Abstracts
Severe cutaneous adverse drug reactions generally require hospitalization, sometimes in intensive care or burns units, for observation of the vital signs and the visceral function. The objective was to describe these reactions in order to facilitate recognition and treatment. This group of drug reactions includes drug hypersensitivity syndrome (DHS), acute generalized exanthematous pustulosis (AGEP), anticoagulant-induced skin necrosis, small-vessel vasculitis (SVV), propylthiouracil hypersensitivity vasculitis and serum sickness disease. DHS has been most relevant due to universal prescription of aromatic anticonvulsant drugs and dapsone use in the treatment of some diseases such as acne and leprosy. AGEP is mostly induced by b-lactam related drugs and presents similar clinical characteristics as generalized pustular psoriasis, thus these must be differentiated. SVV can present an occult systemic illness, with impairment of relevant internal organs, such as kidneys, lungs and hematological system, with elevated morbidity and risk of death.
serum sickness; exanthema; hepatitis, chronic; drug hypersensitivity; pharmaceutical preparations; vasculitis
As reações cutâneas graves adversas à droga são as que geralmente necessitam de internação hospitalar, por vezes em unidade de terapia intensiva ou de queimados, com observação minuciosa dos sinais vitais e da função de órgãos internos. O objetivo é descrever estas reações facilitando o seu reconhecimento e tratamento. Fazem parte deste grupo a Síndrome de Hipersensibilidade à Droga (SHD), a Pustulose Exantemática Generalizada Aguda (PEGA), a Necrose Cutânea induzida por Anticoagulante, as Vasculites de Pequenos Vasos (VPV), a Vasculite de Hipersensibilidade ao Propiltiouracil (VHP) e as Reações tipo Doença do Soro (RDS). A SHD tem-se tornado de elevada relevância clínica devido ao uso amplo dos anticonvulsivantes aromáticos e da dapsona, utilizada no tratamento de doenças como a acne e a hanseníase. A PEGA é determinada principalmente pelos derivados beta-lactâmicos e tem como principal diagnóstico diferencial a psoríase pustulosa generalizada. As VPV tegumentares podem refletir uma doença multissistêmica subjacente, com danos graves em órgãos nobres, como os rins, pulmões e sistema hematológico, com morbidade elevada e possível letalidade. Abordamos as características clínicas e o tratamento destas reações adversas à droga.
doença do soro; exantema; hepatite crônica induzida por droga; hipersensibilidade a drogas; preparações farmacêuticas; vasculite
REVIEW ARTICLE
Severe cutaneous adverse drug reactions - relevant aspects to diagnosis and treatment - Part II* * Work done at Hospital do Servidor Publico Estadual de Sao Paulo, at the Complexo Hospitalar Padre Bento, Guarulhos, State of Sao Paulo, and at the Clinical Research Laboratory 53 (LIM-53) of the Dermatology epartment, Hospital das Clinicas, Faculty of Medicine of the University of Sao Paulo (HC - FMUSP).
Paulo Ricardo CriadoI; Roberta Fachini Jardim CriadoII; Cidia VasconcellosIII; Rodrigo de Oliveira RamosIV; Andréia Christina GonçalvesV
IDermatologist, Master's Degree in Clinical Medicine, Assistant M.D. of the Dermatology Service, Hospital do Servidor Publico Estadual de Sao Paulo, and commissioned doctor at the Clinical Research Laboratory 53 (LIM-53) of the Dermatology Department, Hospital das Clinicas, Faculty of Medicine of the University of Sao Paulo (HC - FMUSP)
IIAllergologist, Master's Degree in Clinical Medicine, Assistant M.D. and lecturer, Dermatology Service of the Sao Paulo Hospital do Servidor Público Estadual, Voluntary Allergologist of the ABC Faculty of Medicine
IIIDermatologist, Ph.D. in Medicine, Assistant M.D. Dermatology Service of the Sao Paulo Hospital do Servidor Público Estadual and LIM-53 of the Hospital das Clinicas, University of Sao Paulo Faculty of Medicine
IVDermatologist in residence, Dermatology Service, Sao Paulo Hospital do Servidor Público Estadual
VPh.D. candidate (sixth-year), Estácio de Sá University (RJ)
Correspondence Correspondence to Paulo Ricardo Criado Rua Xingu 245/182 - Bairro Valparaíso 09060-050 Santo André SP Tel./fax: (11) 4426-8803 E-mail: prcriado@uol.com.br
ABSTRACT
Severe cutaneous adverse drug reactions generally require hospitalization, sometimes in intensive care or burns units, for observation of the vital signs and the visceral function. The objective was to describe these reactions in order to facilitate recognition and treatment. This group of drug reactions includes drug hypersensitivity syndrome (DHS), acute generalized exanthematous pustulosis (AGEP), anticoagulant-induced skin necrosis, small-vessel vasculitis (SVV), propylthiouracil hypersensitivity vasculitis and serum sickness disease. DHS has been most relevant due to universal prescription of aromatic anticonvulsant drugs and dapsone use in the treatment of some diseases such as acne and leprosy. AGEP is mostly induced by b-lactam related drugs and presents similar clinical characteristics as generalized pustular psoriasis, thus these must be differentiated. SVV can present an occult systemic illness, with impairment of relevant internal organs, such as kidneys, lungs and hematological system, with elevated morbidity and risk of death.
Key-words: serum sickness; exanthema; hepatitis, chronic, drug-induced; drug hypersensitivity; pharmaceutical preparations/adverse effects; vasculitis.
INTRODUCTION
We can define severe cutaneous adverse drug reactions (SCADR) as those that usually require hospitalization, often in intensive care or burns units, for meticulous observation of the vital signs and the function of internal organs. This article considers the following reactions: drug hypersensitivity syndrome (DHS), acute generalized exanthematous pustulosis (AGEP), anticoagulant-induced skin necrosis, small-vessel vasculitis, propylthiouracil hypersensitivity vasculitis and serum sickness disease type reactions.
A wide range of systemic manifestations was described in 1950 as dilantin sensitivity syndrome.1,2 This syndrome was renamed anticonvulsant hypersensitivity syndrome in 1988, after observation of its occurrence with other anticonvulsants.1,2 It occurs in a proportion that varies approximately from 1:1,000 to 1:10,000 patients exposed to anticonvulsants and above all among Afro-Americans.1,2
The original description of anticonvulsant hypersensitivity syndrome included occurrence of fever, cutaneous eruptions, leukocytosis and eosinophilia.1,2 The most notable hematological disturbance is atypia of lymphocytes, that suggests lymphomatous transformation, leading to cytological or histological findings of lymphoma-like cells. Presently, there is a tendency to separate hypersensitivity reactions that mimic lymphoma into two syndromes.2 The first being as described above, with abrupt onset and for which the most appropriate denomination is "DRESS", due to the English expression "Drug Rash with Eosinophilia and Systemic Symptoms"; secondly, it can occur in a more insidious manner, the initial manifestation being the appearance of single or multiple nodules, or generalized cutaneous papular plaques, or even, exfoliative erythroderma similar to Sézary syndrome, with mean onset 1 to 11 months after initiating antiepileptic therapy. This insidious form presents cutaneous histological characteristics of pseudolymphoma and remission on withdrawal of the drug, however it increases the possibility of a lymphomatous transformation during the patient's life and should be denominated "drug-induced cutaneous pseudolymphoma or mycosis fungoides-like lesions", as described in 1991.2
DISCUSSION
1. Drug Hypersensitivity Syndrome (Drug Rash with Eosinophilia and Systemic Symptoms) - DRESS
Clinically, this syndrome in its complete form includes severe eruption, fever, lymphadenopathy, hepatitis, hematological abnormalities with eosinophilia and atypical lymphocytes, and can involve other organs. 1,2,3 This multivisceral involvement differentiates it from other cutaneous reactions to common drugs.2 Recognition of this entity is of special importance, given that the mortality rate is about 10% and specific therapy can be necessary.2 This type of reaction is most commonly observed after use of aromatic antiepileptics (phenytoin, carbamazepine and phenobarbital) and sulfonamides, although cases have been reported secondary to allopurinol, gold salts, dapsone, sulfasalazine, thalidomide, lamotrigine, calcium canal blockers, ranitidine, mexiletine, sorbinil, dipyrone and drugs used in the treatment of HIV infection such as indinavir, nevirapine and zalcitabine.2-7 With aromatic anticonvulsants the occurrence is estimated to be one case for every 1,000 to 10,000 individuals exposed to the drug and is more common especially among black patients.2,3 The cross-reaction between various aromatic anticonvulsant drugs is well documented, making the choice of an alternative therapy somewhat difficult.2,3,4
In 1996, to overcome ambiguity in the expression 'Hypersensitivity Syndrome', Bocquet, Bagot & Roujeau2 proposed the adoption of the descriptive acronym 'DRESS' (Drug Rash with Eosinophilia and Systemic Symptoms).
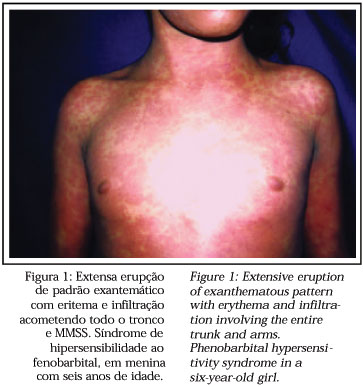
The syndrome develops within two months following introduction of the drug, most frequently between two to six weeks afterwards, or more immediately whenever it is a re-administration.2 Fever and cutaneous eruption are the first signs, especially when related to antiepileptics (87% of cases).2 The eruptions consist of morbilliform exanthema indistinguishable from the exanthemas of other less severe reactions.1,2,3 Face, upper trunk and upper extremities are involved initially, with subsequent progression to the lower extremities.2 An erythrodermal picture can occur.2 The maculo-papular eruption later becomes infiltrated and with certain induration, with emphasis on follicular edematous accentuantion (Figure 1).2 Edema of the face, with periorbital accentuantion is an alert for the diagnosis and can be very intense.2 Vesicles and thin blisters can occur due to edema of the dermis, however here is no epidermic necrosis as in toxic epidermal necrolysis (TEN).2
Occasionally small sterile perifollicular pustules develop as well as nonfollicular pustules, which differ from acute generalized exanthematous pustulosis as they are not found predominantly in the main skinfolds.2 Atypical targets can appear.2 Over time, the eruption becomes purpuric, markedly so in the lower limbs, and desquamation occurs after resolution.2 It can also present as a picture of exfoliative dermatitis, which can be associated with mucous involvement, such as cheilitis, erosions, exanthematous pharynx and enlarged tonsils.2
Histopathological exam of the skin demonstrates lymphocytic infiltration that is dense and diffuse or superficial and perivascular (Figure 2).2,3 Eosinophils or edema in the dermis may or may not be present.2,3 On some occasions there is an infiltrated band of atypical lymphocytes with epidermotropism simulating fungoid mycosis.2,3,4
Lymphadenopathy is common (about 75% of cases), frequently generalized and painful, improving gradually after drug withdrawal.2,4 Two distinct types of lymph nodes involvement may be present: a benign lymphoid hyperplasia pattern with maintenance of the normal lymph node architecture or a pattern with a pseudolymphomatous aspect.2
Various hematological abnormalities are observed, consisting of anemia, pronounced leukocytosis, eosinophilia (30% of cases) and atypical lymphocytes similar to mononucleosis.2,4 EAlthough these findings point the diagnosis towards DRESS, the differentiation can sometimes be difficult between viral infections, such as infection by Epstein-Barr virus, or hematological diseases.2,3 Leukocytosis may be high, even over 50,000 leukocytes/mm3, and eosinophilia may reach values greater than 20,000 cells/mm3.2 The marked eosinophilia may determine potentially fatal involvement of internal organs with pulmonary infiltrate, shock and respiratory distress syndrome with hypotension, myocarditis, pericarditis, interstitial nephritis (11% of cases), involvement of the brain, thyroid, or myositis.1-4 We observed a patient that developed acute pancreatitis and who coursed to a lethal outcome.3
Hepatic involvement constitutes the most common visceral manifestation,1-4 such that hepatomegaly is found at physical exam. Hepatitis with isolated elevation of transaminases is common (51% of cases), however hepatic insufficiency competes as the main cause of mortality, with rates varying from 10 to 38% of deaths.1-4 Hepatic biopsy demonstrates granulomas or infiltration of eosinophils, with the reaction combined with cholestasis and hepatocyte necrosis.2 In the most serious cases there can be massive or focal disseminated necrosis, explaining the hepatic insufficiency.2 An early recognition of the syndrome and prompt discontinuance of the drug may limit the hepatic damage that sometimes continues to progress for several weeks, even after withdrawal of the drug, and take months to resolve.1,2
Diagnosis is difficult since there are incomplete or less characteristic clinical pictures for instance, hepatitis without cutaneous eruption or lung infiltration with eosinophilia in an isolated form.2
The pathophysiological mechanism determining this syndrome has not been fully clarified, though it appears to involve metabolic aspects of the drugs, as well as the triggering of immune events.3
The aromatic anticonvulsants are metabolized by oxidation reactions that transform them into nontoxic hydroxylated metabolites.4 Arenae oxide metabolites mediate these reactions, they may be responsible for toxic interactions with the cytochrome P-450 (CYP) system.4 There may be an alteration in the structure of this cytochrome with the unchaining of an autoimmune aggression against the target organs in which the cytochromes are produced, such as the stomach, liver, intestine and lungs, among those patients predisposed to incapacity for the detoxification of the toxic metabolites.4
The idiosyncratic adverse drug reactions in the liver occur under two categories: those that result from an altered metabolism of the drug, with excessive production of poisonous metabolites in susceptible individuals; and those that involve an aggression directed against hepatocytes, immunologically mediated, triggered by the drug (allergic hepatitis).8 The differences observed between the various individuals in the general population, regarding metabolism of the drugs are due to alterations in the expression of the enzymes involved in their metabolism.8
These differences may be the result of genetic polymorphism (in general, absence of a gene; existence of a mutant; nonfunctional or partially active genes; duplication of genes, etc.) or the expression of a different phenotype.8
Genetic polymorphism can be found in the genes of CYP (CYP2D6, CYP2C19, CYP 2A6, CYP2C9 and CYP2E1), genes of glutathione-S-transferase (GST-M1 and GST-T1) and in the gene of N-acetyltransferase (NAT2).7 In this manner the geno- and phenotypic variability in these enzymes are responsible for the differences in the metabolization of the drugs, generation of reactive intermediate metabolites, constituting a relevant cause of adverse drug reactions.8,9
Another hypothesis that involves the etiopathogenesis of DHS is the proposition that it is mediated by a virus, particularly human herpesvirus type 6 (HHV 6), in a similar way to the association between Epstein-Barr virus and ampicillin or hypersensitivity to sulfa drugs in patients with HIV.4 ADHS differs from other adverse drug reactions in several aspects: limited number of drugs involved, onset of the picture in a relatively late form in relation to the date the medicine was introduced, clinical similarity with many infectious diseases and prolonged period for resolution of the signs and symptoms.1,10 In 1998, Toyama and cols.11 and Suzuki and cols.12 detected a possible connection between viral infection and adverse drug reaction, specifically among those with human herpesvirus 6 (HHV 6) and DHS. These authors suggested that infection by HHV 6 could be involved in the development of DHS in susceptible patients.13 The activation of CD4+ lymphocytes induced by the drug reaction appears to result in the reactivation of a latent infection by HHV 6.10 The infection by HHV 6 in turn modulates the expression of several inflammatory cytokines that propitiate an immune dysfunction.10,13
Hashimoto and cols10 studied 20 patients with DHS using polymerase chain reaction (PCR) method in real time, in a quantitative manner to analyze serum and blood samples from the patients to detect HHV 6, besides measuring the titers of anti-HHV 6 IgG. The authors found a pattern of relationship between the clinical disease (DHS) and infection by HHV 6:10 (i) exposure to the drug with a latency period (sensitization) from 2 to 6 weeks; (ii) first peak of symptoms and signs of DHS with fever, cutaneous eruption, hepatic alterations; and (iii) second peak of clinical signs and symptoms reflecting the typically slow form of DHS resolution, that is simultaneous with increase in the titers of anti-HHV 6 IgG in the 3rd and 4th weeks. This second peak of signs and symptoms is preceded by the detection of DNA of HHV 6 by PCR technique.
Thus, genetic polymorphism, the generation of intermediate reactive metabolites and the reactivation of latent viral infections (HHV-6) could compete for the generation of immune mechanisms in the etiopathogenesis of DHS.
Bocquet and cols.2 proposed criteria for the diagnosis of DRESS, which can be established if there are at least three criteria present: 1) cutaneous drug eruption; 2) hematological abnormalities: eosinophilia > 1,500/mm3 and/or presence of atypical lymphocytes; 3) systemic involvement: adenopathy (> 2 cm in diameter) and/or hepatitis (elevation of the transaminases to at least twice the normal values) and/or interstitial nephritis and/or interstitial pneumonitis and/or carditis.
Carroll and cols.14 in 2001, carried out a review of pediatric cases of DHS. Out of 105 patients studied, with age varying from 1 to 17 years, the authors found the following systemic alterations: hepatic involvement (61% of cases; coagulopathy, hepatitis, hepatic insufficiency, hepatomegaly and hypoalbuminemia), hematological findings (48% of cases; anemia, including aplastic anemia, eosinophilia, leukopenia, leukocytosis, thrombocytopenia), renal involvement (15% of cases; elevation of urea and of creatinine, hematuria, nephritis and proteinuria), pulmonary findings (14% of cases; atelectasis, consolidation and hemorrhages, dyspnea, hypoxia, pulmonary edema and pneumonitis) and other organs (20% of cases; pancreatitis, nausea, vomiting, diarrhea, hyper- and hypothyroidism, myocarditis, splenomegaly, mental deterioration, convulsions and syndrome of inappropriate secretion of antidiuretic hormone). Carroll and cols.14 observed a case with lethal outcome among these children. Most of the cases were triggered by use of antiepileptic drugs followed in frequency by the drugs minocycline, sulphamidic derivatives and dapsone. In pediatric patients it is essential to differentiate this adverse drug reaction from bacterial and viral infections.14
The proposed treatment, after exclusion of infectious causes, is based on the use of systemic corticosteroids (doses > 0.5 mg/kg/day oral prednisone or 60 mg endovenous methylprednisolone every 6 hours) providing a marked improvement in symptoms and in laboratorial parameters.1-4 Recurrence of the cutaneous eruption and hepatitis may occur as the corticosteroid administration is reduced, sometimes requiring prolonged corticosteroid therapy for several months (personal observation).2,3 Transitory hypothyroidism may occur.2 A relationship between acute reaction and subsequent development of lymphoma, even after several years, continues to be a subject of debate.2
The incidence of this syndrome in first degree relatives is high and these individuals should be counseled about the possibility of similar reactions, with the same or a correlated drug.2,4
2. Acute Generalized Exanthematous Pustulosis
Acute generalized exanthematous pustulosis (AGEP) is a clinical entity that appears in the intertriginous areas or in the face as a diffuse erythema (scarlatiniform) with acute installation.15,16 Patients report pruritus or local burning sensation.15 After this picture, the erythema is replaced by hundreds of nonfollicular sterile small pustules (less than five millimeters in diameter) (Figure 3).15 These pustules may sometimes converge and mimic Nikolsky's sign, leading to misdiagnosis as toxic epidermal necrolysis (TEN).15 Intense edema of the face may occur, with purpuric lesions mainly in the legs and the onset of lesions similar to Erythema Multiforme (EM) in the legs.15,16,17 There may be mucous involvement in about 20% of the patients, however it is usually mild and self limited, occurring in just one location.15 The cutaneous symptoms are almost always accompanied by fever over 38°C.15,16,17 Frequently there is leukocytosis in the blood count and eosinophilia may also occur in one third of the patients.15,16,17
Usually this eruption regresses within 4 to 10 days after withdrawal of the drug and in typical cases leaves a lamellar or punctiform desquamation.15,17 Disease prognosis worsens when there is hyperthermia, infection of the lesions and involves elderly individuals, who should be hospitalized.15
The drugs described as a cause of EGEP are most frequently16 os b-lactam (penicillin, cephalosporins), macrolides (azithromycin, erythromycin), cyclines (doxycycline), sulfonamides (trimethoprim, sulfasalazine), chloramphenicol, isoniazid, streptomycin, vancomycin, quinolones (ciprofloxacin, norfloxacin), itraconazole, terbinafine, allopurinol, carbamazepine, phenytoin, diltiazem, nifedipine, chromium picolinate, diclophenac, enalapril, disulfiram, furosemide, hydroxychloroquine, paracetamol, mercury, thalidomide, protease inhibitors7 and bamifylline.17
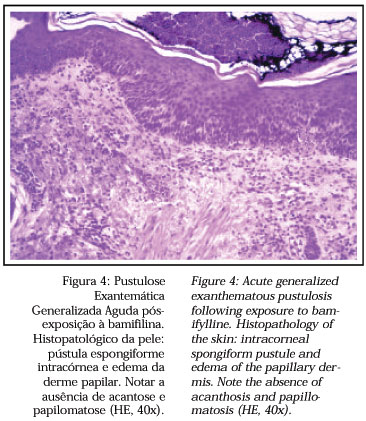
Sidoroff and cols.15 proposed some characteristics that might aid in the differentiation between pustular psoriasis and AGEP. In the latter, a history of psoriasis is rare, the lesions are most frequent in the cutaneous folds, the duration of the fever and of the pustules is short, there is usually a history of recent exposure to the drug, arthritis is rare. Histopathology may show subcorneal and/or intraepidermal spongiform pustules, edema of the papillary dermis, vasculitis, exocytosis of eosinophils and focal necrosis of keratinocytes (Figure 4). On the other hand, in pustular psoriasis a history of psoriasis is common, the involvement is generalized, the duration of the fever and of the pustules is longer, history of drug exposure is less frequent, arthritis occurs in about 30% of the patients and the histopathological exam shows subcorneal and/or intra-epidermal pustules, papillomatosis and acanthosis of the epidermis.
Skin tests for late-phase reactions may be useful tools for identifying the etiological agent of AGEP, when the systemic re-administration is potentially dangerous.16,17 Approximately 50% of the cases of AGEP present positive contact tests for the suspect drug, usually reproducing the lesion in both a clinical and histological form.15,16
Recently, Britschgi and cols.18 demonstrated the high expression of interleukin (IL)-8 in these patients. It is known that IL-8 is a chemokine with potent activity in the recruitment of neutrophils, which is produced by the keratinocytes and mononuclear cells of the cutaneous inflammatory infiltration.18 EThese authors concluded that AGEP might be the expression of a reaction, in which a cell binded to the drug triggers a drug-specific CD4+ and CD8+ immune response, which results in a high expression of IL-8.18
3. Serum sickness
In 1905, Von Piquet and Shick described serum sickness in children treated with horse serum containing diphtheric antitoxin.1 More recently serum sickness has been observed in patients treated with horse antithymocyte globulin or vaccines of rabbit anti-human diploid cells.1 This constitutes a type III hypersensitivity reaction, mediated by immunocomplexes deposited on the walls of the vessels, activation of the complement and recruitment of granulocytes.1 It presents particular cutaneous manifestations: typically, there is erythema in the lateral portion of the fingers and toes, that precedes a more disseminated eruption (occurring in 90% of cases), which frequently is morbilliform (2/3 of the patients) and sometimes urticariform.1,19 The presence of urticaria, leukocytoclastic vasculitis and multiform erythema is rarely observed.19 In half of the cases there is visceral involvement.1 The following clinical findings are common: fever, cutaneous eruption, constitutional symptoms, arthritis and arthralgia.1,19
The disease begins about 8 to 14 days after the initial exposure to the foreign protein.1 The drugs related with this type of manifestation are the heterologous serums and vaccines.1,19 A19 serum sickness-like reactions can also be caused by penicillin, cephalosporin, minocycline, propranolol, streptokinase and non-hormonal anti-inflammatories.1,19 There is no data on the prevalence of this disease in Brazil, however reports of cases of this disease are not rare in the medical literature.
Fractions C3 and C4 of the complement are strongly decreased in serum sickness while they are usually normal in serum sickness-like reactions.1
Treatment of the disease constitutes withdrawal of the drug allied to the use of systemic corticosteroids, in addition to antihistamines for symptomatic relief of pruritus when present.1 Careful observation of the clinical course of the patient's systemic involvement is imperative.1,19
4. Drug-Induced Vasculitis
Several medicines can induce a cutaneous vasculitis type response, the histopathologic definition of which is the presence of inflammation and necrosis in the wall of the cutaneous blood vessels. Clinically it presents as tangible purpura or maculo-papular purpuric eruption.1,20 This disease can also occur in the form of hemorrhagic blisters, urticaria, ulceration, nodules, Raynaud's disease and digital necrosis.1 The same vasculitis process can involve internal organs, such as the kidneys, liver, gastrointestinal tract or the central nervous system and any area of the tegument, including the mucous membranes and the palmar and plantar regions.1,21,22
The disease develops about 7 to 21 days after initiating the drug, however there can be a longer time interval, and any medication instituted within the two months prior to the picture should be considered suspect.1 Given the absence of confirmatory tests for this entity, one should valorize anamnesis and the correlation with drug exposure, that in general occurs one to three weeks before onset of the cutaneous picture. However, the exposure can have occurred in periods as disparate as two days to nine years.22 Withdrawal of the drug leads to a rapid resolution of the picture and systemic corticosteroids can benefit some patients.1 The process is usually solved without sequels.21
The clinical, epidemic and pathological characteristics of drug induced vasculitis have been little reported in the medical literature, since there is no consensus in the definition of this disease, with various revisions using different criteria for inclusion of cases.22 Vasculitis attributed to exposure to medicines is rare, but seemingly account for about 10% to 20% of dermal vasculitis cases.22 It is difficult to quantify the frequency with which drug-induced vasculitis is strictly cutaneous.22 Clinical experience suggests that most of the cases are confined to the skin and have a self-limited course, however it can be associated with varied degrees of systemic symptoms including arthralgia, indisposition and fever.22 Visceral involvement is well described and pathologically heterogeneous.22 Glomerulonephritis and interstitial renal disease, varied degrees of hepatocellular damage and formation of granulomas in the liver have been described, besides involvement of the heart, lungs and central nervous system.22 Furthermore, there are rare cases of drug-induced vasculitis with renal and hepatic involvement in the absence of cutaneous disease.23,24
The drugs most frequently referred to in the literature under the form of case reports or studies on series of patients, as causative of vasculitis are: propylthiouracil, hydralazine, granulocyte colony-stimulating factor (G-CSF), cefaclor, minocycline, allopurinol, D-penicillamine, phenytoin, isotretinoin and methotrexate.25 MMany of the cases of drug-induced vasculitis are not reported in the medical literature, hence there are other drugs that could be important causative agents of this reaction type. To a lesser frequency other drugs have been reported as causal agents of vasculitis:25 several antibiotics, etretinate, didanosine, zidovudine, acebutolol, atenolol, sotalol, propranolol, chlorothiazide, furosemide, diltiazem, nifedipine, methyldopa, captopril, enalapril, lisinopril, losartan, procainamida, quinidine, antithyroid medications, painkillers and antipyretics, levamisole, tamoxifen, arabinoside C, interferon, interleukin-2, sulfasalazine, etaneracept, gold, carbamazepine, antidepressants, zafirlukast, chroamolin, cimetidine, ranitidine, L-tryptophan, radiocontrast, streptokinase, heparin, cumarinic, chlorpromazine, metformin, pimagedine and diphenhydramine.
There are three drugs that cause vasculitis associated to antineutrophil cytoplasmic antibodies (ANCA): hydralazine, propylthiouracil and minocycline.25
About 20% of the patients that use propylthiouracil develop ANCA, a fact that is related to a higher risk of glomerulonephritis.25 A particularly relevant form among the drug induced vasculites is Propylthiouracil (PTU) Hypersensitivity Vasculitis. There are cases with other antithyroid compounds, such as methimazole, thiamazole/methylthiouracil and carbimazole, that similarly to PTU contain a thioamide group and cause allergic cross reactions.24 Although uncommon, nowadays a larger number of case reports of this entity are observed, suggesting cases were previously not reported or that were included among other nosologic entities, since PTU is a drug classically consecrated for the treatment of hyperthyroidism.25
The picture begins after initiating PTU, though the duration of drug use is extremely variable, from 1 week to 13 years, it appears under a classic tetrad of symptoms that include fever, sore throat, arthralgia and cutaneous eruption, there can also be myalgia, fatigue, weight loss, conjunctivitis, rhinitis and hemoptysis.24,26
It courses as a systemic vasculitis, there can be a lupus-like syndrome, Wegener-like granulomatosis or nodular-like polyarthritis with multiple involvement of organs, such as kidneys, articulations, lungs and others associated to cutaneous lesions.24,26 The cutaneous lesions are usually constituted of plaques or acral purpuric nodules arranged in a livedoid pattern, with a preference for the extremities (Figure 5), for the face, mammas and characteristically the lobes and helixes of the ears, which mimics the leprosy type reaction of Lucio's phenomenon.26 Hemorrhagic blisters appear on these lesions that progress to central necrosis of the skin, this can be so extensive that it simulates the clinical presentation of fulminant purpura observed in septic infectious states with disseminated intravascular coagulation (DIC).27
Laboratory tests reveal anemia, leukopenia and platelet depletion in the blood count; increase of erythrocyte sedimentation rate, urea, creatinine, transaminases, bilirubin, hypoalbuminemia, alterations in the coagulation time, prothrombin time, partial activated thromboplastin and immunological abnormalities such as positive ANCA, rheumatoid factor and hypergammaglobulinemia can be found. Positivity can also be present of the anti-SSA, anti-DNA double helix, anticardiolipin, anti-smooth muscle antibodies, antimitochondrial, parietal and antiadrenargic anticells, besides hypocomplementemia, cryoglobulinemia and elevation of C-reactive protein.24-28 Histopathologic study demonstrates a leukocytoclastic vasculitis of the superficial and profound vessels of the dermis. The finding of immunocomplexes deposited in the vascular wall is uncommon, such that some authors have denominated them pauciimmune ANCA-positive vasculitis.24-28 Most of the patients recover completely following withdrawal of PTU, however some develop impairment of the kidneys or other internal organs, or skin (Figure 6) requiring high doses of prednisone for several months.25
The dermatological findings present in patients with drug-induced vasculitis associated to ANCA include plaques and purpuric acral nodules, which appear more commonly in the extremities, face, breasts and ears.26 In addition, the patients report the same signs and symptoms as other small-vessel vasculites associated to ANCA (Wegener's granulomatosis, Churg-Strauss syndrome), including glomerulonephritis, pulmonary hemorrhage and digital gangrene.26 Besides withdrawal of the offending drug, it is generally necessary to use corticosteroids in high doses or in pulse therapy, plasmapheresis and immunosuppressants, for several months.24,26 The mortality rate is approximately 10%.24
5. Anticoagulant-Induced Skin Necrosis
This is a rare and severe adverse effect from treatment with Warfarin, occurring with cutaneous necrosis secondary to the occlusive thrombosis in the vessels of the skin and subcutaneous cellular tissue.1 It usually presents 3 to 5 days after use of the drug, as painful erythematous plaques that course to necrosis, with hemorrhagic blisters or necrotic scars in the rich areas in subcutaneous tissue, such as buttocks, breasts and hip.1 The risk of this disease increases in patients that are female, obese and users of high doses of the medication.1 The necrotic tissue requires débridement and grafts.1 This type of reaction has also been described with the use of heparin.1
CONCLUSIONS
In situations that involve patients with acute adverse drug reaction, certain general principles should be observed:1,29,30,31
1) if possible identify the physiopathological mechanism involved in the reaction;
2) identify as rapidly as possible the drug inducing the reaction and always opt for its withdrawal; in some circumstances the choice is difficult as there is no alternative drug and its use is essential for the maintenance of life;
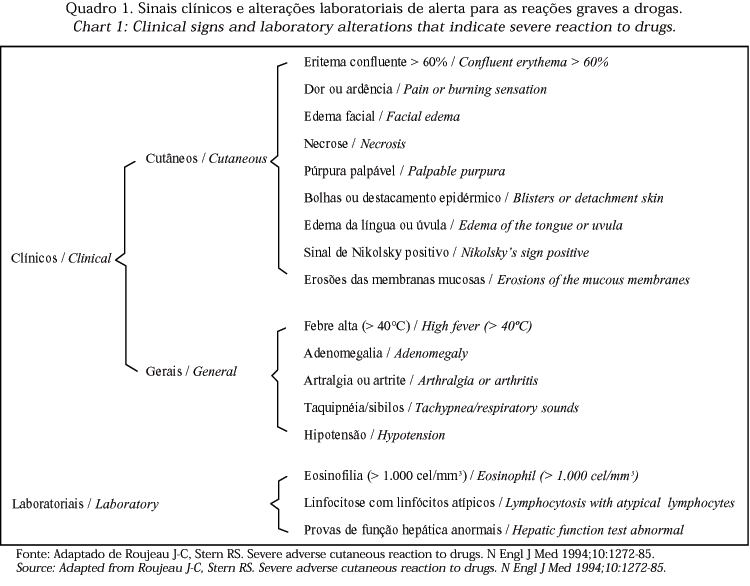
3) a careful and intensive observation is recommended for the occurrence of warning signs regarding the appearance of a potentially severe adverse drug reaction, as suggested in chart 1, especially in relation to mucous, oral, ocular and genital involvement and progression of any present cutaneous eruption;
4) it is imperative that the drug responsible is withdrawn on a permanent basis together with chemically related compounds, this advice is also valid for first-degree relatives who can present the same type of reaction.
NOTE
During the revision phase of this article, a study done at the Mayo Clinic (USA), was published involving the use of infliximab (antagonist for the action of tumor necrosis factor alpha, TNF-a) in 500 patients with Crohn's disease.32 Thirty patients (6%) developed severe adverse reactions that were related to infliximab. Serum sickness-like syndrome occurred in 19 patients, and was attributed to infliximab in 14 of these (2.8%).32 The ability of TNF-a antagonists in causing significant adverse reactions could be related to its interference in the activity of TNF-a or is a direct consequence of these therapeutic agents, due to the presence of immunoglobulin or the fusion protein in its structure, which can be recognized by the immune system as proteins foreign to the organism, rendering them a target of the immune response.33 The new modifiers of the biological response, such as infliximab, constitute a promising new therapeutic arsenal in medicine and in dermatology, however a meticulous pharmacovigilance is necessary, especially as they constitute a new group of drugs that every day are being used in new clinical indications. 
REFERENCES
Received on May 09, 2003.
Approved by the Consultive Council and accepted for publication on December 17, 2003.
- 1. Roujeau J-C, Stern RS. Severe adverse cutaneous reaction to drugs. N Engl J Med. 1994;10:1272-85.
- 2. Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Sem Cutan Med Surgery. 1996;15:250-7.
- 3. Criado PR, Lucena SK, Crivellaro APGS, Criado RFJ, Tebcherani A, Nogueira AT et al Síndrome de hipersensibilidade a anticonvulsivantes: relato de dois casos. Rev Bras Clin Terap. 2002;28:59-63.
- 4. Kennebeck GA. Anticonvulsant Hypersensitivity Syndrome. J Am Board Fam Pract. 2000;13:364-70.
- 5. Jonas D, Chhiap V, Resor S, Appel G, Grossman ME. Phenytoin-like hypersensitivity associated with lamotrigine. J Am Acad Dermatol. 1997;36:1016-8.
- 6. Gupta AK, Kopstein JB, Shear NH. Hypersensitivity reaction to terbinafine. J Am Acad Dermatol. 1997;36:1018-9.
- 7. Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol. 2002;46:284-93.
- 8. Castell JV. Allergic hepatitis: a drug-mediated organ-specific immune reaction. Clin Exp Allergy. 1998;28:13-9.
- 9. Shapiro LE, Shear NH. Mecanisms of drug reaction the metabolic track. Sem Cutan Med Surg. 1996;15 :217-27.
- 10. Hashimoto K, Yasukawa M, Tohyama M. Human herpesvirus 6 and drug allergy, Curr Opin Allergy Clin Immunol. 2003;3:255-60.
- 11. Tohyama M, Yahata Y, Yasukawa M et al Severe hypersensitivity syndrome due to sulfalazine associated with reactivation of human herpesvirus 6. Arch Dermatol. 1998; 134: 1113-7.
- 12. Suzuki Y, Inagi R, Aono T, Yamanishi K, Shiohara T. Human herpesvirus 6 infection as a risk factor for the development of severe drug induced hypersensitivity syndrome. Arch Dermatol. 1998; 134: 1108-12.
- 13. Criado PR, Criado RFJ, Vasconcellos C, Pegas JRP, Cera PC. Drug-induced hypersensitivity syndrome due to anticonvulsants in a two-year-old boy. J Dermatol. In press 2004.
- 14. Carrol MC, Yueng-Yue KA, Esterly NB, Drolet BA. Drug-induced hypersensitivity syndrome in pediatric patients. Pediatrics. 2001;108:485-92.
- 15. Sidoroff A, Halevy S, Bavinck JNB, Vailant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)- a clinical reaction pattern. J Cutan Pathol. 2001;28:113-19.
- 16. Young PC, Turiansky GW, Bonner MW, Benson PM. Acute generalized exanthematous pustulosis induced by chromium picolinate. J Am Acad Dermatol. 1999;41:820-3.
- 17. Galvao C, Criado RFJ, Criado PR, Valente NY, de Mello JF, Fernandes MF. Acute generalized exanthematous pustulosis induced by ingestion of bamifylline. J Eur Acad Dermatol Venereol. 2002;16:634-7.
- 18. Bristschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A et al T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107:1433-41.
- 19. Bigby M, Stern RS, Arndt KA. Allergic Cutaneous Reactions to Drugs. Primare Care. 1989;16:713-27.
- 20. Crowson AN, Magro CM. Recents advances in the pathology of cutaneous drug eruptions. Dermatol Clin. 1999;17:537-60.
- 21. Lotti T, Ghersetich I, Comocchi C, Jorizzo JL. Cutaneous small-vessel vasculitis. J Am Acad Dermatol. 1998;39:667-87.
- 22. Calabrese LH, Duna GF. Drug-induced vasculitis. Curr Opin Rheumatol. 1996;8:34-40.
- 23. Leung ACT, McLay A, Dobbie JW, Jones JM. Phenylbutazone-induced systemic vasculitis with crescentic glomerulonephritis. Arch Intern Med. 1985;145:685-7.
- 24. Rosemberg JL, Edlow D, Sneider R. Liver disease and vasculitis in a patient taking cromolyn. Arch Intern Med. 1978;138:989-91.
- 25. ten Holder AM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130-47.
- 26. Florentino DF. Cutaneous vasculitis. J Am Acad Dermatol. 2003;48:311-40.
- 27. Chastain MA, Russo GG, Boh EE, Chastain JB, Falabella A, Millikan LE. Propylthiouracil hypersensitivity: report of two patients with vasculitis and review of the literature. J Am Acad Dermatol. 1999;41:757-64.
- 28. Park KEJ, Chipps DR, Benson EM. Necrotizing vasculitis secondary to propylthiouracil presenting as purpura fulminans. Rheumatology (Oxford) 1999;38:790-2.
- 29. Gruchalla R. Advances in allergic diseases: An update for the new millennium. Understanding drug allergies. J Allergy Clin Immunol. 2000;105:s637-44.
- 30. Ghislain P-D, Roujeau J-C. Treatment of severe drug reaction: Stevens-Johnson syndrome, Toxic Epidermal Necrolysis and Hypersensitivity syndrome. Dermatology Online Journal [serial the internete] 2002;8:5. Avaliable from: http://dermatology.cdlib.org/DOJvol8num1/reviews/drugxn/ghislain.html
- 31. Criado RFJ, Criado PR, Vasconcellos C. Reações cutâneas graves adversas a drogas: definições, sinais de alerta e opções terapęuticas. Rev bras alerg imunopatol. 2003; 26:110-128.
- 32. Colombel JF, Loftus EV Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD et al The safety profile of infliximab in patients with Cronh´s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004; 126:19-31.
- 33. Weber RW. Adverse reactions to biological modifiers. Curr Opin Allergy Clin Immunol. 2004; 4: 277-283.
Publication Dates
-
Publication in this collection
29 May 2006 -
Date of issue
Oct 2004
History
-
Received
09 May 2003 -
Accepted
17 Dec 2003