Abstracts
The combination of a flow-batch system, quantum dots (QDs) and webcam as detector for the automatic spectrofluorimetric determination of N-acetyl-L-cysteine (NAC) in pharmaceutical formulations is proposed. NAC generates surface interactions that result in enhanced QD fluorescence intensity, which is proportional to analyte concentration. Wavelength selectors are not necessary for excitation and emission process due to the broad excitation spectrum of QDs, the selective and specific reaction between CdTe QDs and NAC, and the mathematical model employed for the treatment of digital images. The limit of detection and relative standard deviation were estimated at 0.14 µg mL-1 and < 1.4% (n = 5), respectively. The accuracy was assessed through recovery test (98.5 to 102.8%).The ruggedness of the method was assessed by comparison of the intra- and inter-day using the iodometric titration method at a 95% confidence level. The system presented satisfactory robustness, high sampling rate (153 h-1), and reduced chemical consumption.
flow-batch system; digital images; CdTe quantum dots; fluorescence; N-acetyl-L-cysteine; pharmaceutical formulations
Neste trabalho é proposta a combinação de um sistema flow-batch, pontos quânticos (QDs) e de uma webcam como detector para determinação espectrofluorimétrica automática de N-acetil-L-cisteina (NAC) em formulações farmacêuticas. O NAC gera interações superficiais com os QDs, que resultam no aumento proporcional da intensidade de fluorescência com a concentração do analito. Seletores de comprimento de onda não são necessários para o processo de excitação e emissão de fluorescência devido ao espectro de excitação alargado, a reação seletiva e específica entre QDs de CdTe e NAC, e o modelo matemático de tratamento das imagens digitais. O limite de detecção e o desvio padrão relativo foram estimados em 0,14 µg mL-1 e < 1,4% (n = 5), respectivamente. A exatidão foi avaliada através do teste de recuperação (98,5-102,8%). A robustez do método foi avaliada por comparação intra- e inter-dias, utilizando o método volumétrico de referência ao nível de 95% de confiança. O sistema apresenta robustez satisfatória, alta frequência analítica (153 h-1) e reduzido consumo de produtos químicos.
ARTICLE
Using webcam, CdTe quantum dots and flow-batch system for automatic spectrofluorimetric determination of N-Acetyl-L-cysteine in pharmaceutical formulations
Marcelo B. Lima; Inakã S. Barreto; Stéfani Iury E. Andrade; Luciano F. Almeida; Mário César U. Araújo* * e-mail: laqa@quimica.ufpb.br
Departamento de Química, Centro de Ciências Exatas e da Natureza (CCEN), Universidade Federal da Paraíba, Caixa Postal 5093, 58051-970 João Pessoa-PB, Brazil
ABSTRACT
The combination of a flow-batch system, quantum dots (QDs) and webcam as detector for the automatic spectrofluorimetric determination of N-acetyl-L-cysteine (NAC) in pharmaceutical formulations is proposed. NAC generates surface interactions that result in enhanced QD fluorescence intensity, which is proportional to analyte concentration. Wavelength selectors are not necessary for excitation and emission process due to the broad excitation spectrum of QDs, the selective and specific reaction between CdTe QDs and NAC, and the mathematical model employed for the treatment of digital images. The limit of detection and relative standard deviation were estimated at 0.14 µg mL1 and < 1.4% (n = 5), respectively. The accuracy was assessed through recovery test (98.5 to 102.8%).The ruggedness of the method was assessed by comparison of the intra- and inter-day using the iodometric titration method at a 95% confidence level. The system presented satisfactory robustness, high sampling rate (153 h1), and reduced chemical consumption.
Keywords: flow-batch system, digital images, CdTe quantum dots, fluorescence, N-acetyl-L-cysteine, pharmaceutical formulations
RESUMO
Neste trabalho é proposta a combinação de um sistema flow-batch, pontos quânticos (QDs) e de uma webcam como detector para determinação espectrofluorimétrica automática de N-acetil-L-cisteina (NAC) em formulações farmacêuticas. O NAC gera interações superficiais com os QDs, que resultam no aumento proporcional da intensidade de fluorescência com a concentração do analito. Seletores de comprimento de onda não são necessários para o processo de excitação e emissão de fluorescência devido ao espectro de excitação alargado, a reação seletiva e específica entre QDs de CdTe e NAC, e o modelo matemático de tratamento das imagens digitais. O limite de detecção e o desvio padrão relativo foram estimados em 0,14 µg mL1 e < 1,4% (n = 5), respectivamente. A exatidão foi avaliada através do teste de recuperação (98,5-102,8%). A robustez do método foi avaliada por comparação intra- e inter-dias, utilizando o método volumétrico de referência ao nível de 95% de confiança. O sistema apresenta robustez satisfatória, alta frequência analítica (153 h1) e reduzido consumo de produtos químicos.
Introduction
In recent years, several works have been developed involving the use of cadmium telluride quantum dots (CdTe QDs). These nanocrystals present attractive optical properties, such as size-tunable, broad absorption, narrow emission bands and good photostability.1-3As the optical properties of CdTe QDs strongly depend on the nature of their surface, modifications of the latter with functional groups or biomolecules and the interactions that it could establish with specific analytes can result in dramatic changes in these properties.4,5 In most CdTe QD applications, the detection is based on signal quenching, although more recently attention has been focused on signal enhancing, mainly associated with QD ability to sensitize distinct chemiluminescent systems.6
N-acetyl-L-cysteine (NAC), or simply acetylcysteine, is a derivative of the amino acid L-cysteine used, primarily, as pharmaceutical drug to reduce the viscosity of pulmonary secretions in respiratory diseases.7 Other potential applications of this drug consist in using it to treat paracetamol overdoses,8 as a heavy metal chelating agent to clear the body of some toxic metals and as nutritional supplement by immunocompromised patients.9
The determination of NAC in pharmaceutical formulations is usually performed by methods based on high performance liquid chromatography (HPLC), as recommended by the United States Pharmacopeia10 or by iodometric titration method, as described by the Brazilian Pharmacopoeia.11 Alternatively, several analytical methodologies with different detection techniques were developed and described in the literature for the quantification of NAC, with the automatic approaches that employ flow systems standing out.12,13
Recently, Frigerio et al.13 developed a multipumping flow system (MPFS) for the determination of NAC in pharmaceutical formulations using CdTe quantum dots. The developed approach was based on NAC ability to establish surface interactions that result in enhanced nanocrystal fluorescence intensity, which is proportional to analyte concentration. This methodology allowed a simplification of the previous systems and brought important advantages in terms of analytical application, such as simplicity of assemblage and operation, compact and straightforward configuration increasing versatility and minimizing solution consumption. However, in this work, as in all others, the employed detection system was the conventional spectrofluorometer.
The use of a webcam that is readily available in the market can be an alternative to a spectrofluorometer. This device does not require wavelength selection, allows simplification of the instrumentation, implements chemometric treatments due to the trivariate nature of the detection (when RGB data is used), as well as the spatial-resolution characteristics inherent in digital images.14
Digital image-based methods have frequently been used as an alternative for quantitative determinations in analytical sciences.15-17 Recent advances in digital image acquisition technology have offered video cameras (webcam) based on charge-coupled devices (CCD), which are capable to capture digital images with up to 24 bits (16.7 million colors). In fact, by using the RGB color system, the primary colors are combined in different intensities with values varying in the range 0-255 (8 bits) per color. Thus, procedures based on digital images are very sensitive, since they include abilities to identify little difference between the colors of images (the analytical signals).18
The flow-batch (FB) system is a promising alternative for the development of novel automatic procedures using CdTe QDs because of its advantageous characteristics, such as sampling throughput, reduced chemical and sample consumption, efficient and fast homogenization.19 These automatic systems use an instantaneous stop chamber drawn upon the useful features of batch and flow methods by using programmed multi-commutation.20 The main component is the mixing chamber (MC) where the whole analytical process, including: fluid addition, sample pretreatment, homogenization, precipitation, extraction, preparation of calibration solutions, and detection, takes place under the total control of the software.21 Classical (discrete) methods can be performed with precision, accuracy and speed similar to other flow analysis methods.22
In this work, for the first time, an automatic flow-batch system for the spectrofluorimetric determination of N-acetyl-L-cysteine in pharmaceutical formulations using CdTe quantum dots and a webcam as detector is proposed. The employed approach takes advantage of the capacity of the organic compound to generate surface interactions that result in enhanced CdTe nanocrystal fluorescence intensity, which is proportional to analyte concentration.13 These nanocrystals were successfully synthesized in aqueous medium using mercaptopropionic acid (MPA).
Experimental
Reagent solutions
All reagents were of analytical grade and freshly distilled and deionized water (> 18 MΩ cm1) was used to prepare all solutions. Reagents were not subjected to any further purification.
A 100 µg mL1N-acetyl-L-cysteine (NAC, Sigma) stock standard solution was prepared by dissolving 10 mg in 100 mL of deionized water and kept in the refrigerator. Standard solutions with concentration of 5.0 to 50.0 µg mL1 of NAC were prepared, on a daily basis, by appropriate dilution of the above stock solution in 15 mmol L1 acetate buffer solution at pH 5.2 to use in titrimetric reference method.
For the synthesis of the CdTe QDs the following reagents were used: sodium borohydride (NaBH4, 99%), tellurium powder (200 mesh, 99.8%), cadmium chloride hemi (pentahydrate) (CdCl2·2.5 H2O, 99%) and 3-mercaptopropionic acid (MPA, 99%) purchased from Sigma-Aldrich. For adjusting the alkalinity of the reaction medium, a 1.0 mol L1 NaOH solution was used.19,23
Sample preparation
Ten commercial pharmaceutical formulations from several manufacturers were purchased from local suppliers in João Pessoa, Paraíba, Brazil. Five formulations in granulated form and five in liquid form containing NAC were analyzed according to the automatic proposed procedure. For the granulated forms, these were accurately weighed, dissolved in water and filtered. For the determination of N-acetyl-L-cysteine content, only dilutions with 15 mmol L1 acetate buffer solution at pH 5.2 were required. The amounts of sample were defined in accordance with the labeled NAC contents of the assayed samples.
Apparatus
A tungsten-halogen lamp (Ocean Optics, model LS-1-LL) was used as radiation source to promote CdTe QD fluorescence. A Philips VGA webcam with a CCD sensor (model SPC900NC) was used in conjunction with LabVIEW2013 (National Instruments) software to control the flow-batch analyzer. The pictures were captured by means of the software written in Delphi (version 3.0). The webcam was connected to the USB port of an Intel Core2Duo 2 Gigabyte microcomputer (PC), and configured to capture 24-bit digital images (16.7 million colors) at a rate of 30 frames s1 and a 640 × 480 pixels spatial resolution.
Four solenoid mini-pumps (Takasago Fluidic Systems, model MLP-200TF, 12VDC, 36453)24 with nominal values of 200 µL were used for the fluidic addition in the mixing chamber of the automatic system. The solenoid mini-pumps were controlled by the microcomputer using an USB interface (USB6009, National Instruments)25 and an external lab-made electronic actuator (Figure 1), which provided the required increase in power (potential difference and current) for the signal sent by the microcomputer.
The circuit of the lab-made electronic actuator is based on an ULN2803 integrated circuit. As described in its data sheet,26 this device is a high-voltage, high-current Darlington transistor array with eight NPN Darlington pairs that feature high-voltage outputs with common-cathode clamp diodes for switching inductive loads. The collector-current rating of each Darlington pair is 500 mA. The Darlington pairs may be connected in parallel for higher current capability.
For the characterization of the synthesized nanocrystals, QDs absorption spectra were obtained by using a UV-Vis spectrophotometer (model 8453, Hewlett-Packard). The fluorescence measurements were performed by a multi-channel CCD spectrophotometer (model USB4000, Ocean Optics) with a tungsten-halogen light source (model LS-1-LL, Ocean Optics). A glass flow cell with 1-cm optical path and 80 µL internal volume (Hellma, Plainview, NY, USA) was used with two 100 µm i.d. optical fibers.
Synthesis of CdTe QDs
CdTe QDs were synthesized as previously described19,23 with some modifications. Briefly, NaHTe solution was prepared by reaction between NaBH4 (1 × 103 mol) and the tellurium powder (0.4 × 103 mol) in N2 saturated water (20 mL). The reaction mixture was heated at 80 ºC for 30 min under N2 flow to get an intense red clear solution. Then, the NaHTe solution obtained was stored for further use at room temperature still under the protection of N2.
The resulting NaHTe solution was transferred to another flask containing CdCl2 (4 × 103 mol) and MPA (7 × 103 mol) in a 100 mL N2 saturated water solution. The pH of the solution was adjusted to 11.5 by addition of 1.0 mol L1 NaOH solution. The Cd2+:Te2:MPA molar ratio was fixed as 1:0.1:1.7. CdTe QD size was tuned by varying the heating time. In order to remove the contaminants, purification of QDs was performed by precipitation in absolute ethanol. The precipitate fractions were subsequently centrifuged, vacuum dried and kept in the refrigerator.
The nanocrystal size for the synthesized CdTe QDs was calculated as shown in equation 1:27
where D is the diameter or size of the nanocrystals (nm) and λ is the wavelength of maximum absorbance corresponding to the first excitonic absorption peak of the crystal.
CdTe QD aqueous solution molar concentration was determined by appraising the extinction coefficient (ε), calculated as shown in equation 2:27
where ΔE is the transition energy corresponding to the first absorption peak expressed in eV. D (nm) is the size of the CdTe QDs. Knowing both ε and the absorbance peak of the nanocrystal solution, the molar concentration was calculated by applying Lambert-Beer's law.
Flow-batch system
A schematic diagram of the proposed flow-batch is shown in Figure 2. The homemade mixing chamber (MC) was built of polytetrafluoroethylene (PTFE). It has a total volume of 2.0 mL and three quartz windows (W1, W2 and W3) mounted at 180º and 90° from each other (1 cm optical path).
The webcam was used in the proposed system as a simple and inexpensive detector for measuring fluorescence emission, just as a photomultiplier tube actuates in spectrofluorometers, i.e., a detector placed at 90 degrees of excitation beam. So, the webcam was placed at a position (see top view of Figure 2) such that the digital images are captured through W1 perpendicular to the radiation beam from the tungsten lamp,which enters W2 and exits thru W3. Although this configuration at 90 degrees does not guarantee complete elimination of an eventual spurious radiation from the excitation source (tungsten-halogen lamp), it is significantly removed by subtracting the blank picture (CdTe QDs without analyte) from pictures of the samples and standard solutions (see "Treatment of digital images and mathematical model" and "Captured digital images" sections). Moreover, this system was mounted onto a suitable support in a black (darkroom) box measuring 15 cm × 12 cm × 9 cm, to preserve the system from the effects of spurious environmental radiation while in operation.
The fluids are added with solenoid mini-pumps with nominal values of 200 µL (µP1 to µP4) per pulse. Teflon® tubes with 0.5 mm internal diameter were used for fluid transport. The mixture of solutions was performed by a stirring bar (SB) located inside the mixing chamber (MC), driven by a magnetic stirrer (MS).The magnetic stirrer is not stopped during all analytical procedure, including the image acquisition stage.
Automatic analytical procedure
Before starting the analytical procedure, solenoid mini-pumps µP1-µP3 are simultaneously switched on (for 5 pulses) and the working solutions (S, QDs, C) are pumped towards the MC to fill the channels between the mini-pumps and the chamber. Then, immediately, the discard µP4 is opened for 3 pulses and then all MC content is emptied by displacing the solution contained in them towards waste. This channel filling procedure is very important and must be carried out whenever there is a change of the reservoir liquids.
The analyzer was operated as described in Table 1 for the N-acetyl-L-cysteine determination using digital images. The solenoid mini-pumps were actuated at 2 Hz, yielding flow rates of 24 mL min1 (µP1 to µP4).
Sample or standard solution (3 pulses, adding 600 µL by µP1) and CdTe quantum dot solution (3 pulses, adding 600 µL by µP2), were added simultaneously. The homogenization was performed by the stirring bar (SB) located inside the MC. Then, ten digital images (pictures) were captured and archived for later post processing. Finally, all content of MC was aspirated towards waste (6 pulses, removing 1200 µL by µP4).
Afterwards, the MC is cleaned by activation of µP3 (6 pulses, adding 1200 µL by µP3), adding 1200 µL of the water while activated, and agitation for cleaning occurs for 2 seconds. Then, µP4 is activated (6 pulses, removing 1200 µL by µP4) to discard the contents of the MC. This cleaning and discard procedure must be done twice to effectively clean the MC.
The procedure for in-line blank preparation is similar to the one described for the sample analysis. The difference is that activation of µP3 (3 pulses, adding 600 µL by µP3) is used instead of the sample or standard solutions.
Treatment of digital images and mathematical model
The treatment of the captured digital images was made by means of a second software also written in Delphi (version 3.0). The routine with the working stages of this software is similar to that used elsewhere14,18 and illustrated in Figure 3.
Initially, the user selects the most homogeneous region in the picture which will define the coordinates of the selected region, and will also be used for all other images. Then the software scans all the pixels column by column to extract the RGB component for each pixel and calculate a mean integer value for each RGB component. These mean values are used in the RGB-based value calculation (analytical response) as described below.
The RGB-based values were calculated by means of a mathematical model developed from the concept of vector norm "||ν||",28 calculated as shown in equation 3:
where
sb,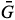 sb and
sb and 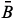 sb result from the difference between the
sb result from the difference between the 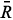 s,
s, 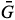 s and
s and 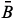 s average values obtained from digital images of the standard solutions and samples and
s average values obtained from digital images of the standard solutions and samples and 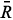 b,
b, 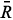 b and
b and 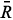 b from the blank.
b from the blank.
As a result, a linear relationship was observed between the analyte concentration (C) in the standard solution (or sample) and RGB data, for which the following equation is valid:
As demonstrated in previous work,14,18 equation 4 provides the basis for building micro flow-batch analysis (µFBA) analytical curves, establishing a linear relationship between ||ν|| (RBG data value adopted as analytical response) and analyte concentration in standard solutions. Furthermore, the vectors associated with the digital images from each analyte should be positioned on the same support line in the RGB three-dimensional space.
Reference method
The experimental results using a flow-batch system for the determination of N-acetyl-L-cysteine in pharmaceutical formulations were compared with those obtained by iodometric titration method, as described in the 5th edition of the Brazilian Pharmacopoeia.11 The method consists in the titration of N-acetyl-L-cysteine solutions with 0.05 mol L1 iodine, in the presence of 1 mL of starch as indicator.
Results and Discussion
Flow-batch parameters
The determination method is based on the interaction of N-acetyl-L-cysteine (NAC) with CdTe QDs. The developed approach was based on NAC ability to establish surface interactions that result in enhanced nanocrystal fluorescence intensity, proportional to analyte concentration, as shown in Figure 4a. The maximum emission peak for the NAC-CdTe QDs is 636 nm (Figure 4a). The interaction with the dots' surface can be explained by complexation of incompletely bonded cadmium ions (Cd2+ surface traps) which contributed to improve dot passivation inducing a quantitative modification of the optical properties.13
A preliminary spectrofluorimetric evaluation was performed for four different sizes of CdTe QDs being 1.82, 2.34, 2.93 and 3.25 nm, corresponding with the fluorescence peaks of 521, 540, 582 and 636 nm, respectively. This study did not reveal significant influence (of the sizes) on the measured fluorescence signal (NAC-CdTe QDs), under equivalent conditions of pH and concentration. Therefore, the 3.25 nm nanocrystals employed at 5 × 104 mol L1 CdTe QDs were chosen for the digital image studies.
Fluorescence emission is closely related to the interaction of NAC with the QDs surface (at pH 5.2). This radiation emission of the samples (Figure 4b) enables the use of a webcam as detector (for the picture capture) due to the selective and specific reaction between CdTe QDs and NAC present in the samples. Moreover, CdTe QDs are characterized by their broad excitation spectrum,29,30 allowing the use of an inexpensive excitation source (tungsten-halogen lamp, for example). Thus, for excitation, a wavelength selector is not necessary for the proposed system and method.
The webcam was only used in the proposed system as a simple and inexpensive detector for measuring fluorescence emission. An eventual spurious radiation from the emission source (tungsten-halogen lamp) is removed with the subtraction of the blank picture. A wavelength selector is also not necessary due to the mathematical model employed for the treatment of digital images.
Volumes of samples of CdTe QD solution to be added inside MC during the automatic procedure were evaluated in order to improve the sensitivity and reproducibility of the analytical signal. Volumes of 600 µL of sample (or standard solution) and reagents were selected as the best compromise between reproducibility and sensitivity considering the minimum volume of the MC (1200 µL), which is necessary to fulfill its optical path with the processed fluid.
Captured digital images
Figure 5 shows the digital images obtained from five standard solutions with different concentrations. The first image of the sequences is the blank solution. All images represent a selected area equal to 50 × 80 pixels. As can be seen in Figure 5, the images present an increase in the intensity for the color orange with analytical concentration in each standard solution.
Each sampled image is a matrix with 640 × 480 pixels and the delimited region by the user is a matrix with about 50 × 80 pixels. The pixel resolution is 87 × 87 dots per inch. The RGB-based value calculations are based on the product 2R × 2G × 2B, where R, G and B are the red, green and blue color components, respectively. These components may assume integer values in a range from 0 to 7, reaching up to 16,777,216 colors.
Other linear relationships between complex color and the ratios B × R1, B × G1, B × (Itot)1 (Itot = RGB) and log (B) were also studied to possibly maximize precision, as already done in other papers.14,17,18 However, in all cases the results were poor when compared to using vector norm for concentration of the complex formed. The statistical quality of the regression was verified by the residuals and analysis of variance (ANOVA). They confirmed the homoscedastic distribution of residuals and a significant linear regression.
Potentially interfering substances
The selectivity of the method using digital images was investigated by studying the effect of excipients usually found in pharmaceutical formulations, such as ethylenediamine-tetraacetic acid (EDTA), sodium chloride (NaCl), sodium benzoate, citric acid and sucrose.12,13 Samples containing NAC at a fixed concentration of 15 µg mL1 and increasing concentrations of the excipient (up to a 100-fold molar ratio) were analyzed by the developed automatic method. A compound was considering as non-interfering if the analytical signal variation was ± 3% when compared to the one obtained in its absence. The results (Table 2) showed that under the system operating conditions, no interfering effect was observed.
FBA applications
For the determination of N-acetyl-L-cysteine (NAC) in pharmaceutical formulations using FB and webcam as detector, the regression equation of the analytical curve was R = 91.995 + 1.2614C, where R is the analytical response and C is the NAC concentration in µg mL1 in the measuring solution. The squared linear correlation coefficient, r2 was 0.998 (n = 5) in the range between 5.0 to 50.0 µg mL1. This curve was statistically validated by analysis of variance (ANOVA), showing no significant lack of fit in the proposed models at a 95% confidence level. The limit of detection (LOD) and the limit of quantification (LOQ) were estimated based on the criteria established by the International Union of Pure and Applied Chemistry (IUPAC)31 and their values were 0.14 and 0.48 µg mL1, respectively.
Table 3 presents the results for the proposed FB, and the reference method for the NAC in pharmaceutical formulations. No statistically significant differences were observed between the results at a confidence level of 95% when applying the paired t-test. The relative standard deviation (RSD%) was less than 1.4% (n = 5).
Importantly, based on the results presented in Table 3, the effect of spurious and environmental radiation was not significant to NAC determination when using a webcam as a detector. This result may have been obtained due to the use of CdTe nanocrystals, which emit a characteristic narrow and well-defined spectral radiation, independent of the excitation wavelength. Also, the use of a black box (darkroom), where the measurements occurred, did not allow environmental radiation interference while capturing the pictures.
Besides the comparison with the reference method, the use of a recovery test is another means of assessing the accuracy of the proposed method. The recoveries were investigated using three different pharmaceutical formulations. The volume of 1.0 mL standard solution with known concentrations of 5.0, 15.0 and 25.0 µg mL1 was added to 9.0 mL of the pharmaceutical products (20.3, 100.3 and 120.2 mg mL1 of NAC), for measurement using the proposed FB system. The recovery values obtained are shown in Table 4. As can be seen, the recoveries obtained for each of the samples were within the 98.5-102.8% range.
Table 5 presents selected analytical features of the proposed FBA and other recent procedures,12,13 as described in the literature, for NAC determination in pharmaceutical formulations. In general, FB (compared to the other methods) presents satisfactory parameters, such as detection limit, relative standard deviation (RSD%), working range and sampling rate. Moreover, the proposed system does use carrier fluid to transport the sample towards the detector, as in previous papers.12,13
Conclusions
In this study, we proposed the use of FB system as a novel strategy for spectrofluorimetric quantitative chemical analysis involving the use of QDs and digital images. The proposed methodology was successfully applied for the automatic spetrofluorimetric determination of N-acetyl-L-cysteine (NAC) in pharmaceutical formulations using CdTe QDs.
NAC generates surface interactions that result in enhanced QD fluorescence intensity, which is proportional to analyte concentration. Wavelength selectors are not necessary for excitation and emission process due to the broad excitation spectrum of QDs, the selective and specific reaction between CdTe QDs and NAC, and the mathematical model employed for the treatment of digital images.
Acknowledgments
The authors would like to thank the Brazilian agencies CNPq and CAPES for the research fellowships and scholarships.
Submitted: March 11, 2014
Published online: June 27, 2014
- 1. Costas-Mora, I.; Romero, V.; Lavilla, I.; Bendicho, C.; TrAC, Trends Anal. Chem. 2014, 57, 64.
- 2. Molina-García, L.; Llorent-Martínez, E. J.; Fernández-de- Córdova, M. L.; Santos, J. L. M.; Rodrigues, S. S. M.; Ruiz-Medina, A.; J. Pharm. Biomed. Anal. 2013, 80, 147.
- 3. Rodrigues, S. S. M.; Lima, A. S.; Teixeira, L. S. G.; Korn, M. G. A.; Santos, J. L. M.; Fuel 2014, 117, 520.
- 4. Chen, S.; Tian, J.; Jiang, Y.; Zhao, Y.; Zhang, J.; Zhao, S.; Anal. Chim. Acta 2013, 787, 181.
- 5. Rodrigues, S. S. M.; Ribeiro, D. S. M.; Molina-Garcia, L.; Medina, A. R.; Prior, J. A. V.; Santos, J. L. M.; Talanta 2014, 122, 157.
- 6. Chen, H.; Lin, L.; Li, H.; Lin, J.; Coord. Chem. Rev. 2014, 263, 86.
- 7. Silva, I. S.; Araújo, M. F. A.; Ferreira, H. A.; Varela Jr., J. J. G.; Tanaka, S. M. C. N.; Tanaka, A. A.; Angnes, L.; Talanta 2011, 83, 1701.
- 8. Tsai, C.; Chang, W.; Weng, T.; Fang, C.; Walson, P. D.; Clin. Ther. 2005, 27, 336.
- 9. Al-Dbass, A. M.; J. King Saud Univ., Sci., in press, DOI: 10.1016/j.jksus.2013.08.006.
- 10. The United States Pharmacopeia, USP 27-NF 22, The United States Pharmacopeial Convention: Rockville, MD, 1990, pp. 27.
- 11. Brazilian Pharmacopoeia, 5th ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2010, p. 563.
- 12. Suarez, W. T.; Vieira, H. J.; Fatibello-Filho, O.; J. Pharm. Biomed. Anal. 2005, 37, 771.
- 13. Frigerio, C.; Abreu, V. L. R. G.; Santos, J. L. M.; Talanta 2012, 96, 55.
- 14. Lima, M. B.; Andrade, S. I. E.; Barreto, I. S.; Almeida, L. F.; Araújo, M. C. U.; Microchem. J. 2013, 106, 238.
- 15. Lopez-Molinero, A.; Liñan, D.; Sipiera, D.; Falcon, R.; Microchem. J. 2010, 96, 380.
- 16. Wongwilai, W.; Lapanantnoppakhun, S.; Grudpan, S.; Grudpan, K.; Talanta 2010, 81, 1137.
- 17. Andrade, S. I. E.; Lima, M. B.; Barreto, I. S.; Lyra, W. S.; Almeida, L. F.; Araújo, M. C. U.; Silva, E. C.; Microchem. J. 2013, 109, 106.
- 18. Lima, M. B.; Andrade, S. I. E.; Silva Neta, M. S.; Barreto, I. S.; Almeida, L. F.; Araújo, M. C. U.; J. Braz. Chem. Soc. 2014, 25, 898.
- 19. Lima, M. B.; Andrade, S. I. E.; Barreto, I. S.; Araújo, M. C. U.; Food Anal. Meth., in press, DOI: 10.1007/s12161-014-9794-x.
- 20. Barreto, I. S.; Andrade, S. I. E.; Lima, M. B.; Silva, E. C.; Araújo, M. C. U.; Almeida, L. F.; Talanta 2012, 94, 111.
- 21. Barreto, I. S.; Lima, M. B.; Andrade, S. I. E.; Araújo, M. C. U.; Almeida, L. F.; Anal. Methods 2013, 5, 1040.
- 22. Diniz, P. H. G. D.; Almeida, L. F.; Harding, D. P.; Araújo, M. C. U.; TrAC, Trends Anal. Chem. 2012, 35, 39.
- 23. Zou, L.; Gu, Z.; Zhang, N.; Zhang, Y.; Fang, Z.; Zhu, W.; Zhong, X.; J. Mater. Chem. 2008, 18, 2807.
-
24http://www.takasago-fluidics.com/pdf/mlp-200p-e.pdf accessed in May 2014.
» link -
25http://www.ni.com/pdf/manuals/371303m.pdf accessed in May 2014.
» link -
26http://www.ti.com/lit/ds/symlink/uln2803a.pdf accessed in May 2014.
» link - 27. Yu, W. W.; Qu, L.; Guo, W.; Peng, X.; Chem. Mater. 2003, 15, 2854.
- 28. Lyra, W. S.; Santos, V. B.; Dionízio, A. G. G.; Martins, V. L.; Almeida, L. F.; Gaião, E. N.; Diniz, P. H. G. D.; Silva, E. C.; Araújo, M. C. U.; Talanta 2009, 77, 1584.
- 29. Frigerio, C.; Ribeiro, D. S. M.; Rodrigues, S. S. M.; Abreu, V. L. R. G.; Barbosa, J. A. C.; Prior, J. A. V.; Marques, K. L.; Santos, J. L. M.; Anal. Chim. Acta 2012, 735, 9.
- 30. Llorent-Martínez, E. J.; Molina-García, L.; Kwiatkowski, R.; Ruiz-Medina, A.; Talanta 2013, 109, 203.
- 31. McNaught, A. D.; Andrew, W.; IUPAC Compendium of Chemical Terminology, 2nd ed.; Royal Society of Chemistry: Cambridge, 1997.
Publication Dates
-
Publication in this collection
30 Sept 2014 -
Date of issue
Sept 2014
History
-
Received
11 Mar 2014 -
Accepted
27 June 2014














