Abstract
Lactobacilli are probiotics with Aflatoxin (AF) detoxification ability, found in fermented products, GIT of animals and environment. Purpose of this study was to investigate the ability of broiler isolates of Lactobacillus against Aflatoxin B1 (AFB1). For this purpose, 5 isolates of Lactobacillus from broiler gut were incubated with 100 ppb AFB1 in aqueous environment and effect of different parameters (cell fractions, time, temperature, pH) on detoxification was determined by HPLC. The ameliorative effect of Lactobacillus salivarius (LS) against AFB1 was studied in broiler. The results revealed that LS (CR. 4) showed the best results (in vitro) as compared to other isolates (L. salivarius (CR. 3, CR, 4), L. agilis (CE. 2.1, CE. 3.1) and L. crispatus (CE. 28). Cell debris of CR. 4 showed significantly higher detoxification (P<0.05). Maximum amount of AFB1 was detoxified at 30°C (97%), pH 4.0 (99%) and 6 h (99.97%). In vivo study showed that AFB1 decreased weight gain (1,269 ± 0.04 gm/ bird), feed consumed (2,161 ± 0.08 gm/ bird), serum total protein (2.42 ± 0.34 gm/ dl), serum albumin (0.5 ± 0.2 2 gm/dl) and antibody titer (4.2 ± 0.83). Liver function enzymes were found (alanine transaminase (ALT): 32 ± 10.7 U/L) and aspartate transaminase (AST): 314.8 ± 27 U/L) elevated in AFB1 fed broilers. Treatment with 1% LS not only decreased the toxic effects of AFB1 (group D) but also improved the overall health of broilers due to its probiotic effects (p<0.05) as compared to control negative (group A). The detoxification ability of LS was better than commercial binder (CB) (0.2% Protmyc). It was concluded that detoxification of AFB1 by Lactobacillus was strain, temperature, pH and time dependent. LS has detoxification ability against AFB1 in vivo.
Keywords:
L. salivarius; Aflatoxin B1; detoxification; HPLC
Resumo
Os lactobacilos são probióticos com capacidade de desintoxicação da Aflatoxina (AF), encontrados em produtos fermentados, TGI de animais e meio ambiente. O objetivo deste estudo foi investigar a capacidade de isolados de frango de corte de Lactobacillus contra a Aflatoxina B1 (AFB1). Para tanto, 5 isolados de Lactobacillus de intestino de frango foram incubados com 100 ppb AFB1 em meio aquoso, e o efeito de diferentes parâmetros (frações celulares, tempo, temperatura, pH) na desintoxicação foi determinado por CLAE. O efeito melhorador de Lactobacillus salivarius (LS) contra AFB1 foi estudado em frangos de corte. Os resultados revelaram que LS (CR. 4) apresentou os melhores resultados (in vitro) em comparação com outros isolados [L. salivarius (CR. 3, CR. 4), L. agilis (CE. 2.1, CE. 3.1) e L. crispatus (CE. 28)]. Detritos celulares de CR. 4 mostraram desintoxicação significativamente maior (P < 0.05). A quantidade máxima de AFB1 foi desintoxicada a 30 °C (97%), pH 4.0 (99%) e 6 h (99,97%). O estudo in vivo mostrou que AFB1 diminuiu o ganho de peso (1,269 ± 0.04 g / ave), alimento consumido (2,161 ± 0.08 g / ave), proteína total sérica (2.42 ± 0.34 g / dl), albumina sérica (0.5 ± 0.22 gm / dl) e título de anticorpo (4.2 ± 0.83). As enzimas da função hepática foram encontradas (alanina transaminase (ALT): 32 ± 10.7 U / L) e aspartato transaminase (AST): 314.8 ± 27 U / L) elevadas em AFB1 alimentados com frangos. O tratamento com 1% LS não só diminuiu os efeitos tóxicos de AFB1 (grupo D), mas também melhorou a saúde geral dos frangos devido aos seus efeitos probióticos (p < 0.05) em comparação com o controle negativo (grupo A). A capacidade de desintoxicação do LS foi melhor do que o aglutinante comercial (CB) (0.2% Protmyc). Concluiu-se que a desintoxicação de AFB1 por Lactobacillus foi dependente da cepa, temperatura, pH e tempo. LS tem capacidade de desintoxicação contra AFB1 in vivo.
Palavras-chave:
L. Salivarius; Aflatoxin B1; desintoxicação; HPLC
1. Introduction
Aflatoxins (AFs) are highly toxic secondary metabolites produced by A. flavus and A. parasiticus (Faria et al., 2017FARIA, C.B., SANTOS, F.C., CASTRO, F.F., SUTIL, A.R., SERGIO, L.M., SILVA, M.V., MACHINSKI JUNIOR, M. and BARBOSA-TESSMANN, I.P., 2017. Occurrence of toxigenic Aspergillus flavus in commercial Bulgur wheat. Food Science and Technology (Campinas), vol. 37, no. 1, pp. 103-111. http://dx.doi.org/10.1590/1678-457x.09316.
http://dx.doi.org/10.1590/1678-457x.0931...
). Among different molecules AFs, Aflatoxin B1 (AFB1) is highly toxic (B1>G1>B2>G2). It is a serious health hazard for birds, animals and human, as it is hepatotoxic, mutagenic, carcinogenic, teratogen and immunosuppressant (Kosztik et al., 2020KOSZTIK, J., MÖRTL, M., SZÉKÁCS, A., KUKOLYA, J. and BATA-VIDÁCS, I.. 2020. Aflatoxin B1 and sterigmatocystin binding potential of Lactobacilli. Toxins, vol. 12, no. 12, pp. 756. http://dx.doi.org/10.3390/toxins12120756. PMid:33266172.
http://dx.doi.org/10.3390/toxins12120756...
).
AFs enters body through ingestion, inhalation and skin penetration. Inside body it is activated by cytochrome P450enzyme system in liver to produce a highly reactive intermediate, AFB1-8,9-epoxide. This highly reactive radical binds to nucleophile sites in DNA forming 8,9-dihydro-8-(N7guanyl)-9-hydroxy-AFB1 adduct. It is a critical step in the initiation of AFB1-induced carcinogenesis (Guo et al., 2020GUO, Y., ZHAO, L., MA, Q. and JI, C., 2020. Novel strategies for degradation of aflatoxins in food and feed: a review. Food Research International, vol. 140, pp. 109878. https://doi.org/10.1016/j.foodres.2020.1098.
https://doi.org/10.1016/j.foodres.2020.1...
).
Many biological or chemical agents are used to reduce toxicity of AFs. These agents are mixed in feed rations to eliminate, detoxify or inactivate toxins. The hot topic for toxin binding, now a day are probiotics (Wang et al., 2020WANG, J., ISHFAQ, M., GUO, Y., CHEN, C. and LI, J., 2020. Assessment of probiotic properties of Lactobacillus salivarius isolated from chickens as feed additives. Frontiers in Veterinary Science, vol. 7, pp. 415. http://dx.doi.org/10.3389/fvets.2020.00415. PMid:32766298.
http://dx.doi.org/10.3389/fvets.2020.004...
). Lactobacilli are probiotics which adsorb AFB1 by cell wall components (Teichoic acid). They adsorbed more than 99% of AFs (Ghazvini et al., 2016GHAZVINI, R. D., KOUHSARI, E., ZIBAFAR, E., HASHEMI, S. J. and NIKNEJAD, A. F., 2016. Antifungal activity and aflatoxin degradation of Bifidobacterium bifidum and Lactobacillus fermentum against toxigenic Aspergillus parasiticus. The Open Microbiology Journal, vol. 10, pp. 197-201. http://dx.doi.org/10.2174/1874285801610010197.
http://dx.doi.org/10.2174/18742858016100...
). L. acidophilus removed 80% whereas L. plantarum removed 85% of the AFB1 in one week from sample at 4 °C. L. casei bind 98% AFB1 from medium and prevented Aflatoxicosis in mice (Liew et al., 2018LIEW, W.P., NURUL-ADILAH, Z., THAN, L.T.L. and MOHD-REDZWAN, S., 2018. The binding efficiency and interaction of Lactobacillus casei Shirota toward Aflatoxin B1. Frontiers in Microbiology, vol. 9, pp. 1503. http://dx.doi.org/10.3389/fmicb.2018.01503. PMid:30042748.
http://dx.doi.org/10.3389/fmicb.2018.015...
). They are well known for their probiotic characters (Chen et al., 2017CHEN, F., ZHU, L. and QIU, H., 2017. Isolation and probiotic potential of Lactobacillus salivarius and Pediococcus pentosaceus in specific pathogen free chickens. Revista Brasileira de Ciência Avícola, vol. 9, no. 2, pp. 325-332. http://dx.doi.org/10.1590/1806-9061-2016-0413.
http://dx.doi.org/10.1590/1806-9061-2016...
). As antibiotics are hazard for birds and humans so they are replaced by probiotics as feed additives. L. salivarius (LS) improved growth rate, body weight gain, feed intake and feed conversion efficiency. They also improve probiotic count in GIT, cholesterol metabolism, mineral metabolism, carcass characteristics and sensory quality of chicken meat (Jadhav et al., 2015JADHAV, K., SHARMA, K. S., KATOCH, S., SHARMA, V. K. and MANE, V. K., 2015. Probiotics in broiler poultry feeds: a review. International Journal of Animal and Veterinary Science, vol. 2, pp. 4-16.). LS increases body weight, average daily gain, dressing percentage, crude protein, fat content of meat and the number of Lactobacillus in ceca (p<0.05) when fed to poultry birds (2×108 CFU/g). They decrease FCR, plasma ammonia content and fecal ammonia (Chen et al., 2017CHEN, F., ZHU, L. and QIU, H., 2017. Isolation and probiotic potential of Lactobacillus salivarius and Pediococcus pentosaceus in specific pathogen free chickens. Revista Brasileira de Ciência Avícola, vol. 9, no. 2, pp. 325-332. http://dx.doi.org/10.1590/1806-9061-2016-0413.
http://dx.doi.org/10.1590/1806-9061-2016...
). It reduced total cholesterol, LDL-cholesterol and triglycerides in broilers. Its administration increases intestinal populations of beneficial bacteria (such as Lactobacilli and bifidobacteria) and decreases harmful bacteria (such as E. coli) (Shokryazdan et al., 2017SHOKRYAZDAN, P., FASELEH JAHROMI, M., LIANG, J.B., RAMASAMY, K., SIEO, C.C. and HO, Y.W., 2017. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS One, vol. 12, no. 5, pp. e0175959. http://dx.doi.org/10.1371/journal.pone.0175959. PMid:28459856.
http://dx.doi.org/10.1371/journal.pone.0...
). LS is expected to show probiotic as well as anti-AFB1 activity in broiler.
2. Materials and Methods
2.1. Source of chemicals
Chemicals were purchased from local suppliers. These include Acetonitrile (ACN) (Sigma Aldrich, CAS # 75-05-8), Man Rogosa Sharp (MRS) broth (Oxoid code: CM0359), Methanol (Sigma Aldrich CAS # 67-56-1), Trifluoroacetic acid (TFA) (Sigma Aldrich, CAS # 76-05-1), Syringe micro filters (Sigma Aldrich, 22 µm, nylon), Aflatoxin B1 (Sigma Aldrich, CAS #1162-65-8).
2.2. Source of Lactobacillus
Five identified Lactobacillus broiler isolates were taken from Institute of biochemistry and biotechnology, University of Veterinary and Animals Sciences, Lahore, in form of glycerol stock. They were activated on MRS agar. Their MRS broth culture was prepared after inoculating and incubating at 37 °C for 24 h.
2.3. Effect of Lactobacillus against AFB1 In-vitro
Activity of Lactobacillus isolates against AFB1 was determined by using method described by Chlebicz and Slizewska (2020)CHLEBICZ, A. and SLIZEWSKA, K., 2020. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics and Antimicrobial Proteins, vol. 12, no. 1, pp. 289-301. http://dx.doi.org/10.1007/s12602-018-9512-x. PMid:30721525.
http://dx.doi.org/10.1007/s12602-018-951...
with minor variations. The experiment was repeated thrice. Bacterial load of working bacterial suspension was counted by spread plate method and expressed in Colony Forming Units (CFU)/mL (Thomas et al., 2015THOMAS, P., SEKHAR, A.C., UPRETI, R., MUJAWAR, M.M. and PASHA, S.S., 2015. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnology Reports (Amsterdam, Netherlands), vol. 8, pp. 45-55. http://dx.doi.org/10.1016/j.btre.2015.08.003. PMid:28352572.
http://dx.doi.org/10.1016/j.btre.2015.08...
). One mL broth culture with 9E11 CFU/mL of isolates were taken and incubated with AFB1 (50 ppb in PBS, pH 7.4, 0.1 M) in separate tubes at 37 °C for 2 h. The tubes were centrifuged; supernatants were dried and AFB1 was estimated by HPLC.
2.4. Effect of whole cell and fractions
Bacterial cell fractions (cell wall and cell soluble) were prepared for all five isolates by using method described by Shrestha et al. (2012)SHRESTHA, P., HOLLAND, T.M. and BUNDY, B.C., 2012. Streamlined extract preparation for Escherichia coli-based cell-free protein synthesis by sonication or bead vortex mixing. BioTechniques, vol. 53, no. 3, pp. 163-174. http://dx.doi.org/10.2144/0000113924. PMid:22963478.
http://dx.doi.org/10.2144/0000113924...
. For this purpose, bacterial cells (9E11 CFU/mL) were centrifuged at 6000 rpm for 15 min and supernatant was discarded. Cells were suspended in 1 mL PBS buffer (pH 7.4, 0.1 M) and sonication at 20 k Hz, keeping in ice with 10 sec sonication and 30 sec cooling. It was repeated thrice and then centrifuged at 12000 rpm, 30 min at 4° C. Pellet (cell debris) and supernatant (cell soluble) were separated in tubes and stored at -20 °C till further use.
Whole viable cells (9E11 CFU/mL), cell debris and cell soluble of all five isolates were separately mixed with 1mL of AFB1 solution (50 ppb in PBS, pH 7.4, 0.1 M). They were incubated at 37 °C for 2 h and centrifuged at 5000 rpm for 10 min. Supernatant were dried in water bath and AFB1 was estimated by HPLC.
2.5. Effect of temperature
One mL of a day-old culture with 9E11 CFU/mL were taken for all five isolates. They were mixed with 1mL of AFB1 (50 ppb in PBS pH 7.4, 0.1 M). Four tubes were prepared for each isolate and were incubated at different temperatures (20, 25, 30, 37 °C) for 2 h. They were centrifuged at 5000 rpm for 10 min. Supernatants were dried in water bath and AFB1 was derived. AFB1 was estimated in samples by HPLC method.
2.6. Effect of pH
One mL of a day-old culture with 9E11 CFU/mL was taken for all five isolates. They were mixed with 1mL of AFB1 (50 ppb in PBS). Four tubes were prepared with pH 4.0, 5.0, 6.0, 7.0 for each isolate. They were incubated at 30 °C for 2 h and centrifuged at 5000 rpm for 10 min. Supernatants were dried in water bath and for better results AFB1was derived. Samples were estimated for AFB1 by HPLC method.
2.7. Effect of incubation time
One mL of a day-old culture with 9E11 CFU/mL was taken for all five isolates. They were mixed with 1mL of AFB1 (50 ppb in PBS). Three tubes were prepared for each isolate and were incubated at 30 °C for 2, 4, 6 h. They were centrifuged at 5000 rpm for 10 min. Supernatants were dried in water bath, and for better results AFB1was derived. Samples were estimated for AFB1 by HPLC method.
2.8. Samples run on HPLC
The dried AFB1 samples were taken and 200 µL N-Hexane, 500 µL TFA were added. They were mixed by vortex mixer and after 5 min 1,950 µL ACN: Water mixture (1:1) was added. Two layers were formed in the tube. From lower layer 1 mL was taken in a vial by passing through syringe filter (nylon, 0.22 µm). These samples were sent to WTO- quality operation laboratory, UVAS, Lahore, for estimation of AFB1 (Hussain et al., 2016HUSSAIN, Z., REHMAN, H., MANZOOR, S., TAHIR, S. and MUKHTAR, M., 2016. Determination of liver and muscle aflatoxin B1 residues and select serum chemistry variables during chronic aflatoxicosis in broiler chickens. Veterinary Clinical Pathology, vol. 45, no. 2, pp. 330-334. http://dx.doi.org/10.1111/vcp.12336. PMid:27044011.
http://dx.doi.org/10.1111/vcp.12336...
).
HPLC system of Agilent 1100 series with C 18 Column and fluorescent detector was used in this study. Mobile phase was ACN: Methanol: Water (20:20:60; v/v/v). Excitation and emission wavelengths were 360 and 440 nm. Flow rate was 1mL/Min and column temperature was 40 °C. Injection volume was 20 µL (Hussain et al., 2016HUSSAIN, Z., REHMAN, H., MANZOOR, S., TAHIR, S. and MUKHTAR, M., 2016. Determination of liver and muscle aflatoxin B1 residues and select serum chemistry variables during chronic aflatoxicosis in broiler chickens. Veterinary Clinical Pathology, vol. 45, no. 2, pp. 330-334. http://dx.doi.org/10.1111/vcp.12336. PMid:27044011.
http://dx.doi.org/10.1111/vcp.12336...
).
2.9. Effect of LS against AFB1 in-vivo
2.9.1. Source of bacteria and growth
LS (strain CR. 4) has shown highest in vitro detoxification (92.3%) of AFB1. It was activated on sterile MRS agar and cultured in MRS broth. Bacterial cells were counted by spread plate method and mixed in feed.
2.9.2. Feeding trail
Fifteen hundred one day old broiler (Arbreaker Breed) chicks were randomly divided into six groups (A-F). Each group was divided into five replicates with 50 units in each replicate. Group A was control negative and group B was control positive with 100 ppb AFB1. Group C received LS with 3x109 CFU/gm feed. Group D was treated with 100 ppb AFB1 along with LS, 3x109 CFU/gm feed. Group E received 0.2% yeast wall based commercial binder (Protmyc). Group F was treated with 100 ppb AFB1 and 0.2% yeast wall based commercial binder (Protmyc). Broiler starter feed mesh #4 by Hi-Tech feeds was used in this research. The experiment trail of 35 days was run. This trail was run in a control shed at Hi-Tech Research and Development center, Lahore, in the months of May-June, 2018 according to method described by Shokryazdan et al. (2017)SHOKRYAZDAN, P., FASELEH JAHROMI, M., LIANG, J.B., RAMASAMY, K., SIEO, C.C. and HO, Y.W., 2017. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS One, vol. 12, no. 5, pp. e0175959. http://dx.doi.org/10.1371/journal.pone.0175959. PMid:28459856.
http://dx.doi.org/10.1371/journal.pone.0...
.
2.9.3. Weight gain, FCR and Serum biochemistry estimation
During trail feed consumed, weight gain and FCR were calculated on weekly basis. At end of trail blood was taken from five birds per replicate (1 mL/ bird) for HI test (Khushi et al., 2014KHUSHI, M., KHUSHI, M.Z., RABBANI, M., ANJUM, A.A., AHMAD, A., NAZIR, J., NAWAZ, M., CHAUDHRY, H.R., ALI, M.A., HANIF, K., YASMEEN, R. and ALTAF, I., 2014. Khushi’s practicals in fundamental immunology. USA: CreateSpace Independent Publishing Platform.). Two birds were selected randomly per replicate for sample collection. For biochemical analysis 5 mL blood/ bird was collected by syringe from wing vein by expert veterinarian in red capped vacutainers. Serum was separated and used for biochemical analysis (Serum total protein, Albumin, ALT, AST and Creatinine) by using Biochemistry analyzer “Micro lab 300 by Merck”. After blood collection bird was slaughtered and liver was collected in formalin containing jars for histopathology (Selvam et al., 2018SELVAM, R., SARAVANAKUMAR, M., SURESH, S., CHANDRASEKERAN, C.V. and PRASHANTH, D., 2018. Evaluation of polyherbal formulation and synthetic choline chlorideon choline deficiency model in broiler: implications on zootechnical parameters, serum biochemistry and liver histopathology. Asian-Australasian Journal of Animal Sciences, vol. 31, no. 11, pp. 1795-1806. http://dx.doi.org/10.5713/ajas.18.0018. PMid:29642669.
http://dx.doi.org/10.5713/ajas.18.0018...
).
2.10. Statistical analysis
From in vitro study, data were obtained in form of concentration of AFB1 in ng/mL left after treatments. Detoxified AFB1 was calculated in form of ng/mL (ppb). Data from in vitro and in vivo study was analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0.05.
3. Results
3.1. Effect of Lactobacillus against AFB1 In vitro
Five Lactobacillus isolates (Table 1) were investigated for their activity against AFB1.When incubated with AFB1 (50 PPB), whole viable cells of CR. 4 removed maximum amount of AFB1 (92.3%) as compared to CE.28 (89.4%), CE. 2.1 (86.6%), CE. 3.1 (81.2%) and CR. 3 (74.6%). Similarly, CR.4 cell soluble removed highest amount of AFB1 (99.7%) as compared to other isolates in study. As cell debris is concerned CR. 4 gave best results by removing 99.9% AFB1. Comparatively cell debris gave best results as compared to cell soluble and whole cells (Table 2).
Different temperatures were observed for their effect on detoxification potential of isolates. At room temperature (20 °C) CR.4 removed highest amount of AFB1 (90.5%). At 25 °C CR.3 removed 97.2% toxin, which is highest at this temperature as compared to other isolates. At 30 °C all three isolates CR.4, CR.3 and CE 3.1 removed 97% AFB1. At physiological pH (37°C), CR.4 removed 92% of toxin. From above results it was concluded that at 30 °C all isolates removed highest amount of AFB1 (Table 3).
Different pH representing different parts of poultry GIT were observed for their effect on detoxification potential of isolates. At pH 4.0 isolate CR.4 removed maximum toxin (99%) as compared to CR. 3 (95.8%), CE.28 (95.7%) and CE. 3.1 (95.9%). At pH 5.0 two isolates CE. 2.1 and CE. 3.1 removed maximum toxin (96%) as compared to CE. 28 (94.4%), CR. 3 and CR. 4 (95%). At pH 6.0 CE. 28 and CR. 4 (93-94%) removed highest level of AFB1 followed by CR. 3 and CE. 2.1 (85-86%). Least adsorption was shown by CE. 3.1 (84%). Isolate CR. 4 removed highest amount of AFB1 (92%) at pH 7.0 as compared to other isolates. As observed in the Table 4, optimum pH for AFB1 removal is strain specific. It is pH 4.0 for L. crispatus, L. salivarius and pH 5.0 for L. agilis.
Effect of time duration on removal of AFB1 by isolates was determined. According to observation after 2 h of incubation CR. 4 removed highest amount of AFB1 (92%) as compared to remaining isolates (CE. 28: 89%, CE. 2.1: 85%, CE. 3.1: 81% and CR. 3: 74%). Incubation of 4 h was best for CR. 4 (99%) for removing AFB1 following other isolates (CE. 28: 93%, CR. 3: 90%, CE. 2.1: 86% and CE. 3.1: 81%). Maximum time incubation was 6 h for which CR. 4 showed best results by removing 99.97% AFB1 followed by other isolates CR. 3 (99.92%), CE. 28 (97%), CE. 2.1 (91.6%) and CE. 3.1 (90.6%). As the comparison of time is concerned amount of AFB1 detoxified increases by time for all isolates (Table 5).
3.2. Effect of LS against AFB1 in vivo
The detoxification and probiotic effects of LS (CR. 4) on feed intake, body weight gain and FCR of broiler chicks were observed. Feed consumed (gm/bird) was significantly different between the groups. Addition of AFB1 in feed reduced feed consumption rate in birds. But the treatment with LS improved this effect. Highest feed consumption was observed in group C (LS, AFB1) with 2,391 ± 0.16 gm/bird. In group D feed consumption was less than group C but more than groups A, E and F. So, it was found that LS treatment not only improved feed consumption but also removed detrimental effects of AFB1 in feed. Even performance of birds with LS (Group C, D) was better than birds which received commercial binder (Group E, F) as shown in Figure 1.
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on feed consumed and weight gain of broiler. Data were analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0.05.
Highest weight gain of birds was observed in group C (1,429 gm/bird) as compared to other groups. Lowest weight gain was in group B (AFB1), E (CB) and F (CB, AFB1) as observed in Figure 1. Feed conversion ratio (FCR) did not show difference between treatment groups. Group C (LS) and D (LS, AFB1) had shown similar FCR (1.67, 1.68) as shown by control group A (1.67). For remaining groups like A (BD, AFB1), E (CB) and F (CB, AFB1) FCR was higher (1.7), as shown in Figure 2.
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on feed conversion ratio (FCR) of broiler. Data were analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0,05.
Antibody titer against ND vaccine was observed in serum of birds (Figure 3). The results showed that titer was low (4.2 ± 0.83) in group B (AFB1). But remaining groups (A, C, D, E and F) had shown titer in normal range (5-7). Highest titer (7 ± 0.7) was observed in group C (LS), which indicated that L. salivarius played role in increasing immune response in broilers.
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on serum Hemagglutination inhibition (HI) titer of broiler. The values of the highest dilutions which causes total inhibition of hemagglutination were calculated as the logarithm to the base 2.
Results of biochemical analysis of serum showed that AFB1 (Group B) resulted in liver damage. As serum level of STP (Serum total protein), SA (Serum Albumin) were decreased as compared to control (Group A) (Figure 4).
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on serum total protein (STP) and albumin (SA) of broiler. Data were analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0,05.
Level of ALT, AST were increased in group B as compared to group A. AFB1 showed slight damage in kidney as level of creatinine was higher in group B as compared to group A. L. salivarius and CB removed toxic effects of AFB1 as shown in Figures 5 -7.
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on serum alanine transaminase (ALT), international unit (IU) level in broiler. Data were analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0,05.
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on serum aspartate transaminase (AST), international unit (IU) level in broiler. Data were analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0,05.
Effect of feeding Aflatoxin B1, Lactobacillus salivarius and Protmic (Yeast based mycotoxin binder) supplementation on serum creatinine (CR) level in broiler. Data were analyzed by one way ANOVA of SPSS 16.0 software. The research design was Complete Randomized design (RCD), and Duncan’s multiple range test was used to compare means. The difference was considered significant if P<0,05.
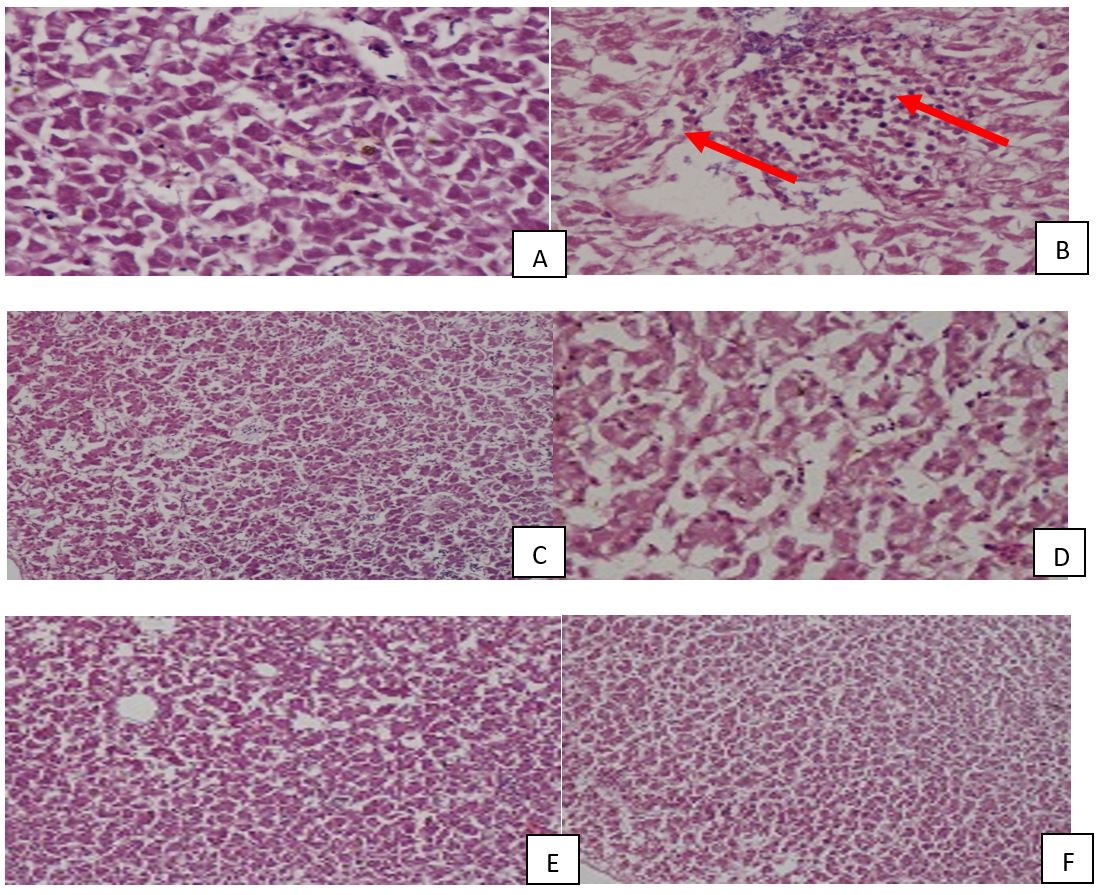
Histo-pathological study of broiler livers showed that AFB1 treatment resulted in infiltration of inflammatory cells, necrosis and degeneration of hepatocytes. Treatment with toxin binders (LS and CB) removed these damaging effects of AFB1 from group D and F (Figures 8, 9).
Effect of feeding Aflatoxin B1 (Group B, D, F), Lactobacillus salivarius (Group C, D) and Protmic (Yeast based mycotoxin binder in Group E, F) on broiler liver. Livers of broiler from group A, C, D, E and F showing normal view, but liver from broiler of group B showing discoloration and lesions. AFB1: Aflatoxin B1, LS: L. salivarius.
Effect of feeding Aflatoxin B1 (Group B, D, F), Lactobacillus salivarius (Group C, D) and Protmic (Yeast based mycotoxin binder in Group E, F) on broiler liver. Results of Liver histology from group: (A) 10x40X, normal, (B) 10x10X, damaged (infiltration of inflammatory cells, necrosis and degeneration of hepatocytes), (C) 10x10X, normal, (D) 40x10X, normal, (E) 10x10X, normal, (F) 10x10X, normal. AFB1: Aflatoxin B1, LS: L. salivarius.
4. Discussion
Previous studies have shown that live Lactobacillus has highest binding efficiency (98%) against AFB1. Stability of the formed complexes depends on strain, treatment, and environmental conditions (Liew et al., 2018LIEW, W.P., NURUL-ADILAH, Z., THAN, L.T.L. and MOHD-REDZWAN, S., 2018. The binding efficiency and interaction of Lactobacillus casei Shirota toward Aflatoxin B1. Frontiers in Microbiology, vol. 9, pp. 1503. http://dx.doi.org/10.3389/fmicb.2018.01503. PMid:30042748.
http://dx.doi.org/10.3389/fmicb.2018.015...
). It was found that Lactobacillus cell wall play important role in detoxification of AFB1through physical binding. Even heat-treated cells can also establish stable complex with AFB1. Solvent extraction procedures released about 90% of bound AFB1 (Liew et al., 2018LIEW, W.P., NURUL-ADILAH, Z., THAN, L.T.L. and MOHD-REDZWAN, S., 2018. The binding efficiency and interaction of Lactobacillus casei Shirota toward Aflatoxin B1. Frontiers in Microbiology, vol. 9, pp. 1503. http://dx.doi.org/10.3389/fmicb.2018.01503. PMid:30042748.
http://dx.doi.org/10.3389/fmicb.2018.015...
). Cell wall teichoic acid (C5H11O7P) n was responsible for AFB1 binding (Liew et al., 2018LIEW, W.P., NURUL-ADILAH, Z., THAN, L.T.L. and MOHD-REDZWAN, S., 2018. The binding efficiency and interaction of Lactobacillus casei Shirota toward Aflatoxin B1. Frontiers in Microbiology, vol. 9, pp. 1503. http://dx.doi.org/10.3389/fmicb.2018.01503. PMid:30042748.
http://dx.doi.org/10.3389/fmicb.2018.015...
). Intracellular metabolites (Enzymes) also play role in detoxification of AFB1. L. fermentum metabolites reduced AF level from 88.8% to 99.8% (Ghazvini et al., 2016GHAZVINI, R. D., KOUHSARI, E., ZIBAFAR, E., HASHEMI, S. J. and NIKNEJAD, A. F., 2016. Antifungal activity and aflatoxin degradation of Bifidobacterium bifidum and Lactobacillus fermentum against toxigenic Aspergillus parasiticus. The Open Microbiology Journal, vol. 10, pp. 197-201. http://dx.doi.org/10.2174/1874285801610010197.
http://dx.doi.org/10.2174/18742858016100...
).
According to previous work dairy strains of Lactic acid bacteria has ability to bind toxins such as AFB1 from liquid media. Lactobacillus species adsorb about 60% of AFB1 within 6 h (Chlebicz and Slizewska, 2020CHLEBICZ, A. and SLIZEWSKA, K., 2020. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics and Antimicrobial Proteins, vol. 12, no. 1, pp. 289-301. http://dx.doi.org/10.1007/s12602-018-9512-x. PMid:30721525.
http://dx.doi.org/10.1007/s12602-018-951...
). Different Lactobacilli species from yogurt have shown AFB1 detoxification (8-31%), depending upon their strains (Zolfaghari et al., 2020ZOLFAGHARI, H., KHEZERLOU, A., EHSANI, A. and YARI KHOSROUSHAHI, A., 2020. Detoxification of Aflatoxin B1 by probiotic Yeasts and Bacteria isolated from dairy products of Iran. Advanced Pharmaceutical Bulletin, vol. 10, no. 3, pp. 482-487. http://dx.doi.org/10.34172/apb.2020.060. PMid:32665910.
http://dx.doi.org/10.34172/apb.2020.060...
). They have ability to reduce level of AFs in media by enzymatic/chemical degradation, metabolic conversion, or adsorption through cell wall components (Sadiq et al., 2019SADIQ, F.A., YAN, B., TIAN, F., ZHAO, J., ZHANG, H. and CHEN, W., 2019. Lactic acid bacteria as antifungal and anti‐mycotoxigenic agents: a comprehensive review. Comprehensive Reviews in Food Science and Food Safety, vol. 18, no. 5, pp. 1403-1436. http://dx.doi.org/10.1111/1541-4337.12481. PMid:33336904.
http://dx.doi.org/10.1111/1541-4337.1248...
). Mainly polysaccharides and peptidoglycan are responsible for AFs detoxification. The structures of these molecules are conserved but minor variations among strains are responsible for difference in detoxification ability (Sadiq et al., 2019SADIQ, F.A., YAN, B., TIAN, F., ZHAO, J., ZHANG, H. and CHEN, W., 2019. Lactic acid bacteria as antifungal and anti‐mycotoxigenic agents: a comprehensive review. Comprehensive Reviews in Food Science and Food Safety, vol. 18, no. 5, pp. 1403-1436. http://dx.doi.org/10.1111/1541-4337.12481. PMid:33336904.
http://dx.doi.org/10.1111/1541-4337.1248...
).
The results of this study are in accordance with several previous investigation, LS administration improved growth rate, body weight gain, feed intake, feed conversion efficiency in broilers (Jadhav et al., 2015JADHAV, K., SHARMA, K. S., KATOCH, S., SHARMA, V. K. and MANE, V. K., 2015. Probiotics in broiler poultry feeds: a review. International Journal of Animal and Veterinary Science, vol. 2, pp. 4-16.); (Shokryazdan et al., 2017SHOKRYAZDAN, P., FASELEH JAHROMI, M., LIANG, J.B., RAMASAMY, K., SIEO, C.C. and HO, Y.W., 2017. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS One, vol. 12, no. 5, pp. e0175959. http://dx.doi.org/10.1371/journal.pone.0175959. PMid:28459856.
http://dx.doi.org/10.1371/journal.pone.0...
). Administration of 40 ppb AFB1 till 21 days has shown insignificant change in mortality, but feed consumed and weight gain are reduced (Liu et al., 2018LIU, N., WANG, W., DENG, Q., GU, K. and WANG, J., 2018. Detoxification of aflatoxin B1 by lactic acid bacteria and hydrated sodium calcium aluminosilicate in broiler chickens. Livestock Science, vol. 208, pp. 28-32. http://dx.doi.org/10.1016/j.livsci.2017.12.005.
http://dx.doi.org/10.1016/j.livsci.2017....
). Lactobacillus increased serum IgG and IgM level (Liu et al., 2018LIU, N., WANG, W., DENG, Q., GU, K. and WANG, J., 2018. Detoxification of aflatoxin B1 by lactic acid bacteria and hydrated sodium calcium aluminosilicate in broiler chickens. Livestock Science, vol. 208, pp. 28-32. http://dx.doi.org/10.1016/j.livsci.2017.12.005.
http://dx.doi.org/10.1016/j.livsci.2017....
). LS are natural part of broiler GIT micro flora. They have shown to induce different cytokine profiles in mononuclear cells in vitro and increased antibody against ND as compared to control birds (Brisbin et al., 2011BRISBIN, J.T., GONG, J., OROUJI, S., ESUFALI, J., MALLICK, A.I., PARVIZI, P., SHEWEN, P.E. and SHARIF, S., 2011. Oral Treatment of Chickens with Lactobacilli influences elicitation of immune responses. Clinical and Vaccine Immunology; CVI, vol. 18, no. 9, pp. 1447-1455. http://dx.doi.org/10.1128/CVI.05100-11. PMid:21734067.
http://dx.doi.org/10.1128/CVI.05100-11...
). AFB1 (800 ppb) when fed to broiler for 28 days resulted in increased ALT and AST level in serum. But total protein and albumin level was significantly low. AFB1 (50 and 100 ppb) when administered for 28 days, has not shown measurable AFB1 residues in tissues. Serum level of ALT, AST, total protein and albumin serve as markers for chronic aflatoxicosis in birds (Hussain et al., 2016HUSSAIN, Z., REHMAN, H., MANZOOR, S., TAHIR, S. and MUKHTAR, M., 2016. Determination of liver and muscle aflatoxin B1 residues and select serum chemistry variables during chronic aflatoxicosis in broiler chickens. Veterinary Clinical Pathology, vol. 45, no. 2, pp. 330-334. http://dx.doi.org/10.1111/vcp.12336. PMid:27044011.
http://dx.doi.org/10.1111/vcp.12336...
). LS decreased liver fibrosis by protecting the intestinal barrier and promoted micro biome health (Shi et al., 2017SHI, D., LV, D.X., FANG, D., WU, W., HU, C., XU, L., CHEN, Y., GUO, J., HU, X., LI, A., GUO, F., YE, J., LI, Y., ANDAYANI, D. and LI, L., 2017. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4 induced liver cirrhosis by protecting the intestinal barrier in rats. Scientific Reports, vol. 7, no. 1, pp. 6927. http://dx.doi.org/10.1038/s41598-017-07091-1. PMid:28761060.
http://dx.doi.org/10.1038/s41598-017-070...
). When 3-300 ppb AFB1 is given to broiler for 42 days, significant damage in liver is observed by histological analysis. According to observations liver was fatty with necrosis, bile duct hyperplasia and aggregation of lymphocyte. Lactobacillus supplemented in feed removed toxic effects AFB1 in broiler (Shlej et al., 2015SHLEJ, A.A., SAAID, J.M. and THLIJ, K.M., 2015. Ability of L. casei and L. acidophilus to protect against the toxicity in broiler fed contaminated diet AFB1. Iraqi Journal of Agriculture Science, vol. 64, no. 4, pp. 652-661.).
In conclusion, the present study described performance parameters and biochemical alterations in AFB1 fed broilers. Improvement in productive performance and serum biochemical parameters in group fed diet supplement with novel poultry gut isolate of LS suggested a decrease in severity of aflatoxin B1 induced toxicity in broilers.
5. Conclusion
Detoxification of AFB1 by Lactobacillus was strain, pH, time and temperature dependent. Cell wall of Lactobacillus played significant role in removing AFB1 by adsorption, but intracellular metabolites also play their role. Optimum reduction was achieved at 30°C, acidic pH (4.0) and 6 h of incubation. L. salivarius plays active role as probiotic in broilers. It detoxifies AFB1 and removes its toxicity. It was concluded that L. salivarius not only improves broiler health but also removes toxic effects of AFB1.
Acknowledgements
I am thankful to Dr Muhammad Athar from Hi-Tech feeds pvt. limited for providing us the facility to run the biological trial.
References
- BRISBIN, J.T., GONG, J., OROUJI, S., ESUFALI, J., MALLICK, A.I., PARVIZI, P., SHEWEN, P.E. and SHARIF, S., 2011. Oral Treatment of Chickens with Lactobacilli influences elicitation of immune responses. Clinical and Vaccine Immunology; CVI, vol. 18, no. 9, pp. 1447-1455. http://dx.doi.org/10.1128/CVI.05100-11 PMid:21734067.
» http://dx.doi.org/10.1128/CVI.05100-11 - CHEN, F., ZHU, L. and QIU, H., 2017. Isolation and probiotic potential of Lactobacillus salivarius and Pediococcus pentosaceus in specific pathogen free chickens. Revista Brasileira de Ciência Avícola, vol. 9, no. 2, pp. 325-332. http://dx.doi.org/10.1590/1806-9061-2016-0413
» http://dx.doi.org/10.1590/1806-9061-2016-0413 - CHLEBICZ, A. and SLIZEWSKA, K., 2020. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics and Antimicrobial Proteins, vol. 12, no. 1, pp. 289-301. http://dx.doi.org/10.1007/s12602-018-9512-x PMid:30721525.
» http://dx.doi.org/10.1007/s12602-018-9512-x - FARIA, C.B., SANTOS, F.C., CASTRO, F.F., SUTIL, A.R., SERGIO, L.M., SILVA, M.V., MACHINSKI JUNIOR, M. and BARBOSA-TESSMANN, I.P., 2017. Occurrence of toxigenic Aspergillus flavus in commercial Bulgur wheat. Food Science and Technology (Campinas), vol. 37, no. 1, pp. 103-111. http://dx.doi.org/10.1590/1678-457x.09316
» http://dx.doi.org/10.1590/1678-457x.09316 - GHAZVINI, R. D., KOUHSARI, E., ZIBAFAR, E., HASHEMI, S. J. and NIKNEJAD, A. F., 2016. Antifungal activity and aflatoxin degradation of Bifidobacterium bifidum and Lactobacillus fermentum against toxigenic Aspergillus parasiticus The Open Microbiology Journal, vol. 10, pp. 197-201. http://dx.doi.org/10.2174/1874285801610010197
» http://dx.doi.org/10.2174/1874285801610010197 - GUO, Y., ZHAO, L., MA, Q. and JI, C., 2020. Novel strategies for degradation of aflatoxins in food and feed: a review. Food Research International, vol. 140, pp. 109878. https://doi.org/10.1016/j.foodres.2020.1098
» https://doi.org/10.1016/j.foodres.2020.1098 - HUSSAIN, Z., REHMAN, H., MANZOOR, S., TAHIR, S. and MUKHTAR, M., 2016. Determination of liver and muscle aflatoxin B1 residues and select serum chemistry variables during chronic aflatoxicosis in broiler chickens. Veterinary Clinical Pathology, vol. 45, no. 2, pp. 330-334. http://dx.doi.org/10.1111/vcp.12336 PMid:27044011.
» http://dx.doi.org/10.1111/vcp.12336 - JADHAV, K., SHARMA, K. S., KATOCH, S., SHARMA, V. K. and MANE, V. K., 2015. Probiotics in broiler poultry feeds: a review. International Journal of Animal and Veterinary Science, vol. 2, pp. 4-16.
- KHUSHI, M., KHUSHI, M.Z., RABBANI, M., ANJUM, A.A., AHMAD, A., NAZIR, J., NAWAZ, M., CHAUDHRY, H.R., ALI, M.A., HANIF, K., YASMEEN, R. and ALTAF, I., 2014. Khushi’s practicals in fundamental immunology. USA: CreateSpace Independent Publishing Platform.
- KOSZTIK, J., MÖRTL, M., SZÉKÁCS, A., KUKOLYA, J. and BATA-VIDÁCS, I.. 2020. Aflatoxin B1 and sterigmatocystin binding potential of Lactobacilli. Toxins, vol. 12, no. 12, pp. 756. http://dx.doi.org/10.3390/toxins12120756 PMid:33266172.
» http://dx.doi.org/10.3390/toxins12120756 - LIEW, W.P., NURUL-ADILAH, Z., THAN, L.T.L. and MOHD-REDZWAN, S., 2018. The binding efficiency and interaction of Lactobacillus casei Shirota toward Aflatoxin B1. Frontiers in Microbiology, vol. 9, pp. 1503. http://dx.doi.org/10.3389/fmicb.2018.01503 PMid:30042748.
» http://dx.doi.org/10.3389/fmicb.2018.01503 - LIU, N., WANG, W., DENG, Q., GU, K. and WANG, J., 2018. Detoxification of aflatoxin B1 by lactic acid bacteria and hydrated sodium calcium aluminosilicate in broiler chickens. Livestock Science, vol. 208, pp. 28-32. http://dx.doi.org/10.1016/j.livsci.2017.12.005
» http://dx.doi.org/10.1016/j.livsci.2017.12.005 - SADIQ, F.A., YAN, B., TIAN, F., ZHAO, J., ZHANG, H. and CHEN, W., 2019. Lactic acid bacteria as antifungal and anti‐mycotoxigenic agents: a comprehensive review. Comprehensive Reviews in Food Science and Food Safety, vol. 18, no. 5, pp. 1403-1436. http://dx.doi.org/10.1111/1541-4337.12481 PMid:33336904.
» http://dx.doi.org/10.1111/1541-4337.12481 - SELVAM, R., SARAVANAKUMAR, M., SURESH, S., CHANDRASEKERAN, C.V. and PRASHANTH, D., 2018. Evaluation of polyherbal formulation and synthetic choline chlorideon choline deficiency model in broiler: implications on zootechnical parameters, serum biochemistry and liver histopathology. Asian-Australasian Journal of Animal Sciences, vol. 31, no. 11, pp. 1795-1806. http://dx.doi.org/10.5713/ajas.18.0018 PMid:29642669.
» http://dx.doi.org/10.5713/ajas.18.0018 - SHI, D., LV, D.X., FANG, D., WU, W., HU, C., XU, L., CHEN, Y., GUO, J., HU, X., LI, A., GUO, F., YE, J., LI, Y., ANDAYANI, D. and LI, L., 2017. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4 induced liver cirrhosis by protecting the intestinal barrier in rats. Scientific Reports, vol. 7, no. 1, pp. 6927. http://dx.doi.org/10.1038/s41598-017-07091-1 PMid:28761060.
» http://dx.doi.org/10.1038/s41598-017-07091-1 - SHLEJ, A.A., SAAID, J.M. and THLIJ, K.M., 2015. Ability of L. casei and L. acidophilus to protect against the toxicity in broiler fed contaminated diet AFB1. Iraqi Journal of Agriculture Science, vol. 64, no. 4, pp. 652-661.
- SHOKRYAZDAN, P., FASELEH JAHROMI, M., LIANG, J.B., RAMASAMY, K., SIEO, C.C. and HO, Y.W., 2017. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS One, vol. 12, no. 5, pp. e0175959. http://dx.doi.org/10.1371/journal.pone.0175959 PMid:28459856.
» http://dx.doi.org/10.1371/journal.pone.0175959 - SHRESTHA, P., HOLLAND, T.M. and BUNDY, B.C., 2012. Streamlined extract preparation for Escherichia coli-based cell-free protein synthesis by sonication or bead vortex mixing. BioTechniques, vol. 53, no. 3, pp. 163-174. http://dx.doi.org/10.2144/0000113924 PMid:22963478.
» http://dx.doi.org/10.2144/0000113924 - THOMAS, P., SEKHAR, A.C., UPRETI, R., MUJAWAR, M.M. and PASHA, S.S., 2015. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnology Reports (Amsterdam, Netherlands), vol. 8, pp. 45-55. http://dx.doi.org/10.1016/j.btre.2015.08.003 PMid:28352572.
» http://dx.doi.org/10.1016/j.btre.2015.08.003 - WANG, J., ISHFAQ, M., GUO, Y., CHEN, C. and LI, J., 2020. Assessment of probiotic properties of Lactobacillus salivarius isolated from chickens as feed additives. Frontiers in Veterinary Science, vol. 7, pp. 415. http://dx.doi.org/10.3389/fvets.2020.00415 PMid:32766298.
» http://dx.doi.org/10.3389/fvets.2020.00415 - ZOLFAGHARI, H., KHEZERLOU, A., EHSANI, A. and YARI KHOSROUSHAHI, A., 2020. Detoxification of Aflatoxin B1 by probiotic Yeasts and Bacteria isolated from dairy products of Iran. Advanced Pharmaceutical Bulletin, vol. 10, no. 3, pp. 482-487. http://dx.doi.org/10.34172/apb.2020.060 PMid:32665910.
» http://dx.doi.org/10.34172/apb.2020.060
Publication Dates
-
Publication in this collection
20 Dec 2021 -
Date of issue
2024
History
-
Received
01 Apr 2021 -
Accepted
25 Aug 2021