Abstracts
The aim of this study was to evaluate the degree of conversion (DC) and the cytotoxicity of photo-cured experimental resin composites containing 4-(N,N-dimethylamino)phenethyl alcohol (DMPOH) combined to the camphorquinone (CQ) compared with ethylamine benzoate (EDAB). The resin composites were mechanically blended using 35 wt% of an organic matrix and 65 wt% of filler loading. To this matrix was added 0.2 wt% of CQ and 0.2 wt% of one of the reducing agents tested. 5x1 mm samples (n=5) were previously submitted to DC measurement and then pre-immersed in complete culture medium without 10% (v/v) bovine serum for 1 h or 24 h at 37 °C in a humidifier incubator with 5% CO2 and 95% humidity to evaluate the cytotoxic effects of experimental resin composites using the MTT assay on immortalized human keratinocytes cells. As a result of absence of normal distribution, the statistical analysis was performed using the nonparametric Kruskal-Wallis to evaluate the cytotoxicity and one-way analysis of variance to evaluate the DC. For multiple comparisons, cytotoxicity statistical analyses were submitted to Student-Newman-Keuls and DC analysis to Tukey's HSD post-hoc test (=0.05). No significant differences were found between the DC of DMPOH (49.9%) and EDAB (50.7%). 1 h outcomes showed no significant difference of the cell viability between EDAB (99.26%), DMPOH (94.85%) and the control group (100%). After 24 h no significant difference were found between EDAB (48.44%) and DMPOH (38.06%), but significant difference was found compared with the control group (p>0.05). DMPOH presented similar DC and cytotoxicity compared with EDAB when associated with CQ.
polymer; reducing agent; degree of conversion; photo-initiator system
O objetivo deste estudo foi avaliar o grau de conversão (GC) e a citotoxicidade de resinas compostas experimentais utilizando o álcool 4-(N,N-dimetilamino) fenil etílico (DMPOH) associado à canforoquinona (CQ) como sistema fotoiniciador (SF) comparado à versão comercial utilizando o benzoato de etilamina (EDAB). Para tanto, as resinas compostas experimentais foram mecanicamente misturadas utilizando (em peso): 35% de matriz orgânica e 65% em peso de partículas de carga. Posteriormente, foram adicionados 0,2% de CQ e 0,2% de um dos agentes redutores testados. Amostras de 5 x 1 mm (n=5) foram previamentes submetidas à análise de GC e posteriormente, esterilizadas e colocadas no meio de cultura completo sem soro fetal bovino estéril por 1 h ou 24 h a 37 °C em encubadora com 5% de CO2 and 95% de umidade para avaliar os efeitos citotóxicos das resinas compostas experimentais utilizando o método MTT emcélulas células humanas imortalizadas de queratinócitos. Os dados de citotoxicidade foram submetidos à análise estatística de Kruskal-Wallis e de GC à análise de variância com um fator. Em virtude da ausência de normalidade, a análise estatística da citotoxicidade foi realizada utilizando-se o teste não-paramétrico de Kruskal-Wallis. Para o GC, os dados foram submetidos à análise de variaância de 1 fator. Posteriormente para múltiplas comparações, os dados de citotoxicidade foram submetidos ao teste Student-Newman-Keuls e o GC ao teste de Tukey's HSD post-hoc (=0.05). Não foi observada diferença estatística entre o GC de DMPOH (49,9%) e EDAB (50,7%). Para os resultados de 1 h não houve diferença na viabilidade celular entre EDAB (99,26%), DMPOH (94,85%) e o grupo controle (100%). Após 24 h, nenhuma diferença estatística foi encontrada entre EDAB (48,44%) e DMPOH (38,06%), entretanto, diferença significativa foi encontrada em relação ao grupo controle (p>0,05). O DMPOH apresentou GC e citotoxicidade semelhante à EDAB quando associado à CQ.
Introduction
The improvements in physical and mechanical properties of resin composites enabled its use to perform direct and indirect restorations (1)1 1. Furuse AY, Mondelli J, Watts DC. Network structures of Bis-GMA/TEGDMA resins differ in DC, shrinkage-strain, hardness and optical properties as a function of reducing agent. Dent Mat 2011;27:497-506.. But, while the yellowing effect remains in the dental composite resins, color stability still will be the major reason for the necessity of replacement of esthetical restorations (2)2 2. Mundim F, Pires-de-Souza F, Garcia L, Consani S. Colour stability, opacity and cross-link density of composites submitted to accelerated artificial aging. Eur J Prosthodont Rest Dent 2010;18:89-93..
The camphorquinone (CQ) is the most widely and successfully photo-initiator used in commercial dental resin composites. As a type II photo-initiator, the CQ needs a co-initiator to react and create free radicals to initiate the reaction of the photo-cured resin composites (3)3 3. Neumann MG, Miranda WG Jr., Schmitt CC, Rueggeberg FA, Correa IC. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J Dent 2005;33:525-532.. Despite their high clinical acceptance, photo-initiator systems based on CQ combined with tertiary amines as a co-initiator presents a yellowing effect on the color of these resin composites causing color change (4)4 4. Alvim HH, Alecio AC, Vasconcellos WA, Furlan M, Oliveira JE, Saad JRC. Analysis of camphorquinone in composite resins as a function of shade. Dent Mat 2007;23:1245-1249..
Color change is mostly caused because tertiary amines have double bonds, which are capable of absorbing UV light and creating higher energy states ( 55 5. Ruyter I, Nilner K, Moller B. Color stability of dental resin materials for crown and bridge veneers. Dent Mat 1987;3:246-251. , 66 6. Heydecke G, Zhang F, Razzoog M. In vitro color stability of double-layer veneers after accelerated aging. J Prosthet Dent2001;85:551-557. ). These molecules can react with oxygen or other aromatic groups and form conjugated systems, also called color centers or chromospheres (7)7 7. Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent 2000;12:300-308., increasing the absorption of visible light, especially in the blue region of the electromagnetic spectrum, causing the yellowing of the material(8)8 8. Darvell B. Materials science for dentistry. 7th ed. Hong Kong: BW Darvel; 2002.. As CQ-based resin composites as photo-initiator causes a yellowing effect caused by the oxidation of the tertiary amine used as co-initiators ( 44 4. Alvim HH, Alecio AC, Vasconcellos WA, Furlan M, Oliveira JE, Saad JRC. Analysis of camphorquinone in composite resins as a function of shade. Dent Mat 2007;23:1245-1249. , 77 7. Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent 2000;12:300-308. , 88 8. Darvell B. Materials science for dentistry. 7th ed. Hong Kong: BW Darvel; 2002. , 99 9. Silami FDJ, Mundim FM, Garcia LFR, Sinhoreti MAC, Pires-de-Souza FCP. Colour stability of experimental composites containing different photoinitiators. J Dent 2012;41:e62-e66. , 1010. Albuquerque PPA, Moreira AL, Moraes RR, Cavalcante LM, Schneider LFJ. Colour stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J Dent 2012;41:e67-e72. , 1111. Ely C, Schneider LFJ, Ogliari FA, Schmitt CC, Corrêa IC, Lima JS, et al.. Polymerization kinetics and reactivity of alternative initiator systems for use in light-activated dental resins. Dent Mat 2012;28:1199-1206. , 1212. Brandt WC, Tomaselli LO, Correr-Sobrinho L, Sinhoreti MAC. Can phenylpropanedione influence Knoop hardness, rate of polymerization and bond strength of resin composite restorations? J Dent 2011;39:438-447. ); this clearly imposes limitations for color stability of those composite restorations.
Alternative co-initiators were also suggested to substitute the tertiary amines on dental composite resins associated with the CQ as the photo-initiator system (13)13. Schroeder WF & Vallo CI. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent Mat 2007;23:1313-1321.. The color stability of the amines is more influenced more by the nature of the branch on the aromatic ring than on the nitrogen atom (14)14. Bowen RL, Argentar H. Amine accelerators for methacrylate resin systems. J Dent Res 1971;50:923-928.. The idea of substituting the usual branches on the aromatic ring of amines by an alcohol to be used a co-initiator, such as the 4-(N,N-dimethylamino)phenethyl alcohol (DMPOH) is applicable( 1313. Schroeder WF & Vallo CI. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent Mat 2007;23:1313-1321. ); its kinetics efficiency during polymerization of filled resin composites is still not established. Also, until evaluating new substances, which would be used as new photoiniators or reducing agents in dentistry, its biocompatibility and chemical reaction effectiveness should be evaluated and confirmed.
Despite these reducing agents are not supposed to be totally biocompatible, as the polymerization occurs, the amount of photo-initiator system is reduced after reacting (15)15. Braden M, Causton BE, Clarke RL. Diffusion in water in composite filling materials. J Dent Res 1976;55:730-732.. However, this polymerization process occurs differently according to the photo-initiator used in the resin composite formulation as well as the co-initiator associated and the degree of conversion evaluation is extremely important in order to provide information about the efficient of the photo-initiator system and also if its capable to affect the biological behavior of the resin material.
Thus, in order to evaluate if the association of the DMPOH would promote acceptable degree of conversion of CQ-based resin composites, the present study evaluated the degree of conversion and the cytotoxicity of photo-polymerized experimental dental composite resins using 4-(N,N-dimethylamino)phenethyl alcohol (DMPOH) combined with CQ as the photoinitiator system compared with commercial version using ethylamine benzoate (EDAB). The tested hypotheses were: (i) DMPOH and EDAB will promote similar degree of conversion; (ii) DMPOH and EDAB will not reduce cells viability; (iii) DMPOH and EDAB will have similar cytotoxicity.
Method and Materials
Experimental Dental Composite Resin Production
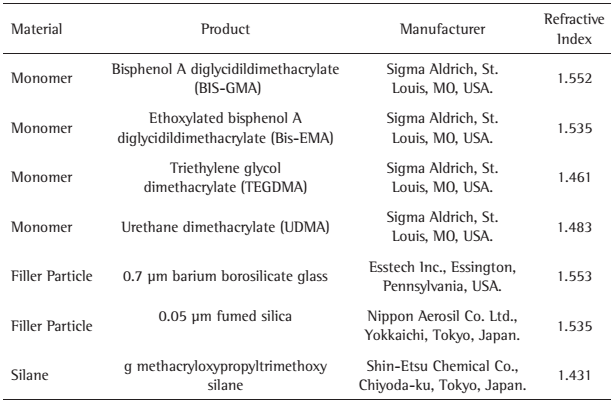
Table 1 shows the experimental dental composite resin was produced blending 35 wt% of an organic matrix (29 wt% Bis-GMA, 32.5 wt% UDMA, 32.5 wt% Bis-EMA, 6 wt% TEGDMA) and 65 wt% of filler content (20 wt% 0.05 μm fumed silica filler and 80 wt% 0.7 μm BaBSiO2 previously treated with 6.5 wt% of -methacryloxypropyltrimethoxy silane) using a centrifugal mixing device (SpeedMixer, DAC 150.1 FVZ-K; Hauschild Engineering, Hamm, Germany), similar to commercial resin-based composites. To this matrix was added: 0.2 wt% of CQ and 0.2 wt% of one of the reducing agents tested (DMPOH or EDAB). 0.01% of 2,6-bis(1,1-dimethylethyl)-4-methylphenol (0.01 wt%) were used as a photo-polymerization inhibitor. All chemical components are shown in Table 1.
Material type, chemical product, manufacturer and refractive index of resin composites composition
Specimen Preparation and Degree of Conversion Measurement
The DC for each resin was measured using Fourier transform infrared spectroscopy (FTIR). The FTIR spectrophotometer was coupled to an attenuated total reflectance (ATR) (Spectrum 100, PerkinElmer, MA, USA) attachment as previously described(16)16. Atai M, Watts DC. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dent Mat 2006;22:785-791.. Absorbance spectra included 16 scans at a resolution of 1 cm-1. Unpolymerized blends were scanned within a Teflon mold (=4 mm x 1 mm thickness) placed on the ATR. The resin blends were photo-activated through a polyester strip with 16 kJ/cm2 using a light-curing unit (Bluephase, Ivoclar Vivadent, Schaan, Liechtenstein). The polymerized samples were scanned 48 h later, and unconverted carbon double bonds were quantified by calculating the ratio derived from the aliphatic C=C (vinyl) absorption (1638 cm-1) to the aromatic C=C absorption (1608 cm-1) signals for both polymerized and unpolymerized samples (n=10). The DC for each resin was calculated according to the follow equation:
where Xa (polymerized) and Xb (unpolymerized) represent the bands of the polymerizable aliphatic double bonds, and Ya (polymerized) and Yb (unpolymerized) represent the bands of the aromatic double bond.
Cell Culture
The immortalized human keratinocytes cells (HaCaT) were obtained from Rio de Janeiro Cell Bank, Rio de Janeiro, RJ, Brazil. The cells were grown flasks containing complete culture medium: Dulbecco's Modified Eagle Medium (DMEM; Gibco(r), Grand Island, NY, USA), supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco(r)), 10,000 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA), and 10 mg/mL streptomycin (Sigma-Aldrich) at 37 ºC in a humidifier incubator with 5% CO2 and 95% humidity. The growth was permitted until the cells achieved the confluence. The cells used in these studies were between 6-8th passages.
Treatment of cells with sterilized disk-shapped specimens
After incubation and semi confluence, the human keratinocytes cells (HaCaT) were trypsinized, washed and re-suspended to a concentration of 104 cells mL-1 and then seeded in a 96-well culture plates (TPP(r) Techno Plastic Products AG, Trasadingen, Switzerland) containing 0.2 mL of complete growth medium and incubated for 24 h at 37 ºC in 5% CO2 and 95% relative humidity to allow attachment. After 24 h, the complete culture medium in the 96-well was removed from the wells, washed twice with 200 µL sterile phosphate-buffered saline (PBS, Sigma-Aldrich) and then 200 µL of complete culture medium without 10% (v/v) of fetal bovine serum (FBS, Gibco(r)) was added into wells. After that, the experimental dental resin composites (disc-shaped specimens - Φ=4 mm x 1 mm thickness) were previously sterilized in ethylene oxide gas and immersed into wells. The cells were further incubated for 1 h or 24 h at 37 oC in a humidifier incubator with 5% CO2 and 95% humidity. The negative control group was placed in the culture medium only.
Cell viability assay (MTT)
Toxic effects of the experimental dental composite resin on cell viability were determining by measuring cell viability using MTT assay (MTT assays Life Technologies Corp., Eugene, OR, USA). This assay is based upon the ability of viable cells to cleave the tetrazolium salt (3- [4, 5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) to a formazan dye. Briefly, the MTT was added to the cells at a final concentration of 0.3 mg/mL and incubated for 4 h at 37 ºC. Purple formazan crystals, produced from the MTT by metabolically active cells, were dissolved with absolute alcohol and its concentration was measured in a spectrophotometer at 570 nm using an ELISA microtiter plate reader (ASYS UVM 340 Biochrom Ltd., Cambridge, UK). Each experimental assay was repeated six times; all assays were performed in triplicate for each evaluated sample.
Results were expressed as percentage (%) control (A570nm in cells exposed to DMEM-agent only) by the following formula:
where, OD means optical density.
Statistical Procedures
As a result of absence of normal distribution, the statistical analysis was performed using the nonparametric Kruskal-Wallis to evaluate the cytotoxicity of each resin blend and one-way analysis of variance to evaluate the degree of conversion. For multiple comparisons, cytotoxicity statistical analyses were submitted to Student-Newman-Keuls and degree of conversion analysis to Tukey's HSD post-hoc test, both at 0.05 significance level.
Results
No significant differences were found between the degree of conversion of any of the reducing agent tested: EDAB (50.7 ±0.3) or DMPOH (49.9 ±0.5). The cell viability (%) median and interquartile range are shown in Table 2. For the cytotoxicity test, after 1 h no significant differences were observed between the cell viability of the control group and the testing groups; these groups showed cell viability reduction after 24 h.
For each of the experimental dental resin-based composite with the different photoinitiator system tested, the median cell viability value (%) ± interquartile range is provided
Discussion
The photo-initiator chemistry of the resin composite is fundamental for efficient polymerization to achieve satisfactory physical-mechanical properties and biological behavior of the polymer in oral condition( 33 3. Neumann MG, Miranda WG Jr., Schmitt CC, Rueggeberg FA, Correa IC. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J Dent 2005;33:525-532. , 77 7. Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent 2000;12:300-308. ). This study tested three hypotheses: the first, that the DMPOH and EDAB would promote similar degree of conversion, was accepted. As the outcomes showed no differences were found between the degree of conversion of DMPOH and EDAB, it indicates that DMPOH is an efficient reducing agent to be used in association with CQ as a photo-initiator system in photo-activated resin composites.
The DMPOH showed to be an efficient reducing agent to be combined with CQ as photoinitiator system in unfilled resins(13)13. Schroeder WF & Vallo CI. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent Mat 2007;23:1313-1321.; however, its efficiency in filled resins was not previous explored. According to Rayleigh's scattering theory, it is expected that light transmission into a restoration might be reduced, especially in highly filled materials(17)17. Cook WD. Factors affecting the depth of cure of UV-polymerized composites. J Dent Res 1980;59:800-808., once greater refractive index difference between the inorganic fillers and the resin matrix lead to a greater light scattering, due to multiple reflections and refraction at the resin matrix and filler interfaces( 1818. Lee YK, Powers JM. Influence of background color on the color changes of resin composites after accelerated aging. Am J Dent 2007;20:27-30. , 1919. Ota M, Ando S, Endo H, Ogura Y, Miyazaki M, Hosoya Y. Influence of refractive index on optical parameters of experimental resin composites. Acta Odontol Scand 2012;70:362-367. , 2020. Fugita K, Nishiyama N, Nemoto K, Okada T, Ikemi T. Effect of base monomer's refractive index on curing depth and polymerization conversion of photo-cured resin composites. Dent Mat J 2005;24:403-408. ). In addition, the light that passes through the resin composite is absorbed and scattered to a greater degree as depth increases, thus further minimizing its effectiveness in promoting curing( 2121. Vargas MA, Cobb DS, Schmit JL. Polymerization of composite resins: argon laser vs conventional light. Oper Dent 1998;23:87-93. , 2222. Watts DC, Cash AJ. Analysis of optical transmission by 400-500 nm visible light into aesthetic dental biomaterials. J Dent 1994;22:112-117. ). As observed in this study, the degree of conversion promoted by DMPOH in association with CQ in filled resin composites is not so high as in unfilled resins ( 1313. Schroeder WF & Vallo CI. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent Mat 2007;23:1313-1321. ), but similar to the monomer conversion of promoted by the conventional photo-initiator system of resin composites (EDAB).
The second hypothesis, that the DMPOH and EDAB would not reduce cells viability was rejected, since both reducing agents tested (EDAB and DMPOH) decreased cell viability after 24 h of exposition; DMPOH presented similar reduced cells viability values compared with the commercial reducing agent, EDAB. The similar degree of conversion of both resin composites also indicates that after polymerization, the amount of unreacted substances was the same in both resin composites regardless the amine used as a co-initiator. As the polymeric chain is formed, some of these reagents stay trapped within that chain. So, despite these reagents are not consumed, they are not able to be in direct contact with the oral environment, except by water lixiviating according to the degradation of the polymer chain over time (15)15. Braden M, Causton BE, Clarke RL. Diffusion in water in composite filling materials. J Dent Res 1976;55:730-732.. However, as the polymer chain was the same for both composites, probably the same amounts of unreacted substances were lixiviated providing similar biological incompatibility as found in the results.
Biological incompability is caused due to cytotoxic effects of unreacted and/or leachable substances, such as monomers and photo-initaitors, when in contact with human oral tissues ( 2323. Porto ICCM, Andrade AKM, Guenes GMT, Ribeiro AIAM, Braz R, Castro CMMB. In vitro potential cytotoxicity of an adhesive system to alveolar macrophages. Braz Dent J 2009;20:195-200. , 2424. Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res 1995;74:1602-1606. ). Despite some monomers presents higher cytotoxicity compared with other, such as the BisGMA, the cytotoxicity effect is not only influenced only by individual components, but also by the synergistic or antagonistic interactions among them (24)24. Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res 1995;74:1602-1606.. Thus, unreacted and/or leachable resin monomers released from resin dental materials during the monomer-polymer conversion may be directly responsible and a major reason for cytotoxicity of resin materials (25)25. Ferracane JL. Elution of leachable components from composites. J Oral Rehabil 1994;21:441-452..
As the monomers are not able to be totally converted into polymers, its suggested that the less polymerized resin-based materials release the more amount of monomers through the dentin to the pulp causing higher citotoxic effects. As the monomeric matrix and monomer-polymer conversion were the same for both resin composites regardless the co-initiator combined to the CQ, similiar citotoxic effects were expected.
The third tested hypothesis, that DMPOH and EDAB would have similar cytotoxicity, was accepted, as both reducing agents, DMPOH and EDAB, had similar cytotoxicity by showing no significant difference in the cells viability after 1 h and 24 h. As the photo-activated samples of both reducing agent-based resin composites showed similar DC (%), the similar cytotoxicity is probably explained once cells viability was not differently affected by the different reducing agents tested (DMPOH and EDAB) as previous explained. Further some cytotoxicity is expected due to the fact that the non-body agents are not usually completely biocompatible with body cells (23)23. Porto ICCM, Andrade AKM, Guenes GMT, Ribeiro AIAM, Braz R, Castro CMMB. In vitro potential cytotoxicity of an adhesive system to alveolar macrophages. Braz Dent J 2009;20:195-200..
Thus, within the limitations in the experimental design, the following conclusion can be drawn: DMPOH presented similar cytotoxicity compared with EDAB reducing agent when combined to CQ, being a possible co-initiator substitute to amines in CQ-based resin composites without compromising its cytotoxicity.
Acknowledgements
This study was supported by FAPESP(grant #2013/04241-2).
-
11. Furuse AY, Mondelli J, Watts DC. Network structures of Bis-GMA/TEGDMA resins differ in DC, shrinkage-strain, hardness and optical properties as a function of reducing agent. Dent Mat 2011;27:497-506.
-
22. Mundim F, Pires-de-Souza F, Garcia L, Consani S. Colour stability, opacity and cross-link density of composites submitted to accelerated artificial aging. Eur J Prosthodont Rest Dent 2010;18:89-93.
-
33. Neumann MG, Miranda WG Jr., Schmitt CC, Rueggeberg FA, Correa IC. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J Dent 2005;33:525-532.
-
44. Alvim HH, Alecio AC, Vasconcellos WA, Furlan M, Oliveira JE, Saad JRC. Analysis of camphorquinone in composite resins as a function of shade. Dent Mat 2007;23:1245-1249.
-
55. Ruyter I, Nilner K, Moller B. Color stability of dental resin materials for crown and bridge veneers. Dent Mat 1987;3:246-251.
-
66. Heydecke G, Zhang F, Razzoog M. In vitro color stability of double-layer veneers after accelerated aging. J Prosthet Dent2001;85:551-557.
-
77. Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent 2000;12:300-308.
-
88. Darvell B. Materials science for dentistry. 7th ed. Hong Kong: BW Darvel; 2002.
-
99. Silami FDJ, Mundim FM, Garcia LFR, Sinhoreti MAC, Pires-de-Souza FCP. Colour stability of experimental composites containing different photoinitiators. J Dent 2012;41:e62-e66.
-
10Albuquerque PPA, Moreira AL, Moraes RR, Cavalcante LM, Schneider LFJ. Colour stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J Dent 2012;41:e67-e72.
-
11Ely C, Schneider LFJ, Ogliari FA, Schmitt CC, Corrêa IC, Lima JS, et al.. Polymerization kinetics and reactivity of alternative initiator systems for use in light-activated dental resins. Dent Mat 2012;28:1199-1206.
-
12Brandt WC, Tomaselli LO, Correr-Sobrinho L, Sinhoreti MAC. Can phenylpropanedione influence Knoop hardness, rate of polymerization and bond strength of resin composite restorations? J Dent 2011;39:438-447.
-
13Schroeder WF & Vallo CI. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent Mat 2007;23:1313-1321.
-
14Bowen RL, Argentar H. Amine accelerators for methacrylate resin systems. J Dent Res 1971;50:923-928.
-
15Braden M, Causton BE, Clarke RL. Diffusion in water in composite filling materials. J Dent Res 1976;55:730-732.
-
16Atai M, Watts DC. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dent Mat 2006;22:785-791.
-
17Cook WD. Factors affecting the depth of cure of UV-polymerized composites. J Dent Res 1980;59:800-808.
-
18Lee YK, Powers JM. Influence of background color on the color changes of resin composites after accelerated aging. Am J Dent 2007;20:27-30.
-
19Ota M, Ando S, Endo H, Ogura Y, Miyazaki M, Hosoya Y. Influence of refractive index on optical parameters of experimental resin composites. Acta Odontol Scand 2012;70:362-367.
-
20Fugita K, Nishiyama N, Nemoto K, Okada T, Ikemi T. Effect of base monomer's refractive index on curing depth and polymerization conversion of photo-cured resin composites. Dent Mat J 2005;24:403-408.
-
21Vargas MA, Cobb DS, Schmit JL. Polymerization of composite resins: argon laser vs conventional light. Oper Dent 1998;23:87-93.
-
22Watts DC, Cash AJ. Analysis of optical transmission by 400-500 nm visible light into aesthetic dental biomaterials. J Dent 1994;22:112-117.
-
23Porto ICCM, Andrade AKM, Guenes GMT, Ribeiro AIAM, Braz R, Castro CMMB. In vitro potential cytotoxicity of an adhesive system to alveolar macrophages. Braz Dent J 2009;20:195-200.
-
24Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res 1995;74:1602-1606.
-
25Ferracane JL. Elution of leachable components from composites. J Oral Rehabil 1994;21:441-452.
Publication Dates
-
Publication in this collection
Nov-Dec 2014
History
-
Received
03 June 2014 -
Accepted
14 Oct 2014