
,1818. Olson DR, Guiot G, Derome P. The symptomatic empty sella: prevention and correction via the transsphenoidal approach. J Neurosurg. 1972;37(5):533-7. doi:10.3171/jns.1972.37.5.0533
https://doi.org/10.3171/jns.1972.37.5.05...
. Gradual dose reduction of the dopamine agonist and systematic visual field evaluation may be attempted as a medical treatment to recover visual field defects1919. Gkekas N, Primikiris P, Georgakoulias N. Untethering of herniated left optic nerve after dopamine agonist treatment for giant prolactinoma. Acta Neurochir (Wien). 2013;155(3):495-6. doi:10.1007/s00701-012-1613-9
https://doi.org/10.1007/s00701-012-1613-...
. Alternatively, surgical treatment should consist of chiasmapexy or sellar packing22. Shrivastava RK, Arginteanu MS, King WA, Post KD. Giant prolactinomas: clinical management and long-term follow up. J Neurosurg. 2002;97(2):299-306. doi:10.3171/jns.2002.97.2.0299
https://doi.org/10.3171/jns.2002.97.2.02...
.
In conclusion, the giant prolactinoma is a rare subtype of macroprolactinoma with diagnostic criteria still not clearly defined. It is proposed that the most important criterion is the maximum tumor diameter greater than 4 cm. Regarding the presenting features, this series reports the first case of a giant prolactinoma presenting as a true incidentaloma, to the best of our knowledge. Also it reminds the importance of considering unusual presentations resulting from massive extrasellar tumor extension. Finally, pharmacological treatment with dopamine agonists is an effective anti-secretory and anti-proliferative option and its use may result in massive tumor shrinkage which may complicate with CSF leak.
References
-
1Molitch ME, Thorner MO, Wilson C. Management of prolactinomas. J Clin Endocrinol Metab 1997;82(4):996-1000. doi:10.1210/jcem.82.4.3845
» https://doi.org/10.1210/jcem.82.4.3845 -
2Shrivastava RK, Arginteanu MS, King WA, Post KD. Giant prolactinomas: clinical management and long-term follow up. J Neurosurg. 2002;97(2):299-306. doi:10.3171/jns.2002.97.2.0299
» https://doi.org/10.3171/jns.2002.97.2.0299 -
3Corsello SM, Ubertini G, Altomare M, Lovicu RM, Migneco MG, Rota CA et al. Giant prolactinomas in men: efficacy of cabergoline treatment. Clin Endocrinol (Oxf). 2003;58(5):662-70. doi:10.1046/j.1365-2265.2003.01770.x
» https://doi.org/10.1046/j.1365-2265.2003.01770.x -
4Colao A, Sarno AD, Cappabianca P, Briganti F, Pivonello R, Somma CD et al. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocrinol. 2003;148(3):325-31. doi:10.1530/eje.0.1480325
» https://doi.org/10.1530/eje.0.1480325 -
5Schaller B. Gender-related differences in prolactinomas: a clinicopathological study. Neuro Endocrinol Lett. 2005;26(2):152-9.
-
6Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170(6):R213-27. doi:10.1530/EJE-14-0013
» https://doi.org/10.1530/EJE-14-0013 -
7Haller BL, Fuller KA, Brown WS, Koenig JW, Eveland BJ, Scott MG. Two automated prolactin immunoassays evaluated with demonstration of a high-dose “hook effect” in one. Clin Chem. 1992;38(3):437-8.
-
8Navarro-Bonnet J, Martínez-Anda JJ, Balderrama-Soto A, Pérez-Reyes SP, Pérez-Neri I, Portocarrero-Ortiz L. Stroke associated with pituitary apoplexy in a giant prolactinoma: a case report. Clin Neurol Neurosurg. 2014;116:101-3. doi:10.1016/j.clineuro.2013.09.039
» https://doi.org/10.1016/j.clineuro.2013.09.039 -
9Sunil B, Reddy A, Bryant N, Young DW, Ashraf AP. Invasive giant prolactinoma presenting as a nasal polyp. J Pediatr. 2013;162(2):435-435.e1. doi:10.1016/j.jpeds.2012.08.029
» https://doi.org/10.1016/j.jpeds.2012.08.029 -
10Care RH, Sunkaraneni VS, Theaker J, Harries PG. Rapidly progressing giant invasive prolactinoma. J Laryngol Otol. 2012;126(8):840-3. doi:10.1017/S0022215112001296
» https://doi.org/10.1017/S0022215112001296 -
11Afshar F, Thomas A. Bromocriptine induced cerebrospinal fluid rhinorrhoea. Surg Neurol. 2004;18(1):61-3. doi:10.1016/0090-3019(82)90020-9
» https://doi.org/10.1016/0090-3019(82)90020-9 -
12Lam G, Mehta V, Zada G. Spontaneus and medically induced cerebrospinal fluid leakage in the setting of pituitary adenomas: review of the literature. Neurosurg Focus. 2012;32(6):E2. doi:10.3171/2012.4.FOCUS1268
» https://doi.org/10.3171/2012.4.FOCUS1268 -
13Gallardo E, Schächter D, Cáceres E, Becker P, Colin E, Martínez C et al. The empty sella: results of treatment in 76 successive cases and high frequency of endocrine and neurological disturbances. Clin Endocrinol (Oxf). 1992;37(6):529-33. doi:10.1111/j.1365-2265.1992.tb01484.x
» https://doi.org/10.1111/j.1365-2265.1992.tb01484.x -
14Hamlyn PJ, Baer R, Afshar F. Transsphenoidal chiasmopexy for long standing visual failure in the secondary empty sella syndrome. Br J Neurosurg. 1988;2(2):277-9. doi:10.3109/02688698808992681
» https://doi.org/10.3109/02688698808992681 -
15Jones SE, James RA, Hall K, Kendall-Taylor P. Optic chiasmal herniation: an under recognized complication of dopamine agonist therapy for macroprolactinoma. Clin Endocrinol (Oxf). 2000;53(4):529-34. doi:10.1046/j.1365-2265.2000.01039.x
» https://doi.org/10.1046/j.1365-2265.2000.01039.x -
16Czech T, Wolfsberger S, Reitner A, Görzer H. Delayed visual deterioration after surgery for pituitary adenoma. Acta Neurochir (Wien). 1999;141(1):45-51. doi:10.1007/s007010050265
» https://doi.org/10.1007/s007010050265 -
17Guinto G, Valle R, Nishimura E, Mercado M, Nettel B, Salazar F. Primary empty sella syndrome: the role of visual system herniation. Surg Neurol. 2002;58(1):42-7. doi:10.1016/S0090-3019(02)00766-8
» https://doi.org/10.1016/S0090-3019(02)00766-8 -
18Olson DR, Guiot G, Derome P. The symptomatic empty sella: prevention and correction via the transsphenoidal approach. J Neurosurg. 1972;37(5):533-7. doi:10.3171/jns.1972.37.5.0533
» https://doi.org/10.3171/jns.1972.37.5.0533 -
19Gkekas N, Primikiris P, Georgakoulias N. Untethering of herniated left optic nerve after dopamine agonist treatment for giant prolactinoma. Acta Neurochir (Wien). 2013;155(3):495-6. doi:10.1007/s00701-012-1613-9
» https://doi.org/10.1007/s00701-012-1613-9
Publication Dates
-
Publication in this collection
July 2016
History
-
Received
05 Jan 2016 -
Reviewed
06 May 2016 -
Accepted
16 May 2016