Abstracts
OBJETIVE: To study the relationship between blood lead levels and cognitive abilities of children exposed to this metal. METHOD: This is a cross-sectional study that included 134 children aged 6 to 8.5 years old from 3 schools with different risks of lead exposure located in El Callao (Peru). Cognitive assessments were made by means of the Graphic Test of Reasoning (GTR) and the Kohs Block Design Test (KBDT). Blood lead levels and other laboratory tests were performed. RESULTS: Children with lead > 10 ug/dl presented greater prevalence of low scores in the Graphic Test of Reasoning (18.9% vs. 7.1%, p = 0.049) and in the Kohs Block Design Test (KBDT) (39.6% vs. 18.6%, p = 0.01) compared with those with lower lead blood levels. A deficit of 1 category in the Graphic Test of Reasoning was associated with an increase in lead blood level of 16.78 ug/dl (assuming a linear relationship) and from 1 to 5.19 ug/dl (logarithmic model). For each 10 ug/dl of increase in lead levels, the Kohs Block Design Test decreases in 6.24 units (12.91 in males and 0.216 in females) (linear model), and an increase from 1 to 10 ug/dl corresponds to a drop of 16.44 points in the Kohs Block Design Test (31.19 in males and 3.98 in females) (logarithmic model). Considering the Graphic Test of Reasoning subscales, lead levels correlated negatively with the areas of numerical problems (rho = -0.445, p < 0.001), numerical relationships (rho = -0.365, p < 0.001), inferences (rho = -0.281, p = 0.002) and similarities (rho = -0.250, p = 0.005). CONCLUSIONS: Lead levels were non-linearly associated with lower cognitive abilities, especially in males, being the numerical reasoning the most affected area.
Lead; Intelligence; Children; Toxicology; Environment
OBJETIVO: Estudar a relação entre níveis séricos de chumbo e funções cognitivas em crianças expostas a esse metal. MÉTODO: Este é um estudo transversal que incluiu 134 crianças de 6 a 8,5 anos de idade, de três escolas localizadas em El Callao (Peru), e com riscos diferentes de exposição ao chumbo. As avaliações de suas funções cognitivas foram realizadas utilizando-se o Teste Gráfico de Raciocínio e o Teste dos Cubos de Kohs. Foram medidos os níveis séricos de chumbo RESULTADOS: As crianças com níveis séricos de chumbo > 10 ug/dl apresentaram maior prevalência de baixa pontuação no Teste Gráfico de Raciocínio (18,9% vs. 7,1%, p = 0,049) e também no Teste dos Cubos de Kohs (39,6% vs. 18,6%, p = 0,01), quando comparadas com aquelas com níveis séricos menores. Um déficit de uma categoria no Teste Gráfico de Raciocínio foi associado com um aumento no nível sérico de chumbo de 16,78 ug/dl (assumindo uma relação linear) e de 1 a 5,19 ug/dl (modelo logarítmico). Para cada 10 ug/dl de aumento no nível sérico de chumbo, o Teste dos Cubos de Kohs apresentou diminuição de 6,24 unidades (12,91 para os meninos e 0,216 para as meninas) (modelo linear) e um aumento de 1 a 10 ug/dl correspondeu a uma diminuição de 16,44 pontos no Teste dos Cubos de Kohs (31,19 para os meninos e 3,98 para as meninas) (modelo logarítmico). Considerando as subescalas do Teste Gráfico de Raciocínio, os níveis séricos de chumbo correlacionaram-se negativamente com as áreas de problemas numéricos (rho = -0,445, p < 0,001), relações numéricas (rho = -0,365, p < 0,001), inferências (rho = -0,281, p = 0,002) e semelhanças (rho = -0,250, p = 0,005). CONCLUSÕES: Níveis séricos de chumbo foram associados de uma maneira não-linear com déficits das habilidades cognitivas, especialmente entre os meninos, sendo o raciocínio numérico a área mais afetada.
Chumbo; Inteligência; Crianças; Toxicologia; Meio ambiente
ORIGINAL ARTICLE
Lead levels and cognitive abilities in Peruvian children
Níveis de chumbo e funções cognitivas em crianças peruanas
Johann M Vega-DienstmaierI; Joel E Salinas-PiélagoII; María del Rosario Gutiérrez-CamposIII; Ricardo D Mandamiento-AyquipaIV; María del Carmen Yara-HokamaV; Johny Ponce-CanchihuamánVI; Jorge Castro-MoralesI
IDepartment of Psychiatry and Mental Health, Universidad Peruana Cayetano Heredia, Lima, Peru
IIHermilio Valdizán Hospital, Universidad Peruana Cayetano Heredia, Lima, Peru
IIIDirection of Health I-Callao, Health Ministry, Lima, Peru
IVRegional Education Direction of Callao, Education Ministry, Lima, Peru
VSan Martín de Porres Private University, Lima, Peru
VIUniversidad Peruana Cayetano Heredia, Lima, Peru
Correspondence Correspondence Johann M Vega-Dienstmaier Av. José Pardo 1142-702, Lima 18, Peru E-mail: jvegad@amauta.rcp.net.pe/ johannvega@yahoo.com
ABSTRACT
OBJETIVE: To study the relationship between blood lead levels and cognitive abilities of children exposed to this metal.
METHOD: This is a cross-sectional study that included 134 children aged 6 to 8.5 years old from 3 schools with different risks of lead exposure located in El Callao (Peru). Cognitive assessments were made by means of the Graphic Test of Reasoning (GTR) and the Kohs Block Design Test (KBDT). Blood lead levels and other laboratory tests were performed.
RESULTS: Children with lead > 10 ug/dl presented greater prevalence of low scores in the Graphic Test of Reasoning (18.9% vs. 7.1%, p = 0.049) and in the Kohs Block Design Test (KBDT) (39.6% vs. 18.6%, p = 0.01) compared with those with lower lead blood levels. A deficit of 1 category in the Graphic Test of Reasoning was associated with an increase in lead blood level of 16.78 ug/dl (assuming a linear relationship) and from 1 to 5.19 ug/dl (logarithmic model). For each 10 ug/dl of increase in lead levels, the Kohs Block Design Test decreases in 6.24 units (12.91 in males and 0.216 in females) (linear model), and an increase from 1 to 10 ug/dl corresponds to a drop of 16.44 points in the Kohs Block Design Test (31.19 in males and 3.98 in females) (logarithmic model). Considering the Graphic Test of Reasoning subscales, lead levels correlated negatively with the areas of numerical problems (rho = -0.445, p < 0.001), numerical relationships (rho = -0.365, p < 0.001), inferences (rho = -0.281, p = 0.002) and similarities (rho = -0.250, p = 0.005).
CONCLUSIONS: Lead levels were non-linearly associated with lower cognitive abilities, especially in males, being the numerical reasoning the most affected area.
Keywords: Lead; Intelligence; Children; Toxicology; Environment
RESUMO
OBJETIVO: Estudar a relação entre níveis séricos de chumbo e funções cognitivas em crianças expostas a esse metal.
MÉTODO: Este é um estudo transversal que incluiu 134 crianças de 6 a 8,5 anos de idade, de três escolas localizadas em El Callao (Peru), e com riscos diferentes de exposição ao chumbo. As avaliações de suas funções cognitivas foram realizadas utilizando-se o Teste Gráfico de Raciocínio e o Teste dos Cubos de Kohs. Foram medidos os níveis séricos de chumbo
RESULTADOS: As crianças com níveis séricos de chumbo > 10 ug/dl apresentaram maior prevalência de baixa pontuação no Teste Gráfico de Raciocínio (18,9% vs. 7,1%, p = 0,049) e também no Teste dos Cubos de Kohs (39,6% vs. 18,6%, p = 0,01), quando comparadas com aquelas com níveis séricos menores. Um déficit de uma categoria no Teste Gráfico de Raciocínio foi associado com um aumento no nível sérico de chumbo de 16,78 ug/dl (assumindo uma relação linear) e de 1 a 5,19 ug/dl (modelo logarítmico). Para cada 10 ug/dl de aumento no nível sérico de chumbo, o Teste dos Cubos de Kohs apresentou diminuição de 6,24 unidades (12,91 para os meninos e 0,216 para as meninas) (modelo linear) e um aumento de 1 a 10 ug/dl correspondeu a uma diminuição de 16,44 pontos no Teste dos Cubos de Kohs (31,19 para os meninos e 3,98 para as meninas) (modelo logarítmico). Considerando as subescalas do Teste Gráfico de Raciocínio, os níveis séricos de chumbo correlacionaram-se negativamente com as áreas de problemas numéricos (rho = -0,445, p < 0,001), relações numéricas (rho = -0,365, p < 0,001), inferências (rho = -0,281, p = 0,002) e semelhanças (rho = -0,250, p = 0,005).
CONCLUSÕES: Níveis séricos de chumbo foram associados de uma maneira não-linear com déficits das habilidades cognitivas, especialmente entre os meninos, sendo o raciocínio numérico a área mais afetada.
Descritores: Chumbo; Inteligência; Crianças; Toxicologia; Meio ambiente
Introduction
Lead intoxication has been recognized thousands of years before, but its occurrence has been almost exclusively limited to people who directly worked with this element. However, since the 19th century the use of leaded paint allowed large amounts of lead at the reach of children. Later in the 20th century lead began to be used in gasoline contributing to a greater dissemination of this metal in the air, dust and soil. On the other hand, young children are especially at risk to be intoxicated by lead due to their frequently hand-to-mouth activity and to their greater gastrointestinal absorption of this element in comparison to adults.1
The relationship between lead exposure and intellectual deficits is supported by numerous studies. The most solid evidence is provided by two meta-analysis2-3 and one systematic review.4 There is a considerable amount of prospective studies that control probable confounding variables, such as those made in Port Pirie (Australia), that involved the follow-up of children under the age of 11-13 years.5-9 Other important studies include the works accomplished in Yugoslavia, in which children were followed up to 7 years of age;10-11 the study of Canfield et al.,12 who found that blood lead levels, especially those below 10 ug/dl, were inversely associated with the intellectual quotient of the children of 3 and 5 years of age; and other relevant research.13-14
The lead level above which intellectual alterations may take place is controversial. For some authors, there is no sufficient evidence to endorse that low values of lead (for example, levels < 10 or 20 ug/dl) cause problems.15 Nevertheless, others support the idea that cognitive changes at levels lower than 10 ug/dl12 and even smaller values such as 5 ug/dl16 are important. The present limit value according to the Centres for Disease Control and Prevention (CDC) is 10 ug/dl, but even this authorized mark has been put in doubt.17
In Peru, in the 'Puerto Nuevo' slums, located in El Callao, the country's main port and the place where mineral deposits for export are located, 93.4% of the children under 6 years of age have lead levels > 10 ug/dl, which is explainable by the environmental unsafe conditions of the warehouses where minerals are deposited. Indeed, it constitutes a serious public health issue.18
The objective of this study is to assess the relationship between lead levels and cognitive abilities in children, and to evaluate if this relationship differs with gender or according to diverse cognitive areas.
Method
The present work is a cross-sectional, descriptive and correlational study. One hundred thirty-four children of both sexes, aged 6 years to 8 years and 6 months, from 3 public schools of El Callao (María Reiche, Divina Pastora and Gálvez Barnechea) were studied. The first and the second schools pertain to Puerto Nuevo, a zone with high lead exposure due to the presence of deposits of this element in the vicinity; and the last one is located in La Punta, a zone of minor exposure to the mineral (some 5 miles from the lead deposits).
The cognitive evaluation was accomplished by means of the Graphic Test of Reasoning (GTR) and the Kohs Block Design Test (KBDT). The GTR19 is an adaptation of the California test of mental maturity, designed for children 6 to 14 years of age and has 6 subscales: similarities, similarities and differences, analogies, inferences, numerical relationships and numerical problems. The test classifies the children's intelligence in the following 7 categories (for the benefit of the statistical analysis represented by values in parentheses): 'far below' (-3), 'inferior' (-2), 'inferior average' (-1), 'average' (0), 'superior average' (1), 'superior' (2) and 'very superior' (3).
The KBDT20 allows the following classification, according to the scores in parentheses: 'idiocy' (< 25), 'imbecility' (between 25 and < 50), 'moron' (between 50 and < 75), 'borderline' (between 75 and 85), 'inferior average' (between > 85 and < 90), 'average' (between 90 and < 110), 'normal brilliant' or 'superior average' (between 110 and < 120), 'superior' (between 120 and < 130) and 'very superior' (from 130 and higher).
The KBDT has been used to study visual-spatial abilities21 and is a good predictor of the lecture ability in children.22
The reason to choose these cognitive testing instruments in a multiethnic country such as Peru is supported by the notion that graphic tests are less culturally biased than those which require 'literal' or 'verbal' capabilites associated to Western types of thinking models. In addition, both the GTR and the KBDT are standardized in this country, which is not the case for the Wechsler series.
The parents of the studied subjects gave their informed consent authorizing to take blood samples and to appliy the psychological tests to their children. Additionally, they registered general data and filled a checklist of the symptoms that their kids might have had as a result of lead exposure. Two psychologists (MY and RM), trained in the administration of the GTR and KBDT, applied these tests to the whole sample. The following laboratory measurements were performed using the blood samples: blood lead level (Pb) by voltammetry by anodic detachment with the Lead Care Equipment, haemoglobin, hematocrit, erythrocyte count, ferritin, hemogram, creatinine, blood ureic nitrogen (BUN) and proteins (albumin and globulins).
Cognitive tests and blood samples were obtained at schools between September and December 2002.
Spearman coefficients were used to assess correlations among values of lead, cognitive tests and anaemia markers. The association of the presence of lead levels > 10 ug/dl with dichotomic variables was studied using chi-square test and with continuous numeric variables using t-Student test. Linear and logistic regression analyses were performed to study the relation between cognitive scores and the main assessed factors (lead levels and anaemia markers).
This research was approved by the Ethics Committee of the Universidad Peruana Cayetano Heredia.
Results
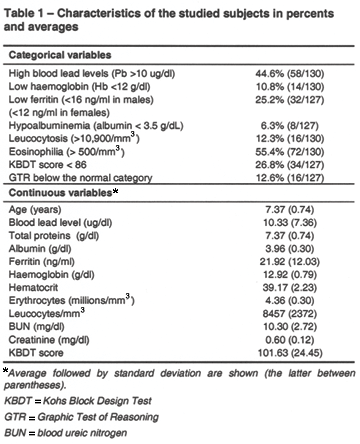
There were 154 children aged 6 to 8.5 years in the 3 studied schools. Of these subjects, 11 were excluded as their parents did not accept to participate in the study and 9 for not being present when our team went to the schools for the evaluations. Therefore, 134 children were included: 55 boys (41.0%) and 79 girls (59.0%), from the following primary school grades: 1rst, 61 (45.9%); 2nd, 47 (35.3%); and 3rd, 25 (18.8%). Of these 134 subjects, blood lead levels could be obtained in 130; 127 adequately completed cognitive tests, and 123 had both measures. The features of studied children are described in Table 1.
Correlations between laboratory and psychological tests were studied by Spearman coefficients. We found negative correlations between lead blood levels and blood cells counts and haemoglobin (erythrocytes: rho = - 0.288, p = 0.001; haemoglobin: rho = -0.3, p = 0.001; hematocrit: rho = -0.221, p = 0.011), KBDT scores (rho = -0.229, p = 0.011), GTR scores (rho = -0.417, p < 0.001), and the following GTR subscales scores: numeric problems (rho = -0.445, p < 0.001), numeric relationships (rho = -0.365, p < 0.001), inferences (rho = -0.281, p = 0.002), and similarities (rho = -0.25, p=0.005). In addition, there were correlations between blood cell counts and haemoglobin and some cognitive measures: GTR positively correlated with erythrocytes (rho = 0.229, p = 0.011), haemoglobin (rho = 0.196, p = 0.03) and hematocrit (rho = 0.179, p = 0.047); numeric problems with erythrocytes (rho = 0.229, p = 0.011), haemoglobin (rho = 0.208, p = 0.021) and hematocrit (rho = 0.205, p = 0.023); and numeric relationships with erythrocytes (rho = 0.198, p = 0.028), haemoglobin (rho = 0.207, p = 0.022) and hematocrit (rho = 0.191, p = 0.034). There were no significant correlations between ferritin and cognitive measures or lead blood levels.
In Table 2, symptoms referred by parents and results of the cognitive tests were compared considering lead levels (lower or higher than 10 ug/dl). This table shows that high lead levels are associated with a greater prevalence of behaviour problems, coordination and gait alterations, loss of appetite, paleness and irritability. Otherwise, psychological tests show lower scores of cognitive capabilities in children that have lead levels > 10 ug/dl.
In a stepwise logistic regression in which we studied the ability of each GTR subscale and current age to predict the presence of blood lead > 10 ug/dl, we obtained a model with the following factors: analogies (odd ratio (OR) = 1.41, 95% confidence interval for OR (CI) = 0.97-2.04), numerical relationships (OR = 0.66, CI = 0.46-0.95), numerical problems (OR = 0.65, CI = 0.48-0.89) and age (OR = 1.92, CI = 1.07-3.43). We included age in the regression as the scores of GTR subscales are not values corrected for current age, finding that a high lead level was associated independently from current age, with lower scores in numerical relationships and numerical problems areas. Similarly, the differences in scores of the GTR areas showed in Table 2 could not be attributed to the influence of current age, as children with high lead levels are precisely the older ones, and higher ages would predict higher not lower scores.
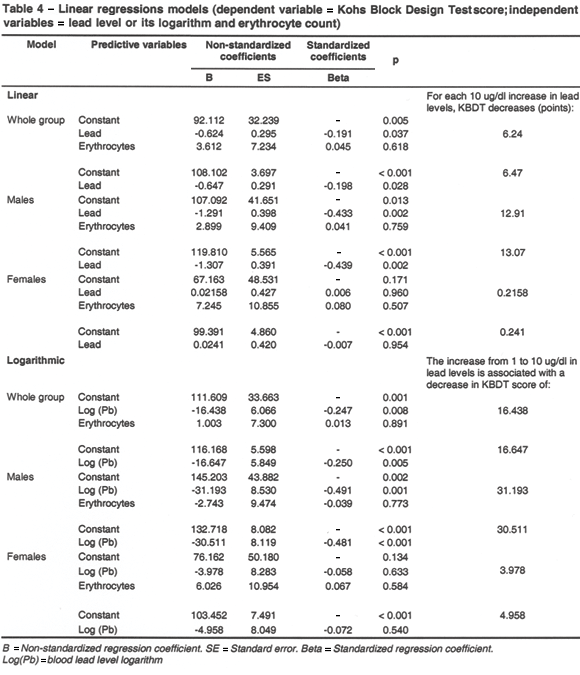
The relationships between intelligence tests and values of lead blood level and erythrocytes count, studied by means of regressions, are shown in Tables 3 and 4; and Figures 1 and 2. Of note, the relationship between higher lead levels and lower cognitive test scores occurs clearly, considering the whole group and the male subgroup, but not similarly for females. On the other hand, if we consider the logarithm of the blood lead concentration as a factor, this value correlates better with scores of cognitive tests than lead level itself.
Based on the results of Tables 3 and 4, it is possible to construct equations to estimate the variations in cognitive tests, taking into account the changes in blood lead levels, in a way that would allow us to compare our findings with those of other studies. These equations are, for the linear model:
CS = k + (Bpb)(Pb) + (Bh) (H)
And, for the logarithmic model:
CS = k + (Blgpb)(log(Pb)) + (Bh) (H)
Where: CS = cognitive score; k = constant; Pb = blood lead level; H = erythrocytes count (in millions/mm3); Bpb = non-standardized regression coefficient for lead; Blgpb = non-standardized regression coefficient for log (lead); and Bh = non-standardized regression coefficient for erythrocytes count.
For example, according to the results of table 4 and using the logarithmic model, in order to estimate the KBDT score, we have the formula: KBDT = 111.609 - 16.438 (log(Pb)) + 1.003 (H).
Considering the Kolmogorov-Smirnov test, KBDT is normally distributed (p > 0.05); however, GTR is not (p < 0.05), thus the results of the model for this variable should be cautiously interpreted.
Discussion
We have found that the prevalence of scores below the normal range, according to the cognitive tests, in children with lead levels > 10 ug/dl is more than twice as much as those of the group with lower lead levels. The limit of 10 ug/dl was chosen for being the value established by the CDC as the mark for high lead levels, although, as we will see below, the existence of a safe range of lead concentrations in which toxicity does not take place is debatable.17
We have found a significant inverse correlation between lead levels and cognitive tests scores, especially those of the GTR. Cognitive tests scores, also, correlate positively with the values of erythrocytes count, haemoglobin concentration and hematocrit; a fact that must be taken into account given the well-known association between iron deficiency and cognitive alterations.23 Iron administration might enhance the cognitive functions in children. Regarding that, a study conducted aiming to demonstrate that possibility, specifically, in a zone near to this study's, found that the ingestion of cookies enriched with heminic iron improved the intellectual status of pre-school children.24
The relationships between cognitive functions, lead and anaemia are more complex, considering that lead can produce anaemia; and that iron deficiency increases the intestinal absorption of lead.1,25 Anaemia is thus a possible confounding factor in the relationship between lead and intelligence, that we have to take into account. Within the variables related to anemia, the one that had the highest correlation with cognitive scores was erythrocyte count; nevertheless, this correlation reached statistical significance only for the GTR. On the other hand, we did not find correlation between ferritin levels and cognitive scores.
Considering lead blood levels and erythrocytes count as independent variables and cognitive scores as the dependent variable, in a linear regression analysis, lead keeps its predictive ability for GTR and KBDT values. The results of the regression allow the estimation of the scores of the cognitive tests based on the lead levels. However, the relationship between lead and intelligence seems to be not linear, being better explained by a logarithmic model, as it can be observed in Tables 3 and 4, and Figures 1 and 2. The standardized coefficient, which indicates the importance of the association between the independent variable and the dependent one (intelligence), improves when the logarithm of the lead is considered in the regression instead of the lead value itself. For example, it changes from -0.275 to -0.317 for the GTR and from -0.191 to -0.247 for the KBDT. Other authors also use logarithmic models10,13-14 or other non-linear models12 to study the relationship between lead and intelligence.
The fact that the relationship between lead and intelligence is not linear has important relevance, as it suggests that at lower lead levels, the concentration changes of this metal are associated with greater variations in cognitive functions; accordingly, even at low lead values (< 10 ug/dl). According to our logarithmic model, an elevation from 1 to 10 ug/dl corresponds to a drop of 16.44 points in the KBDT and of 1-2 categories in the GTR; this same variation of lead levels, in the study of Canfield et al.,12 using a non-linear model, was related to a drop of 7.4 points of intelligence. This last work also found that including only the subgroup of children with lead levels < 10 ug/dl, the relationship between lead and intelligence was stronger than when taking the whole group into account. A convincing mechanism that explains the shape of the dose-effect relationship between lead and IQ has not been proposed.1
In other studies, a variation in lead level from 10 to 30 ug/dl was associated with a drop in intelligence of 4.4-5.3 points,5 2.5-4.5 points10 or 4.3 points.11 In our work, this variation was associated with a decrease of 7.84 points (logarithmic model) or 12.48 points (linear model) in the KBDT and with a drop of less than 1 category (logarithmic model) or slightly more than 1 category (linear model) in the GTR.
The decrease in intelligence associated with an increase in lead from 10 to 20 ug/dl is 2.6 points according to a meta-analysis;3 and 1-2 points,4 5.8 points26 or 3 points,8 considering other research. The corresponding drops in our study are 4.95 (logarithmic model) or 6.24 points (linear model) in the KBDT and less than 1 category in the GTR.
Accordingly, the drops in intelligence associated with variations in blood lead concentration are more important within the range from 0 to 10 ug/dl, a margin currently considered as 'safe'. Based on research findings which relate lead and cognitive functions, the limit above which lead levels have been considered 'elevated' has decreased in several occasions in the last decades, from 60 ug/dl in the 1960's to 10 ug/dl nowadays. However, in this very moment, the existence of a safety margin for blood lead concentrations is debatable.17
The relationship between lead and intelligence found in our study is stronger than evidence reported by other studies. This can be explained by the fact that in our study it was not possible to assess some confounding factors such as the child's home environment, the parents' intelligence, and the mother's tobacco use during pregnancy.15,27 On the other hand, the tests administered in our study, which demand visual competencies, could be altered in a more marked way by the effect of lead.
Dental lead concentration is strongly associated with alterations in certain WISC-R subscales, particularly the block design test.7 This same subscale was one of the most highly correlated with lead levels in other work.8 The study in Port Pirie found a strong inverse correlation between blood lead levels and visual-motor integration in children aged 7 years and it was proposed that this function could be a more sensitive marker of the effects of lead on child development than the intellectual quotient.6 Similarly, Bellinger et al.13 observed that the inverse association between blood lead and cognitive function was especially prominent for visual-spatial and visual-motor abilities. According to Wasserman et al.,11 perceptive-motor abilities are more sensitive to lead exposure than those related to speech. Moreover, other works have also found associations between lead and visual-motor deficiencies.28-29
There are diverse results regarding the areas which are more affected by elevated lead levels. We found, within the GTR, that high lead levels were especially associated with deficiencies in abilities for numerical problems, numerical relationships, and inferences. Other works support our finding that mathematical abilities are particularly affected by lead. In a study,8 after adjusting the analysis for confounding variables, the intelligence subscale that had the most significant inverse association with lead levels was the arithmetic one. Similarly, in children and adolescents of the United States, whose lead levels were below 10 ug/dl in 97.9% of the subjects, after adjusting for confounding variables, it was found an inverse relationship between lead levels and arithmetic, reading, non-verbal reasoning, and short-term memory scores. For the arithmetic and reading scores, the relationship remained even at lead levels below 5 ug/dl.16 On the other hand, a Yugoslavian study found that at the age of 4 and 7 years, lead concentration was more strongly associated with the executive subscales than with verbal ones.10
Our results show that the correlation between lead levels and cognitive scores is greater in males than in females. The lead effects may differ by gender, but the findings are contradictory. In Port Pirie study, the girls were more sensitive to lead effects on cognitive functions than boys.9 In the same way, in Taiwan it was found that the intelligence measured by the Raven's Coloured Progressive Matrices, correlated with lead levels especially among girls.30 In Port Pirie, it was estimated that in children aged 11 to 13 a hypothetical lead increase from 10 to 30 ug/dl would be associated with a higher elevation in externalisation behaviour problems in males than in girls; while the internalisation behaviour problems would increase more in girls than in boys.31
The significantly higher prevalence of behaviour problems and irritability in children with elevated lead levels is consistent with the reported association of lead exposure with behaviour problems,31 antisocial and delinquent behaviour,32 and destructive trends in preschool children.33
This research has the following limitations: it is a transversal study, thus it does not consider the duration of lead exposure and can not demonstrate that lead levels cause a deficit in cognitive abilities, only shows an association between these two variables. It is possible that children with cognitive problems have behaviours predisposing them to be more exposed to lead. Furthermore, the study did not evaluate other factors related to intelligence such as poverty, the parents' intellectual quotient, the child's home environment, mother tobacco use during pregnancy and previous maternal care. Some of these confounders could predispose to low cognitive abilities and a greater exposure to lead. Other problem was the number of subjects (31 of 154) that did not enter in the statistical analysis of the relationship between lead and cognitive scores due to lack of consent, absence of the children, damage of the blood samples and incomplete cognitive tests; then selection biases could have been occurred.
Conclusions
Summing-up, our study found an inverse relationship between blood lead levels and cognitive test scores, especially in male children. This relationship is better explained by a logarithmic than a linear model, the greater variations appearing at lead levels below 10 ug/dl. The most affected areas are those related to numerical reasoning.
Acknowledgements
We appreciate the financial support given by the Universidad Peruana Cayetano Heredia; and the collaboration of the psychologists Carlos Marchena and Merle Santos; Dr. Santiago Stucchi, and Dr. César Arellano, all of them from the Peruvian Specialized Institute of Mental Health.
Submitted: 30 March 2005
Accepted: 17 August 2005
Financing: Universidad Peruana Cayetano Heredia
Conflict of interests: None
The study was a project of the Department of Psychiatry and Mental Health, Universidad Peruana Cayetano Heredia.
- 1. Bellinger DC. Lead. Pediatrics. 2004;113(Suppl 4):1016-22.
- 2. Needleman HL, Gatsonis CA. Low-level lead exposure and the IQ of children. A meta-analysis of modern studies. JAMA 1990;263(5):673-8.
- 3. Schwartz J. Low-level lead exposure and children's IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65(1):42-55.
- 4. Pocock SJ, Smith M, Baghurst P. Environmental lead and children's intelligence: a systematic review of the epidemiological evidence. BMJ 1994;309(6963):1189-97.
- 5. Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, Tong SL. Environmental exposure to lead and children's intelligence at the age of seven years. The Port Pirie Cohort Study. N Engl J Med. 1992;327(18):1279-84.
- 6. Baghurst PA, McMichael AJ, Tong S, Wigg NR, Vimpani GV, Robertson EF. Exposure to environmental lead and visual-motor integration at age 7 years: the Port Pirie Cohort Study. Epidemiology. 1995;6(2):104-9.
- 7. McMichael AJ, Baghurst PA, Vimpani GV, Wigg NR, Robertson EF, Tong S. Tooth lead levels and IQ in school-age children: the Port Pirie Cohort Study. Am J Epidemiol. 1994;140(6):489-99.
- 8. Tong S, Baghurst P, McMichael A, Sawyer M, Mudge J. Lifetime exposure to environmental lead and children's intelligence at 11-13 years: the Port Pirie cohort study. BMJ. 1996;312(7046):1569-75.
- 9. Tong S, McMichael AJ, Baghurst PA. Interactions between environmental lead exposure and sociodemographic factors on cognitive development. Arch Environ Health. 2000;55(5):330-5.
- 10. Factor-Litvak P, Wasserman G, Kline JK, Graziano J. The Yugoslavia Prospective Study of environmental lead exposure. Environ Health Perspect. 1999;107(1):9-15.
- 11. Wasserman GA, Liu X, Lolacono NJ, Factor-Litvak P, Kline JK, Popovac D, Morina N, Musabegovic A, Vrenezi N, Capuni-Paracka S, Lekic V, Preteni-Redjepi E, Hadzialjevic S, Slavkovich V, Graziano JH. Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environ Health Perspect. 1997;105(9):956-62.
- 12. Canfield RL, Henderson CR Jr., Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348(16):1517-26.
- 13. Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C. Low-level lead exposure and children's cognitive function in the preschool years. Pediatrics. 1991;87(2):219-27.
- 14. Schnaas L, Rothenberg SJ, Perroni E, Martinez S, Hernandez C, Hernandez RM. Temporal pattern in the effect of postnatal blood lead level on intellectual development of young children. Neurotoxicol Teratol 2000;22(6):805-10.
- 15. Kaufman AS. Do low levels of lead produce IQ loss in children? A careful examination of the literature. Arch Clin Neuropsychol. 2001;16(4):303-41.
- 16. Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations < 10 microg/dL in US children and adolescents. Public Health Rep. 2000;115(6):521-9.
- 17. Rogan WJ, Ware JH. Exposure to lead in children - how low is low enough? N Engl J Med. 2003;348(16):1515-6.
- 18. López-Sandoval J. Contribution to the study of the lead intoxication in children under 6 years of age in the "Puerto Nuevo" Slums of the Constitutional Province of El Callao, from June to Sep (1998). Lima: Universidad Nacional Mayor de San Marcos; 1999.
- 19. Aranda-Avendaño VF, Jiménez-Urdaniva CA. Standardization of the California test of mental maturity. Pre-Primary Series. Lima: Universidad Nacional Mayor de San Marcos; 1974.
- 20. Kohs SC. Intelligence measurement: a psychological and statistical study based upon the Block-Design Test. New York: Macmillan; 1923.
- 21. Wigg CM, Duro LA. The Kohs' blocks test as an important instrument to investigate the visuo-spatial impairments in myotonic dystrophy. Part I. Quantitative and qualitative analysis. Arq Neuropsiquiatr 1999;57(3A):547-55.
- 22. Catheline-Antipoff N, Battista M, Vernazza A. Can successful reading acquirements be predicted? Arch Pediatr. 1996;3(2):112-6.
- 23. Walter T. Impact of iron deficiency on cognition in infancy and childhood. Eur J Clin Nutr. 1993;47(5):307-16.
- 24. Salinas-Pielago JE, Vega-Dienstmaier JM, Rojas-Oblitas M. Effect of biscuits fortified with haem iron on the intellectual status of pre-school children. Rev Neurol. 1998;27(157):400-4.
- 25. Klaasen CD. Heavy metals and their antagonists. In: Goodman Gilman A, Goodman LS, Rall TW, Murad F, editors. The pharmacological bases of therapeutics. Buenos Aires: Médica Panamericana; 1988.
- 26. Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: long-term follow-up study. Pediatrics. 1992;90(6):855-61.
- 27. Wasserman GA, Factor-Litvak P. Methodology, inference and causation: environment lead exposure and childhood intelligence. Arch Clin Neuropsychol. 2001;16(4):343-52.
- 28. Song HQ. Study of total lead exposure on a hazard to the health of children. Zhonghua Yu Fang Yi Xue Za Zhi. 1993;27(2):91-3.
- 29. Hansen ON, Trillingsgaard A, Beese I, Lyngbye T, Grandjean P. A neuropsychological study of children with elevated dentine lead level: assessment of the effect of lead in different socio-economic groups. Neurotoxicol Teratol 1989;11(3):205-13.
- 30. Rabinowitz MB, Wang JD, Soong WT. Dentine lead and child intelligence in Taiwan. Arch Environ Health. 1991;46(6):351-60.
- 31. Burns JM, Baghurst PA, Sawyer MG, McMichael AJ, Tong SL. Lifetime low-level exposure to environmental lead and children's emotional and behavioral development at ages 11-13 years. The Port Pirie Cohort Study. Am J Epidemiol. 1999;149(8):740-9.
- 32. Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275(5):363-9.
- 33. Wasserman GA, Staghezza-Jaramillo B, Shrout P, Popovac D, Graziano J. The effect of lead exposure on behavior problems in preschool children. Am J Public Health. 1998;88(3):481-6.
Publication Dates
-
Publication in this collection
24 Mar 2006 -
Date of issue
Mar 2006
History
-
Received
30 Mar 2005 -
Accepted
17 Aug 2005