ABSTRACT
Mikania is a pantropical genus of Asteraceae with ca. 450 species distributed mainly in South America. Although most of its species occur in forested phytophysiognomies, significant richness is found in the mountaintop grasslands known as campos rupestres in Brazil. Recent botanical exploration of campos rupestres areas outside their core distribution, namely Serra do Padre Ângelo, Pico da Aliança, and Sete Salões State Park, all located in the eastern part of the state of Minas Gerais and within the Atlantic Forest phytogeographic domain, led to the description of several new plant species. After fieldwork and study of herbarium specimens, we recognize two new species of Mikania endemic to Pico da Aliança and Serra do Padre Ângelo. Mikania semirii is related to Mikania phaeoclados and differs by leaf indumentum, subinvolucral bract shape and size and position on the peduncle. Mikania funkiae is related to Mikania glauca and Mikania obtusata, and differs by the petiole length, leaf shape, texture, and margins. We provide full descriptions, illustrations, distribution maps, composite color figures, preliminary conservation status assessments, and comments on the taxonomy and ecology of these two species. These findings highlight the continued importance of floristic and taxonomic work on the rich eastern Brazilian flora.
Keywords:
Asteraceae; Campos rupestres; Compositae; critically endangered; Mikania; Mikaniinae; Neotropical flora; taxonomy
Introduction
Mikania is a pantropical genus of Asteraceae with around 450 species distributed mainly in South America (King & Robinson 1987King RM, Robinson H. 1987. The Genera of the Eupatorieae (Asteraceae). Monographs in Systematic Botany from the Missouri Botanical Garden 22: 1-581.; Robinson et al. 2009Robinson H, Schilling E, Panero JL. 2009. Eupatorieae. In: Funk VA, et al. (eds.). Systematics, Evolution, and Biogeography of the Compositae. International Association for Plant Taxonomy. p. 171-189.; Godoy et al. 2017Godoy SM, Silva JFM, Paula GBN, Ruas PM, Goés BD, Ruas CF. 2017. Phylogenetic relationships of BrazilianMikaniaspecies (Asteraceae, Eupatorieae) based on multilocus DNA markers. Botanical Journal of the Linnean Society 184: 326-346.). It is the largest genus of tribe Eupatorieae and the only member of subtribe Mikaniinae. Mikania has traditionally been recognized as a natural group, mostly due to its conserved morphology. Most species have lianescent habit, capitula with a variously positioned subinvolucral bract, four involucral bracts and four florets in each capitulum, and five-ribbed cypselae, rarely ten-ribbed (Ritter & Miotto 2005Ritter MR, Miotto STS. 2005. Taxonomia de Mikania Willd. (Asteraceae) no Rio Grande do Sul, Brasil. Hoehnea 32: 309-359.; Hind & Frisby 2014Hind DJN, Frisby S. 2014. Mikania manomoi (Compositae: Eupatorieae: Mikaniinae), a new, but epappose, species from the Cerro Manomo, Santa Cruz, Eastern Bolivia. Kew Bulletin 69: 9502. ; Contro & Nakajima 2017Contro FL, Nakajima JN. 2017. Flora da Serra do Cipó, Minas Gerais: Asteraceae - Eupatorieae. Boletim de Botânica da Universidade de São Paulo 35: 113-162.).
Although Mikania is mostly distributed in forested environments, 41 of the 198 species currently recognized in Brazil (Ritter et al. 2020Ritter MR, Gandara A, Simão-Bianchini R, Souza-Buturi FO, Abreu VHR. 2020.Mikania in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://reflora.jbrj.gov.br/reflora/floradobrasil/FB5344. 06 Jun. 2021.
http://reflora.jbrj.gov.br/reflora/flora...
) occur in campos rupestres (highland rocky fields). This phytophysiognomy is associated to sandy soils and rock outcrops in the Espinhaço Range (ER), a mountain chain extending over 1,000 km in a North-South axis along the central portion of the States of Minas Gerais and Bahia (Giulietti et al. 1997Giulietti AM, Pirani JR, Harley RM. 1997. Espinhaço range region, eastern Brazil. In: Davis SD et al. (eds). Centres of plant diversity: A guide and strategy for their conservation. WWF/IUCN, Cambridge. vol. 3. p. 397-404.; Alves & Kolbeck 2010Alves RJV, Kolbek J. 2010. Can campo rupestre vegetation be floristically delimited based on vascular plant genera? Plant Ecology 207: 67-79.; Fernandes 2016Fernandes GW. 2016. The megadiverse Rupestrian Grassland. In: Fernandes GW (ed.), Ecology and Conservation of Mountaintop Grasslands in Brazil, Springer, p. 3-14.). The ER is situated in the ecotone among three phytogeographical domains: the Atlantic Forest to the east, the Caatinga to the north, and the Cerrado to the west. Other large disjunct areas of campos rupestres are found within the Cerrado domain in western Minas Gerais and in the State of Goiás, within the Amazon Forest domain, mainly in the State of Pará, and in isolated patches in the State of Mato Grosso do Sul and in eastern Bolivia (Fernandes 2016Fernandes GW. 2016. The megadiverse Rupestrian Grassland. In: Fernandes GW (ed.), Ecology and Conservation of Mountaintop Grasslands in Brazil, Springer, p. 3-14.; Silveira et al. 2016Silveira FAO, Negreiros D, Barbosa NP, et al. 2016. Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant and Soil 403: 129-152.; Zappi et al. 2019Zappi DC, Moro MF, Walker B, et al. 2019. Plotting a future for Amazonian canga vegetation in a campo rupestre context. PLOS ONE 14: e0219753.; Miola et al. 2021Miola DTB, Ramos VDV, Silveira FAO. 2021. A brief history of research incampo rupestre: identifying research priorities and revisiting the geographical distribution of an ancient, widespread neotropical biome. Biological Journal of the Linnean Society 133: 464-480.).
Recently, smaller disjunct patches of campos rupestres were discovered on quartzitic outcrops that are entirely inserted in the Atlantic Forest phytogeographic domain (Gonella et al. 2015Gonella PM, Rivadavia F, Fleischmann A. 2015. Drosera magnifica (Droseraceae): the largest New World sundew, discovered on Facebook. Phytotaxa 220: 257-267.; Lopes et al. 2016Lopes LE, Marçal BF, Chaves AV. 2016. The patchy distribution of the Pale-throated Serra-Finch Embernagra longicauda (Aves: Thraupidae) in the eastern Brazilian mountaintops: the overlooked campos rupestres of the Rio Doce valley. North-Western Journal of Zoology 12: 373-376.; Siniscalchi et al. 2016Siniscalchi CM, Loeuille BFP, Pirani JR. 2016. A new species of Chresta (Vernonieae, Asteraceae) endemic to the Mata Atlântica Domain, Brazil. Phytotaxa 244: 80-88. ; Mello-Silva 2018Mello-Silva R. 2018. Land of Giants. Remarkable botanical findings highlight a new area for conservation in Brazil. Rodriguésia 69: 933-937.). These areas located in the Rio Doce valley in eastern Minas Gerais, namely Serra do Padre Ângelo, Pico da Aliança, and Sete Salões State Park, possess some floristic elements related to the ER (Siniscalchi et al. 2016Siniscalchi CM, Loeuille BFP, Pirani JR. 2016. A new species of Chresta (Vernonieae, Asteraceae) endemic to the Mata Atlântica Domain, Brazil. Phytotaxa 244: 80-88. ; Mello-Silva 2018Mello-Silva R. 2018. Land of Giants. Remarkable botanical findings highlight a new area for conservation in Brazil. Rodriguésia 69: 933-937.; Andrino & Gonella 2021Andrino CO, Gonella PM. 2021. An escape from the Espinhaço Range: a new species of Paepalanthus subg. Xeractis (Eriocaulaceae) from the campos rupestres of Serra do Padre Ângelo, Minas Gerais, Brazil. Plant Ecology and Evolution 154(1): 137-149. ; Antar et al. 2021Antar GM, Harley RM, Pastore JFB, Gonella PM, Sano PT. 2021a. Hyptidendron pulcherrimum Antar & Harley, sp. nov. (Hyptidinae, Lamiaceae), a new narrowly endemic species from Minas Gerais, Brazil. Adansonia 43: 1-8.a), but also to the granitic inselbergs typically found in the surrounding Atlantic Forest (Antar et al. 2021bAntar GM, Siniscalchi CM, Gonella PM, Monge M, Loeuille B. 2021b. Novelties in Lepidaploinae (Asteraceae, Vernonieae) from the easternmost campos rupestres of Minas Gerais, Brazil: Two new species and a range expansion. Plant Ecology and Evolution 154: 121-136.; Mezzonato-Pires et al. 2021Mezzonato-Pires AC, Ribeiro RS, Gonella PM. 2021. Maracujá on the rocks: a new Passiflora species (Passifloraceae sensu stricto) from the rupicolous ecosystems of the Brazilian Atlantic rainforest. Willdenowia 51: 371-381.). With an increasing number of taxonomic novelties described in recent years, these areas corroborate the elevated species richness and endemism of the campos rupestres (Giulietti et al. 1997Giulietti AM, Pirani JR, Harley RM. 1997. Espinhaço range region, eastern Brazil. In: Davis SD et al. (eds). Centres of plant diversity: A guide and strategy for their conservation. WWF/IUCN, Cambridge. vol. 3. p. 397-404.; Fernandes 2016Fernandes GW. 2016. The megadiverse Rupestrian Grassland. In: Fernandes GW (ed.), Ecology and Conservation of Mountaintop Grasslands in Brazil, Springer, p. 3-14.; Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TN, Pirani JR. 2019. Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733. ), but also highlight the botanical sampling deficit in Brazil. Biological knowledge shortfalls, mainly related to taxonomy and geographical distribution of species (the Linnean and Wallacean shortfalls, respectively; Lomolino 2004Lomolino MV. 2004. Conservation biogeography. In: Lomolino MV, Heaney LR (eds.) Frontiers of biogeography: new directions in the geography of nature. Sinauer Associates, Sunderland, MA. p. 293-296.), have been identified in recent times; their effects and the spatial accuracy of biodiversity documentation in the Atlantic Forest domain have been recently discussed and analyzed by Colli-Silva et al. (2020)Colli-Silva M, Reginato M, Cabral A, Forzza RC, Pirani JR, Vasconcelos TNC. 2020. Evaluating shortfalls and spatial accuracy of biodiversity documentation in the Atlantic Forest, the most diverse and threatened Brazilian phytogeographic domain. Taxon 69: 567-577..
In the present work, we describe two new species of Mikania endemic to the campos rupestres of Pico da Aliança (1440 m a.s.l.) and Pico do Padre Ângelo (1550 m a.s.l.) in the State of Minas Gerais, which were identified after recent botanical expeditions in these areas. We provide full descriptions, illustrations, and preliminary conservation assessments, as well as taxonomic and ecological comments for each species.
Materials and methods
The morphological descriptions were based on herbarium specimens studied in the following herbaria: BHCB, MBML, RB, and SPF (acronyms according to Thiers, continuously updatedThiers B. Continuously updated. Index Herbariorum: a global directory of public herbaria and associated staff. New York Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/. 15 Jul. 2020.
http://sweetgum.nybg.org/ih/...
). Fieldwork was carried out in Serra do Padre Ângelo and Pico da Aliança from 2013 to the present. A 10-60 × magnification stereomicroscope was used to analyze morphological features of the specimens, with measurements based on rehydrated or dry herbarium material. Terminology follows Harris & Harris (2001Harris JG, Harris MW. 2001. Plant identification terminology: an illustrated glossary. 2nd. edn. Spring Lake, Spring Lake Publishing. ) for general morphology and Hickey (1973Hickey LJ. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17-33.) for leaf shape, as well as King & Robinson (1987King RM, Robinson H. 1987. The Genera of the Eupatorieae (Asteraceae). Monographs in Systematic Botany from the Missouri Botanical Garden 22: 1-581.), Ritter & Miotto (2005Ritter MR, Miotto STS. 2005. Taxonomia de Mikania Willd. (Asteraceae) no Rio Grande do Sul, Brasil. Hoehnea 32: 309-359.), Oliveira et al. (2016Oliveira CT, Nakajima JN, Pirani JR. 2016. A new Mikania (Eupatorieae, Asteraceae) from the southwestern Minas Gerais, Brazil. Phytotaxa 243: 291-296.) and Antar et al. (2021Antar GM, Oliveira CT, Pirani JR. 2021c. Mikania mellosilvae sp. nov. (Asteraceae: Eupatorieae) from the Atlantic Forest of Brazil and lectotypification of Mikania candolleana. Nordic Journal of Botany: e03349.c) for Asteraceae-related terms.
The Geospatial Conservation Assessment (GeoCAT) tool (Bachman et al. 2011Bachman S, Moat J, Hill AW, de la Torre J, Scott B. 2011. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 150: 117-126.) was used along with IUCN (2012)IUCN. 2012. IUCN red list categories and criteria version 3.1. 2nd edition. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK. https://www.iucn.org/content/iucn-red-list-categories-and-criteria-version-31. 18 Sep. 2020
https://www.iucn.org/content/iucn-red-li...
criteria to infer conservation status. The IUCN default values were used for Extent of Occurrence (EOO) and Area of Occupancy (AOO) analyses in GeoCAT. The distribution map was produced in QGIS version 3.14.15 (QGIS Development Team 2020QGIS Development Team. 2020. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. 20 Nov. 2020
http://qgis.osgeo.org...
) using layers downloaded from FBDS (2021)FBDS - Fundação Brasileira pra o Desenvolvimento Sustentável. 2021. Repositório público de mapas e shapefiles para download. http://geo.fbds.org.br. 21 Jan. 2021
http://geo.fbds.org.br...
, IBGE (2021)IBGE. 2021. Instituto Brasileiro de Geografia e Estatística. https://mapas.ibge.gov.br/bases-e-referenciais/bases-cartograficas/malhas-digitais. 21 Jan. 2021
https://mapas.ibge.gov.br/bases-e-refere...
and SISEMA (2021)SISEMA - Sistema Estadual de Meio Ambiente e Recursos Hídricos. 2021. Infraestrutura de Dados Espaciais do Sistema Estadual de Meio Ambiente e Recursos Hídricos. Belo Horizonte: IDE-Sisema. http://idesisema.meioambiente.mg.gov.br. 21 Jan. 2021.
http://idesisema.meioambiente.mg.gov.br...
.
Results
Taxonomic treatment
Mikania semirii C.T.Oliveira & Antar, sp. nov. (Figs. 1, 2, 3)
Type: BRAZIL. Minas Gerais. Conselheiro Pena. Pico do Padre Ângelo. Cume do pico. 19º 19′ 15″ S, 41º 34′ 43″ W, 1500 m, 3/VIII/2014, C.T. Oliveira et al. 1000 (holotype SPF, isotypes to be distributed to ALCB, BHCB, CEN, HUEFS, HUFU, K, G, M, MBM, NY, P, RB, US, W)
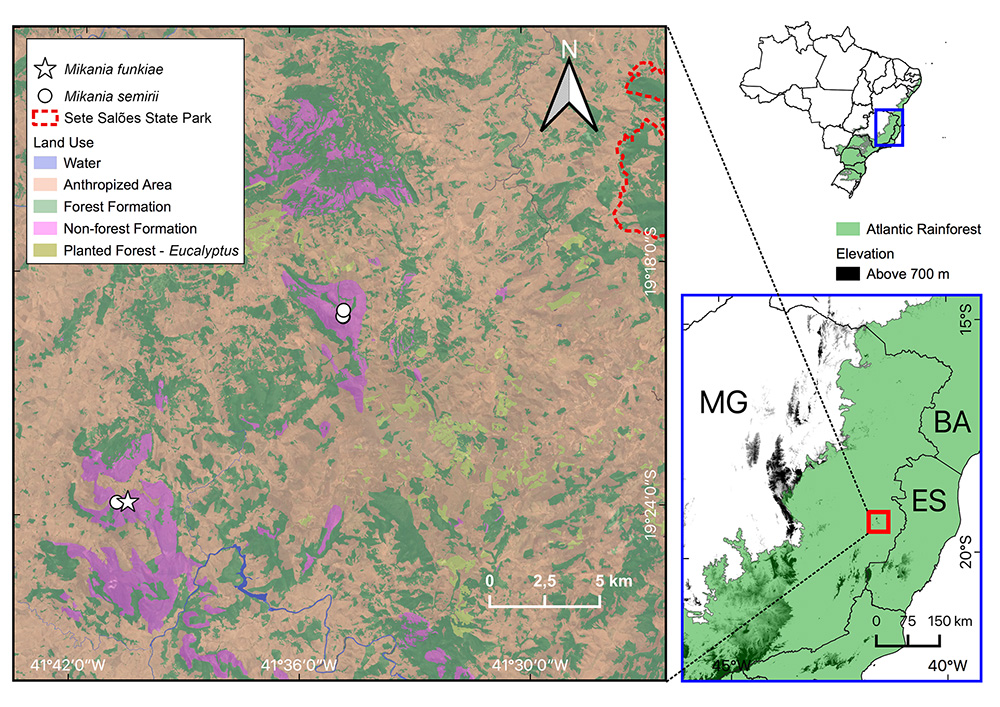
Geographic distribution of Mikania semirii C.T.Oliveira & Antar (white circles) and Mikania funkiae C.T.Oliveira & Antar (white star) in eastern Minas Gerais, with land use projected over a satellite image and highlighting the limits of the only protected area in the region (Sete Salões State Park). In the reference maps to the right, the green shadowing represents the Legal Atlantic Forest area. State acronyms, BA: Bahia, ES: Espírito Santo, MG: Minas Gerais.
Mikania semirii C.T.Oliveira & Antar A. Leafy flowering branch. B. Leaf abaxial surface C. Capitulum D. Subinvolucral bract. E. Outer involucral bract. F. Inner involucral bract. G. Floret, with the pappus partially removed for a clearer view. H. Cypsela with pappus. Illustration by Klei Souza, based on C.T. Oliveira et al. 1000 (SPF).
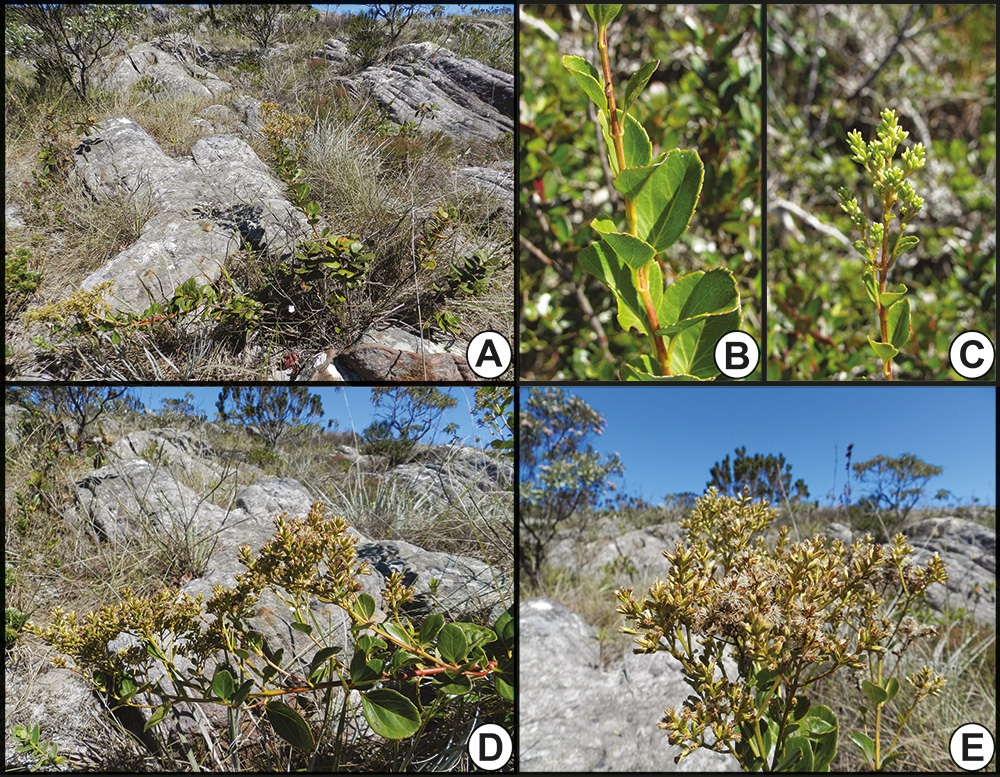
Mikania semirii C.T.Oliveira & Antar A. Inflorescence detailing the pinkish involucral bracts. B. Inflorescence. C. Leaves. D. Part of inflorescence, detailing whitish involucral bracts. E. Habit and habitat. A-B. Photos by Caetano T. Oliveira. C-E. Photos by. P.M. Gonella.
Mikania semirii resembles Mikania phaeoclados as both share the climbing habit and ovate leaves with cordate or rounded base and serrate margins, but differ in leaf indumentum (adaxially glabrous except for pubescent or glabrescent primary and secondary veins with simple curved trichomes, abaxially glabrescent with scattered, small simple trichomes in M. semirii vs. adaxially strigose, abaxially tomentose), shape and position of subinvolucral bracts (4.0-6.1 × 2.2-3.0 mm, elliptic, located below the capitulum in M. semirii (Fig. 2C) vs. 1.6-2.6 × 0.6-0.9 mm, oblong-lanceolate, located at the base of the peduncle) and peduncle size (sessile to 1 mm long in M. semirii vs. 1.1-3.5 mm long).
Perennial vines; stems ± cylindrical, canaliculate, villous, with long, curved, simple, uniseriate, entangled, eglandular trichomes, becoming cylindrical, striate and glabrescent when older, nodes expanded. Leaves opposite, petiole 2.6-8.9 mm long, pubescent to villous with simple curved eglandular trichomes, blade 3.1-6.0 × 1.9-3.0 cm, chartaceous, ovate or wide ovate, apex acute, with a mucro ca. 0.5 m long, base cordate or rounded, sometimes unequal, margins ciliate with long simple eglandular trichomes, revolute to slightly revolute, entire near base to 1/3 of leaf, upper 2/3 serrulate, with 4-9 swollen, acuminate teeth, venation actinodromous, main vein and secondary veins slightly prominent, adaxial surface glabrous except for pubescent or glabrescent primary and secondary veins with simple curved trichomes, mostly near base, abaxial surfaces glabrescent with scattered small, simple trichomes. Capitulescence a terminal thyrsoid, sessile to subsessile, peduncles ca. 1 mm long, villous with curved trichomes; subinvolucral bracts 1, 4.0-6.1 × 2.2-3.0 mm, elliptic, apex acute or rounded, ciliate, just below capitulum. Capitula discoid, 7.3-8.0 mm long; involucre cylindrical, 2.1-2.7 mm diam.; involucral bracts 4, 5.7-6.9 × 1.3-1.7 mm, whitish or pinkish, 2 inner bracts slightly larger than 2 outer, persistent, oblong or narrow obovate, stiffly chartaceous, apex obtuse, ciliate, venation parallel, ± conspicuous. Florets 4, bisexual, fertile; corolla 4.7-5.1 mm long, white, tube 1.4-2.0 mm long, always longer than limb, limb 1.6-2.1 mm long, lobes 5, 1.1-1.4 × 0.8-0.9 mm, deltoid, apex shortly acuminate or acute, divergent relative to tube, incurved; anthers 1.4-1.6 mm long, straw-colored to vinaceous, apical anther appendages ovate, apex acute, base rounded; style 6.7-7.4 mm long, style branches 2.5-3.2 mm long, papillose. Cypselae 3.0-3.5 × 0.8-0.9 mm, carbonized, sparsely glandular-punctate, mostly near apex and base, 5-ribbed, ribs white, ciliate with twin hairs; carpopodium present, annuliform, pale, glabrous; pappus bristles ca. 40, persistent, uniseriate, barbellate, slightly unequal, 3.1-3.5 mm long, longer than cypselae, cream colored.
Etymology: The species epithet honors the late botanist Dr João Semir (1937-2018), professor of the Botany Department of UNICAMP, Campinas, São Paulo, Brazil. Dr Semir was a synantherologist who greatly contributed to the systematic knowledge of Asteraceae in Brazil, especially in Vernonieae, but also in Bignoniaceae, Malvaceae, and Orchidaceae. Dr Semir mentored several Brazilian botanists and is recognized as a pioneer of floristic studies of the campos rupestres.
Preliminary Conservation Status: Critically Endangered: CR B2ab(i, ii, iii). Mikania semirii was found exclusively on summits (at elevations above 1400 m a.s.l.) of two of the highest peaks located in the eastern Minas Gerais quartzitic formations, Pico do Padre Ângelo (part of Serra do Padre Ângelo) and Pico da Aliança, ca. 12 km distant from each other. At both sites, the number of individuals observed was less than 20, but exploration of the habitat is hindered by the rugged relief. Similar habitats and elevations may be further found at Pico do Pinhão and the virtually inaccessible Pico do Sossego (both part of Serra do Padre Ângelo). All these areas are susceptible to invasion by alien grasses, most remarkably “capim-gordura”, Melinis minutiflora (Gonella et al. 2015Gonella PM, Rivadavia F, Fleischmann A. 2015. Drosera magnifica (Droseraceae): the largest New World sundew, discovered on Facebook. Phytotaxa 220: 257-267.), which can be further aggravated by the lack of management and control. Invasion by alien species can be also intensified by wildfires, which are a common practice for pasture renovation in the surrounding areas that had their original vegetation extensively modified (Fig. 1). None of the occurrence areas are included in protected areas, therefore leaving the species habitat susceptible to human disturbance (including predatory collection of plants, garbage dumping, trampling, fire, etc.). Similar to other endemic species of Serra do Padre Ângelo, the population of M. semirii was affected by an anthropogenic fire of large proportions in late 2020 (Andrino & Gonella 2021Andrino CO, Gonella PM. 2021. An escape from the Espinhaço Range: a new species of Paepalanthus subg. Xeractis (Eriocaulaceae) from the campos rupestres of Serra do Padre Ângelo, Minas Gerais, Brazil. Plant Ecology and Evolution 154(1): 137-149. ; Antar et al. 2021Antar GM, Siniscalchi CM, Gonella PM, Monge M, Loeuille B. 2021b. Novelties in Lepidaploinae (Asteraceae, Vernonieae) from the easternmost campos rupestres of Minas Gerais, Brazil: Two new species and a range expansion. Plant Ecology and Evolution 154: 121-136.b; Kollmann & Gonella 2021Kollmann LJC, Gonella PM. 2021. Novelties in Begonia (Begoniaceae) from the campos rupestres of Serra do Padre Ângelo, Minas Gerais, Brazil: a new species and a new record. Phytotaxa 510: 69-77.). Additionally, mountaintop-restricted endemics are severely threatened by climatic change, as they cannot migrate to higher elevations to remain in their climatic envelopes. Based on the restricted range (AOO of 8 km2) and the listed threats, M. semirii should be listed as Critically Endangered based on IUCN criteria B2ab(i, ii, iii) (IUCN 2012IUCN. 2012. IUCN red list categories and criteria version 3.1. 2nd edition. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK. https://www.iucn.org/content/iucn-red-list-categories-and-criteria-version-31. 18 Sep. 2020
https://www.iucn.org/content/iucn-red-li...
).
Distribution and Habitat: Mikania semirii is probably endemic to Serra do Padre Ângelo and Pico da Aliança in the municipalities of Conselheiro Pena and Alvarenga, respectively, in the State of Minas Gerais (Fig. 1). It is found in the highest areas of these peaks, near the summits, growing in campos rupestres vegetation, in sandy soils with organic matter among rock outcrops, from 1430 to 1500 m a.s.l.
Phenology: Mikania semirii was found fertile in the dry season, in June, July, and August.
Additional Specimens Examined (Paratypes): BRAZIL. Minas Gerais: Alvarenga, Pico da Aliança, cume do pico, 19° 23' 44" S, 41° 40' 40" W, 1430 m alt., 4/VIII/2014, C.T. Oliveira et al. 1011 (SPF); Conselheiro Pena, Pico do Padre Ângelo, no topo do pico, 19°19'14.2"S, 41°34'43.7"W, 1530 m alt., 8/VII/2014, P.M. Gonella et al. 681 (SPF); ibid., Serra do Padre Ângelo, Pico do Padre Ângelo, platô do topo do pico, 19° 19' 05.04" S, 41°3 4' 42.26" W, 1480 m alt., 11/VI/2020, P.M. Gonella et al. 1423 (SPF); ibid., 21/VIII/2020, P.M. Gonella 1566 (SPF).
Affinities and morphological notes: Mikania semirii presents a unique combination of the following features: lianescent habit; ovate leaf blade, with cordate or rounded base, adaxially glabrous and abaxially glabrescent, with serrulate, revolute to slightly revolute margins. The subinvolucral bracts are elliptic and located directly beneath the capitulum, the peduncles are sessile to 1 mm long and the involucral bracts pinkish or whitish.
The new species is morphologically similar to M. phaeoclados which also occurs in the State of Minas Gerais, but their distributions do not overlap.
Mikania funkiae C.T.Oliveira & Antar, sp.nov. (Figs. 1, 4, 5)
Type: BRAZIL. Minas Gerais. Alvarenga. Pico da Aliança. Cume do pico. 19º 23’ 44’’ S, 41º 40’ 40’’ W, 1430 m, 4/VIII/2014, C.T. Oliveira et al. 1008 (holotype SPF, isotypes to be distributed to BHCB, RB)
Mikania funkiae C.T.Oliveira & Antar A. Leafy flowering branch. B. Leaf abaxial surface. C. Capitulum. D. Subinvolucral bract. E. Outer involucral bract. F. Inner involucral bract. G. Floret, with the pappus partially removed for a clearer view. H. Style arms. I. Cypsela with pappus. Illustration by Klei Souza, based on C.T. Oliveira et al. 1008 (SPF).
Mikania funkiae C.T.Oliveira & Antar A. Habit and habitat. B. Leaves. C. Young Inflorescence (capitulescence). D. Branch bearing leaves and inflorescence. E. Branch bearing inflorescence. A, D and E. Photos by Caetano T. Oliveira. B-C. Photos by P.M. Gonella.
Mikania funkiae is morphologically similar to Mikania glauca, as both share a similar shrubby habit, and glabrous leaves with slightly revolute margins. However, they differ in that M. funkiae possesses coriaceous leaves without wax (vs. leaves chartaceous, mostly waxy), petiole 0.3-0.6 cm long (vs. petiole absent to 1 mm long), leaf margins with 3-4 pairs of obtuse teeth above the mid portion of the blade (vs. blade entire or sinuate) and leaf shape oblate or wide elliptic (vs. wide elliptic or suborbiculate).
Shrub erect, ca. 60 cm tall, perennial; stems glabrous, angulose, canaliculate, becoming ± cylindrical and striate when older. Leaves opposite, petiole 0.3-0.6 cm long, glabrous, blade 3.3-5.7 × 2.4-5.0 cm, coriaceous, orbiculate, oblate or wide elliptic, apex obtuse, base attenuate or cuneate, margins eciliate, revolute, entire to mid portion of blade, 3-4 toothed distally, teeth obtuse, venation actinodromous, primary and secondary veins ±prominent, adaxial surface glabrous to glabrescent with few simple eglandular trichomes, shiny, abaxial surface glabrous and punctate with small glands sunken in blade. Capitulescence a terminal raceme, sessile or shortly pedunculate, peduncle up to 2 mm long, villous with curved trichomes; subinvolucral bracts 1, 1.8-2.6 × 0.6-0.9 mm, narrow elliptic or narrow oblong, apex rounded, ciliate, just beneath capitulum or slightly lower on peduncle. Capitula discoid, 4.8-5.6 mm tall; involucre greenish, cylindrical, 2.1-2.5 mm diam.; involucral bracts 4, 3.0-3.8 × 1.2-1.5 mm, 2 inner bracts slightly larger than 2 outer, persistent, oblong or narrow obovate, stiffly chartaceous, apex obtuse, ciliate, venation parallel, inconspicuous. Florets 4, unisexual, fertile; corolla 3.1-3.5 mm long, white, tube 1.3-1.7 mm long, always longer than limb, limb 0.9-1.2 mm long, lobes 5, 0.8-1.0 × 0.5-0.6 mm, deltate, apex acute or shortly acuminate, divergent relative to tube, reflexed; anthers 0.7-0.8 mm long, straw-colored, apical anther appendages ovate, apex acute, base rounded; style 3.3-4.5 mm long, style branches 1.5-1.7 mm long, papillose. Cypselae 1.7-2.1 × 0.4-0.6 mm, carbonized, punctate with scattered glands, mostly near apex and base, 5-ribbed, white, setulate, with twin hairs; carpopodium present, present, annuliform, pale, glabrous; pappus bristles ca. 40, uniseriate, barbellate, 3.1-3.5 mm long, longer than cypselae, whitish.
Etymology: The species epithet honors the late botanist and synantherologist Dr Vicki Ann Funk (1947-2019), who was a Senior Researcher and Curator at the Smithsonian National Museum of Natural History, Washington D.C., United States of America. Dr Funk greatly contributed to the knowledge of Asteraceae as well as to the understanding of phylogenetic relationships and biogeography of the family.
Preliminary Conservation Status: Critically Endangered: CR B2ab(i, ii, iii). Mikania funkiae is currently known only from the type collection at the summit of Pico da Aliança (Fig. 1), where less than 20 individuals have been observed, which suggests it is a rare and microendemic species. The taxon is not protected by any conservation area, and is vulnerable to the same threats listed above for M. semirii, therefore being assessed as Critically Endangered based on criteria B2ab(i, ii, iii) of IUCN (IUCN 2012IUCN. 2012. IUCN red list categories and criteria version 3.1. 2nd edition. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK. https://www.iucn.org/content/iucn-red-list-categories-and-criteria-version-31. 18 Sep. 2020
https://www.iucn.org/content/iucn-red-li...
).
Distribution and Habitat: Mikania funkiae is probably endemic to the summit of Pico da Aliança, in the municipality of Alvarenga, State of Minas Gerais. It is found growing in campos rupestres in dry sandy soils among rocks, at around 1400 m a.s.l. (Fig. 1).
Phenology: Mikania funkiae was found fertile in August.
Affinities and morphological notes: Mikania funkiae has the following unique combination of characters: shrubby habit, opposite, petiolate leaves that are coriaceous, orbiculate, oblate or wide elliptic, with slightly revolute margins, and sessile or shortly pedunculate inflorescence, with peduncles up to 2 mm long. The new species is morphologically similar to Mikania glauca, which also occurs in Minas Gerais State but not recorded in the same region. Both species differ by the features indicated in the diagnosis. Mikania funkiae is also similar to M. obtusata based on the shrubby habit, opposite, glabrous leaves with attenuate base and slightly revolute margins, and similar petiole length. However, the new species differs from M. obtusata by the presence of 3-4 pairs of obtuse teeth above the mid portion of the blade (vs. margins entire, repand), coriaceous leaf texture (vs. chartaceous) and oblate or broad-elliptic blade (vs. elliptic).
Final remarks
Species of Mikania are usually widely distributed and without a very specific niche. A few species are microendemics and/or exclusive of restricted habitats, such as some Cerrado and campo rupestre taxa (e.g. M. nelsonii, Hind 1993Hind DJN. 1993. Notes on the Compositae of Bahia, Brazil: I. Kew Bulletin 48: 245-277.; M. fasciculata,Oliveira et al. 2016Oliveira CT, Nakajima JN, Pirani JR. 2016. A new Mikania (Eupatorieae, Asteraceae) from the southwestern Minas Gerais, Brazil. Phytotaxa 243: 291-296.; M. cipoensis, Ritter et al. 2020Ritter MR, Gandara A, Simão-Bianchini R, Souza-Buturi FO, Abreu VHR. 2020.Mikania in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://reflora.jbrj.gov.br/reflora/floradobrasil/FB5344. 06 Jun. 2021.
http://reflora.jbrj.gov.br/reflora/flora...
). The distribution pattern documented herein for M. semirii and M. funkiae, restricted to the summits of two mountaintops within the Atlantic Forest domain, has not been previously reported for the genus. Further collection efforts are needed to confirm the restricted distribution of these two new taxa, as well as ongoing phylogenetic studies that may shed light on their evolution and origin.
The present results highlight a well-documented gap in botanical collections in Brazil and South America as a whole (Mori et al. 2011Mori SA, Berkov A, Gracie CA, Hecklau EF. 2011. Tropical Plant Collecting - From the Field to the Internet. 1st edn. Florianópolis, TECC Editoria.; BFG 2015BFG - The Brazil Flora Group. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.; Morim & Lughadha 2015Morim MP, Nic Lughadha EM. 2015. Flora of Brazil Online: Can Brazil’s botanists achieve their 2020 vision? Rodriguésia 66: 1115-1135.; Oliveira et al. 2017Oliveira U, Soares-Filho BS, Paglia AP, et al. 2017. Biodiversity conservation gaps in the Brazilian protected areas. Scientific Reports 7: 9141., 2019Oliveira U, Soares-Filho BS, Santos AJ, et al. 2019. Modelling highly biodiverse areas in Brazil. Scientific Reports 9: a6355.). Even though the Atlantic Forest is one of the most intensively collected Brazilian phytogeographic domains (Oliveira et al. 2019Oliveira U, Soares-Filho BS, Santos AJ, et al. 2019. Modelling highly biodiverse areas in Brazil. Scientific Reports 9: a6355.), the few expeditions carried out so far in the underexplored areas of Serra do Padre Ângelo and Pico da Aliança have revealed 24 new taxa in different angiosperm families, including the two new species proposed herein. This study highlights the need for more botanical expeditions and sampling effort in these mountains, as well as the importance of active conservation plans for these localities.
Acknowledgments
We thank Benoit Loeuille, Carolina Siniscalchi, Reginaldo Vasconcelos, Lucian Medeiros and Ednilson Caetano Ribeiro and his family for helping with fieldwork; the curators of the visited herbaria; two anonymous reviewers for their contributions; and Klei Sousa for providing the illustrations. This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. CTO thanks FAPESP (grants 2011/18385-0 and 2012/12325-9) for the financial support. GMA thanks CAPES, IdeaWild and American Society of Plant Taxonomists for financial support. PMG thanks The Mohamed bin Zayed Species Conservation Fund (grant 192522325) and IdeaWild. JRP thanks CNPq for financial support (grant 307655/2015-6).
References
- Alves RJV, Kolbek J. 2010. Can campo rupestre vegetation be floristically delimited based on vascular plant genera? Plant Ecology 207: 67-79.
- Andrino CO, Gonella PM. 2021. An escape from the Espinhaço Range: a new species of Paepalanthus subg. Xeractis (Eriocaulaceae) from the campos rupestres of Serra do Padre Ângelo, Minas Gerais, Brazil. Plant Ecology and Evolution 154(1): 137-149.
- Antar GM, Harley RM, Pastore JFB, Gonella PM, Sano PT. 2021a. Hyptidendron pulcherrimum Antar & Harley, sp. nov. (Hyptidinae, Lamiaceae), a new narrowly endemic species from Minas Gerais, Brazil. Adansonia 43: 1-8.
- Antar GM, Siniscalchi CM, Gonella PM, Monge M, Loeuille B. 2021b. Novelties in Lepidaploinae (Asteraceae, Vernonieae) from the easternmost campos rupestres of Minas Gerais, Brazil: Two new species and a range expansion. Plant Ecology and Evolution 154: 121-136.
- Antar GM, Oliveira CT, Pirani JR. 2021c. Mikania mellosilvae sp. nov. (Asteraceae: Eupatorieae) from the Atlantic Forest of Brazil and lectotypification of Mikania candolleana Nordic Journal of Botany: e03349.
- Bachman S, Moat J, Hill AW, de la Torre J, Scott B. 2011. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 150: 117-126.
- BFG - The Brazil Flora Group. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.
- Colli-Silva M, Vasconcelos TN, Pirani JR. 2019. Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.
- Colli-Silva M, Reginato M, Cabral A, Forzza RC, Pirani JR, Vasconcelos TNC. 2020. Evaluating shortfalls and spatial accuracy of biodiversity documentation in the Atlantic Forest, the most diverse and threatened Brazilian phytogeographic domain. Taxon 69: 567-577.
- Contro FL, Nakajima JN. 2017. Flora da Serra do Cipó, Minas Gerais: Asteraceae - Eupatorieae. Boletim de Botânica da Universidade de São Paulo 35: 113-162.
- FBDS - Fundação Brasileira pra o Desenvolvimento Sustentável. 2021. Repositório público de mapas e shapefiles para download. http://geo.fbds.org.br 21 Jan. 2021
» http://geo.fbds.org.br - Fernandes GW. 2016. The megadiverse Rupestrian Grassland. In: Fernandes GW (ed.), Ecology and Conservation of Mountaintop Grasslands in Brazil, Springer, p. 3-14.
- Godoy SM, Silva JFM, Paula GBN, Ruas PM, Goés BD, Ruas CF. 2017. Phylogenetic relationships of BrazilianMikaniaspecies (Asteraceae, Eupatorieae) based on multilocus DNA markers. Botanical Journal of the Linnean Society 184: 326-346.
- Giulietti AM, Pirani JR, Harley RM. 1997. Espinhaço range region, eastern Brazil. In: Davis SD et al (eds). Centres of plant diversity: A guide and strategy for their conservation. WWF/IUCN, Cambridge. vol. 3. p. 397-404.
- Gonella PM, Rivadavia F, Fleischmann A. 2015. Drosera magnifica (Droseraceae): the largest New World sundew, discovered on Facebook. Phytotaxa 220: 257-267.
- Harris JG, Harris MW. 2001. Plant identification terminology: an illustrated glossary. 2nd. edn. Spring Lake, Spring Lake Publishing.
- Hickey LJ. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17-33.
- Hind DJN. 1993. Notes on the Compositae of Bahia, Brazil: I. Kew Bulletin 48: 245-277.
- Hind DJN, Frisby S. 2014. Mikania manomoi (Compositae: Eupatorieae: Mikaniinae), a new, but epappose, species from the Cerro Manomo, Santa Cruz, Eastern Bolivia. Kew Bulletin 69: 9502.
- IBGE. 2021. Instituto Brasileiro de Geografia e Estatística. https://mapas.ibge.gov.br/bases-e-referenciais/bases-cartograficas/malhas-digitais 21 Jan. 2021
» https://mapas.ibge.gov.br/bases-e-referenciais/bases-cartograficas/malhas-digitais - IUCN. 2012. IUCN red list categories and criteria version 3.1. 2nd edition. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK. https://www.iucn.org/content/iucn-red-list-categories-and-criteria-version-31 18 Sep. 2020
» https://www.iucn.org/content/iucn-red-list-categories-and-criteria-version-31 - King RM, Robinson H. 1987. The Genera of the Eupatorieae (Asteraceae). Monographs in Systematic Botany from the Missouri Botanical Garden 22: 1-581.
- Kollmann LJC, Gonella PM. 2021. Novelties in Begonia (Begoniaceae) from the campos rupestres of Serra do Padre Ângelo, Minas Gerais, Brazil: a new species and a new record. Phytotaxa 510: 69-77.
- Lomolino MV. 2004. Conservation biogeography. In: Lomolino MV, Heaney LR (eds.) Frontiers of biogeography: new directions in the geography of nature. Sinauer Associates, Sunderland, MA. p. 293-296.
- Lopes LE, Marçal BF, Chaves AV. 2016. The patchy distribution of the Pale-throated Serra-Finch Embernagra longicauda (Aves: Thraupidae) in the eastern Brazilian mountaintops: the overlooked campos rupestres of the Rio Doce valley. North-Western Journal of Zoology 12: 373-376.
- Mello-Silva R. 2018. Land of Giants. Remarkable botanical findings highlight a new area for conservation in Brazil. Rodriguésia 69: 933-937.
- Mezzonato-Pires AC, Ribeiro RS, Gonella PM. 2021. Maracujá on the rocks: a new Passiflora species (Passifloraceae sensu stricto) from the rupicolous ecosystems of the Brazilian Atlantic rainforest. Willdenowia 51: 371-381.
- Miola DTB, Ramos VDV, Silveira FAO. 2021. A brief history of research incampo rupestre: identifying research priorities and revisiting the geographical distribution of an ancient, widespread neotropical biome. Biological Journal of the Linnean Society 133: 464-480.
- Mori SA, Berkov A, Gracie CA, Hecklau EF. 2011. Tropical Plant Collecting - From the Field to the Internet. 1st edn. Florianópolis, TECC Editoria.
- Morim MP, Nic Lughadha EM. 2015. Flora of Brazil Online: Can Brazil’s botanists achieve their 2020 vision? Rodriguésia 66: 1115-1135.
- Oliveira CT, Nakajima JN, Pirani JR. 2016. A new Mikania (Eupatorieae, Asteraceae) from the southwestern Minas Gerais, Brazil. Phytotaxa 243: 291-296.
- Oliveira U, Soares-Filho BS, Paglia AP, et al 2017. Biodiversity conservation gaps in the Brazilian protected areas. Scientific Reports 7: 9141.
- Oliveira U, Soares-Filho BS, Santos AJ, et al 2019. Modelling highly biodiverse areas in Brazil. Scientific Reports 9: a6355.
- QGIS Development Team. 2020. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org 20 Nov. 2020
» http://qgis.osgeo.org - Ritter MR, Miotto STS. 2005. Taxonomia de Mikania Willd. (Asteraceae) no Rio Grande do Sul, Brasil. Hoehnea 32: 309-359.
- Ritter MR, Gandara A, Simão-Bianchini R, Souza-Buturi FO, Abreu VHR. 2020.Mikania in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://reflora.jbrj.gov.br/reflora/floradobrasil/FB5344 06 Jun. 2021.
» http://reflora.jbrj.gov.br/reflora/floradobrasil/FB5344 - Robinson H, Schilling E, Panero JL. 2009. Eupatorieae. In: Funk VA, et al (eds.). Systematics, Evolution, and Biogeography of the Compositae. International Association for Plant Taxonomy. p. 171-189.
- Silveira FAO, Negreiros D, Barbosa NP, et al 2016. Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant and Soil 403: 129-152.
- Siniscalchi CM, Loeuille BFP, Pirani JR. 2016. A new species of Chresta (Vernonieae, Asteraceae) endemic to the Mata Atlântica Domain, Brazil. Phytotaxa 244: 80-88.
- SISEMA - Sistema Estadual de Meio Ambiente e Recursos Hídricos. 2021. Infraestrutura de Dados Espaciais do Sistema Estadual de Meio Ambiente e Recursos Hídricos. Belo Horizonte: IDE-Sisema. http://idesisema.meioambiente.mg.gov.br 21 Jan. 2021.
» http://idesisema.meioambiente.mg.gov.br - Thiers B. Continuously updated. Index Herbariorum: a global directory of public herbaria and associated staff. New York Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ 15 Jul. 2020.
» http://sweetgum.nybg.org/ih/ - Zappi DC, Moro MF, Walker B, et al 2019. Plotting a future for Amazonian canga vegetation in a campo rupestre context. PLOS ONE 14: e0219753.
Publication Dates
-
Publication in this collection
02 May 2022 -
Date of issue
2022
History
-
Received
01 Nov 2021 -
Accepted
26 Jan 2022