Abstracts
The advent of AIDS has brought new challenges to Dermatology. Antiretroviral therapy dramatically changed the morbidity and mortality associated with HIV / AIDS, but contributed to the emergence of other new situations that require adequate approach by the dermatologist. The HIV / AIDS Associated Lipodystrophy Syndrome is multifactorial in origin, but it is strongly associated with the use of antiretroviral drugs. It includes changes in body fat distribution, with or without metabolic changes. The loss of facial fat, called facial lipoatrophy, is one of the most stigmatizing signs of the syndrome. This condition, often revealing of the disease, brought back the stigma of AIDS. It is necessary that the specialists working with patients with HIV / AIDS identify these changes and seek treatment options, amongst which stands out the implant with polymethylmethacrylate, which is available for the treatment of HIV / AIDS facial lipoatrophy in the Brazilian Public Health System
Ambulatory surgical procedures; HIV; HIV-associated lipodystrophy syndrome; Polymethyl methacrylate; Therapeutics
O advento da AIDS trouxe novos desafios para a Dermatologia. A terapia antirretroviral mudou drasticamente a morbimortalidade associada à infecção pelo HIV/AIDS, mas contribuiu para o surgimento de outras novas situações que exigem abordagem adequada do dermatologista. A Síndrome Lipodistrófica Associada ao HIV/AIDS tem origem multifatorial, mas está fortemente associada ao uso dos antirretrovirais. Compreende alterações na distribuição da gordura corporal, acompanhada ou não de alterações metabólicas. A perda da gordura da face, chamada lipoatrofia facial, é dos sinais mais estigmatizantes da síndrome. Esta condição, muitas vezes reveladora da doença, trouxe de volta o estigma da AIDS. É necessário que os especialistas que atuam com pacientes com HIV/AIDS identifiquem estas alterações e busquem opções de tratamento, dentre as quais se destaca o implante com polimetilmetacrilato, que é disponibilizado para tratamento da lipoatrofia facial associada ao HIV/AIDS no Sistema Único de Saúde
HIV; Polimetil metacrilato; Procedimentos cirúrgicos ambulatórios; Síndrome de lipodistrofia associada ao HIV; Terapêutica
CONTINUED MEDICAL EDUCATION
HIV-Associated facial lipoatrophy: from the advent to current knowledge*
Flávia Machado Gonçalves SoaresI; Izelda Maria Carvalho CostaII
IMaster in Health Sciences by the Universidade de Brasília (UnB) - Physician of the Health Office of Distrito Federal - Brasília (DF), Brasil
IIPhD in Dermatoloy by the Universidade de São Paulo (USP) - Assistant Professor of Dermatology at the Universidade de Brasília (UnB) - Brasília (DF), Brasil
Mailing address
ABSTRACT
The advent of AIDS has brought new challenges to Dermatology. Antiretroviral therapy dramatically changed the morbidity and mortality associated with HIV / AIDS, but contributed to the emergence of other new situations that require adequate approach by the dermatologist. The HIV / AIDS Associated Lipodystrophy Syndrome is multifactorial in origin, but it is strongly associated with the use of antiretroviral drugs. It includes changes in body fat distribution, with or without metabolic changes. The loss of facial fat, called facial lipoatrophy, is one of the most stigmatizing signs of the syndrome. This condition, often revealing of the disease, brought back the stigma of AIDS. It is necessary that the specialists working with patients with HIV / AIDS identify these changes and seek treatment options, amongst which stands out the implant with polymethylmethacrylate, which is available for the treatment of HIV / AIDS facial lipoatrophy in the Brazilian Public Health System.
Keywords: Ambulatory surgical procedures; HIV; HIV-associated lipodystrophy syndrome; Polymethyl methacrylate; Therapeutics
INTRODUCTION
Since AIDS is now a chronic and manageable disease it is vital to recognize and treat the conditions associated with the disease itself or with the adverse effects of the antiretroviral drugs. The facial lipoatrophy associated with HIV/AIDS has become epidemic, and all involved with care of this group of patients must recognize the signs of the lipodystrophic syndrome, as well as the designated treatment, which should always be incorporated to the therapeutic arsenal of patients with HIV/AIDS.
1. ACQUIRED IMMUNODEFICIENCY SYNDROME
1.1 BACKGROUND
In June 1981 the Morbidity and Mortality Weekly Report (MMWR), of the Centers for Disease Control (CDC), organization responsible for epidemiologic surveillance in the United States, released an article reporting five cases of pneumonia caused by the then called Pneumocystis carinii in young, previously healthy men who had in common the fact that they were homosexuals.1 A month later the MMWR released another historical article about the epidemy of Acquired Immunodeficiency Syndrome (AIDS) reporting, this time, an outbreak of Kaposi's sarcoma and Pneumocystis carinii pneumonia amongst male homosexual communities in New York and Los Angeles. 2 In December 1981, they came to the conclusion that it was an infeccious disease. The seach for the immunosupression causative agent, invariably confirmed in all cases, became then a priority.
The first indication that Aids could be caused by a retrovirus came about in 1983, when a virus with reverse transcriptase activity was isolated. This virus is currently nominated as the human immunodeficiency virus type 1 (HIV-1). In 1986, another retrovirus was isolated, and it was nominated as the human immunodeficiency virus type 2 (HIV-2). 3
The modes of transmission of AIDS became known even before the identification of its ethiologic agent, by means of epidemiologic investigation. That was when the notion of risk group occurred, which contributed to the increasing discrimination against homosexuals and drug users and to the development of a heavy stigma for the AIDS carriers. Later on a tendency to the spreading of the disease to the whole population in some affected countries was noted. 4
Sexual preferences were identified as an important mode of transmission of HIV since the first investigations of the disease. It is estimated that 75% to 85% of the HIV infections all over the world have occurred via sexual transmission. Another mode of transmission of HIV, perinatal or vertical, can happen intra-uterus, during labor, or though breastfeeding. The mother/child transmission rates vary from 15 to 35%, in the absence of anti-retroviral therapy. The transmission of HIV through blood is the most efficient. The development of increasingly sensitive serologic tests for the detection of the HIV enormously reduced the risk of transmission via contaminated blood. Sharing injection devices amongst injection drug users has a great potential for the dissemination of HIV, and in some countries it represents the main risk factor for the infection with the virus. Transplants, dialysis, and other inhospital procedures, like artificial insemination, have all been reported on the literature as modes of transmission of the HIV. 4
The first definition of a case of AIDS was published on the MMWR, in September 1982, based exclusively on the presence of opportunistic infections, when a test that could identify the HIV was not yet available. The test to indentify the presence of the virus becomes a fundamental item for the case definition from 1985. In 1993, the CDC expanded its case definition including people infected with the HIV and with CD4+ cell count of less than 200 cells/mm3 of blood, and increased the preexistent list of AIDS indicative diseases. The case definition developed by the CDC was adopted and modified by various countries, including Brazil. The first case definition of AIDS adopted by Brazil was in 1987. 5
1.2 IMMUNOPATHOGENESIS
HIV infection features and the function of the human beings' immune system started to be elucidated during the nineties. The integration of the viral genoma to the infected cells, especially T cells, is the cause of the main alterations of the gene expression of the host cell, leading to the destruction of these cells as well as of the non-infected cells. The patients develop a progressive decline of the function and number of T helper cells, coupled with hypergammaglobulinemia. An imbalance of the cytokines network of the infected patients could be responsible, at least in part, to the immunologic abnormalities that lead to AIDS. The viral infection also causes change to the nonimmunological homeostasis, with ramifications to the immunological process, like the elevation of the level of P substance that, in its turn, increases the HIV expression on the monocytes. Other changes to the basic cell processing features also occur, like the cell regulation cycle, resulting in prematurely programmed cell death. 6
Three important populations of cells are infected by HIV: T helper lymphocytes or CD4+, monocytes and macrophages. The major part of the viral replication occurs on the CD4+ lymphocytes on the peripheral blood or on lymphoid tissue. The hallmark of the HIV infection is a selective depletion of CD4+ lymphocytes. 6
The natural history of HIV infection varies from person to person. The plasmatic levels of HIV-1 ribonucleic acid (RNA) and the CD4+ lymphocyte count are the most important variables that establish the progression rate. The resulting deterioration of the immune system, which happens in most patients with HIV infection, is the development of the clinically evident disease or AIDS. 5
In the absence of treatment the typical duration of seroconversion up to the development of AIDS is ten years. In the San Francisco Clinical Cohort, 54% of the patients had progression to Aids in 11 years. Survival after the CD4+ count drops to 200 cells/mm3 is, on average, 38 to 40 months. Some patients progress quickly to the development of Aids, in three to five years. On the other hand, the so called long term non-progressors, around 1% of the infeccted ones, can have nomal CD4+ cell count and low viral load for more than 10 to 20 years. In the absence of treatment the median survival after the diagnosis of clinically defined Aids is around 7 months. 7
1.3 EPIDEMIOLOGY
The number of HIV infected people in the world is still unknown. The World Health Organization (WHO) and the United Nations Programs on HIV/AIDS (UNAIDS) estimate that, in 2008, there were 33,4 million people living HIV/AIDS worldwide. In 2008 only 2, 7 million people were infected and there were 2 million deaths associated to HIV. In Latin America, in 2008, it was estimated that there were 2 million people living with HIV/AIDS, and 170.000 new infected people.8 It is estimated that 2/3 of the infected ones in Latin America live in Brazil. 9
Of all the cases notified in the Americas up to 2001, 67, 19% occurred in the United States, followed by Brazil, with 17, 99%. In Brazil, from the beginning of 1980 to 31 December 2003, 348.250 cases were registered. 65, 3% of the cases were concentrated on the Southeast region. Although initially restricted to a group of men who had sex with men, the epidemy reached injectable drug users and the general population, with an increase on the number of infected women. From 1990, a change on the epidemiologic profile was observed, resulting in the heterosexualization, feminization, povertyzation and interiorization of the epidemy. The masculine/feminine ratio that was 25/1 in 1985 went to 1 woman per 1, 6 men in 1996. It is estimated that the highest proportion of the transmission amongst women occur via sexual contact with their partners. However, unprotected sex amongst men is still an important fact, accounting for around half of sexully transmitted infections in Brazil. 9,10
Based on these data it is possible to say that the AIDS epidemy is still in the process of stabilization in Brazil, although at high levels, having reached, in 2003, 18,4 cases per 100 thousand population and, in 2008, 18,2 cases per 100 thousand population. However, the epidemy is still growing amongst women. In 1986, the sex ratio (M/F) was 15,1/1 and from 2002 it reached a ration of 1,5/1. Until June 2007, around 474 thousand cases of the disease were identified in Brazil. 10-12
The deaths from the disease up to June 2008 add to around 192 thousand. The mortality rates increased until the middle of the nineties. Since 2000 this rate has stabilized at around 6,4 deaths per 100 thousand population. In 2008 the mortality rate from AIDS in Brazil was 6,1/100 thousand population. 11,12
1.4 ANTIRETROVIRAL THERAPY
The natural history of HIV infection has been considerably changed by the anti-retroviral therapy (ARVT), which slows the progression of the infection to its final stage, when the defining manifestations of AIDS occur. Together with the prevention campaigns, the antiretrovirals (ARV) seem to be contributing to the stabilization of the progress of this epidemy in Brazil and worldwide, leading to a decrease on the incidence of AIDS and to a reduction on the mortality rate of around 50% over the past years. 12
According to WHO, 42% of the HIV infected people in the world receive ARV. In Latin America the overall coverage is higher than the world average, at 54%. 8
Presently, the ARVT consists of 17 medications that are divided in four classes: nucleoside analog reverse-transcriptase inhibitors (NARTIs), that act on the reverse-transcriptase enzyme, incorporating themselves to the DNA chain originated by the virus, rendering this chain defective and preventing the viral reproduction; the non-nucleoside analog reversetranscriptase inhibitors (NNARTIs), that directly block the effect of the enzyme, its multiplication and the development of the infestation in the body; the protease inhibitors (PI), which prevent the production of new copies of cells infected with HIV and the fusion inhibitors (FI), that prevent the virus from entering the cell. 13
In order to fight the HIV it is necessary to use at least two medications of different classes, and the majority of the patients receive three to four antiretroviral medications. However, many medications can not be used together because they interact amongst themselves potentializing the toxic effects or inhibiting their role. 13
The use of schemes containing association of three antiretroviral medications overcame the emergency of viral resistance, easily observed with monotherapy or therapy with two drugs using nucleoside analog reverse-transcriptase inhibitors. The scheme combining three antiretroviral drugs containing protease inhibitors was named Highly Active Antiretroviral Therapy (HAART) in the United States and highly active antiretroviral treatment or simply "cocktail" in Brazil. 13
With the advent of the ARVT it was thought that the virus could be erradicated, but studies have demonstrated the impossibility of complete elimination of the HIV from the organism. Therefore, in view of the current knowledge, patients with AIDS will have to use antiretrovirals all their life. The criteria used to start the therapy take into consideration the clinical status of the patient and the CD4+ lymphocyte count. The measure of the viral load is important for the adequate choice of the various available schemes, as well as for the follow-up of the therapeutic result, which aims to reduce the viral load to levels below 50 copies of RNA/mL, measured by polymerase chain reaction (PCR) or similar. 7
There is a well established relationship between the number of circulating CD4+ lymphocytes and sus-ceptibility to infection. Besides the diseases caused by immunosupression, the HIV can cause disease via direct damage to certain organs or to immunologic pathways. Various studies have shown that the response to the high potency antiretroviral combined therapy, when measured by the CD4+ count and the HIV viral load, is associated with decreased progression of the disease and mortality incidence. These studies also showed that the biggest reductions of the viral load are associated with the biggest improvements of the clinical results. Even modest reductions of plasmatic HIV RNA are associated with reduced subsequent risk of opportunistic infections. 7
The understanding of the viral dynamics and the development of laboratory tests capable of measuring the amount of virus circulating in the plasma (viral load), as well as the CD4+ count, made it possible to reliablly and objectively monitor the progress and the treatment of the infection by the HIV.
The successful treatment of the infected patients depend on the intervention with antiretrovirals, and specific therapeutic and prophylactic measures that will prevent direct damage to organs by the HIV itself, will not allow or will decrease the immunologic decline and will reduce the probability that opportunistic infections and neoplasms cause morbidity and mortality. 7
Brazil is one of the first countries to adopt health policies that significantly improve the care of people with HIV/AIDS. Amongst these policies, universal and free access of the population to medications used for the AIDS treatment stands out. Approximately 181 thousand patients are under treatment with the 16 antiretrovirals plus 100mg thalidomide, distributed by the Unified Health System (UHS). These medications slow the development of AIDS and improve the quality of life for those with the virus. They work by reducing the viral load and rebuilding the immunologic system. As a result of this health policy a significant reduction on the mortality and on the number of hospital treatments for opportunistic infections has been observed. 12
The introduction of the antiretroviral therapy as it is currently known led to the increment of survival rates of HIV seropositive patients, but it is also associated with the advent of new and important problems. Martinez and collaborators showed that the redistribution of body fat and the metabolic anomalies, the then called lipodystrophy syndrome, are amongst the most prevalent and worrying side effects of ARVT. 14
2. HIV/AIDS ASSOCIATED LIPODYSTROPHY
2.1 BACKGROUND
From 1996 a series of new anatomic and metabolic changes started to be reported in HIV/AIDS patients, particularly in those under the highly active antiretroviral therapy. The patients presented with peripheric fat atrophy as well as accumulation of central fat. At the same time, it was observed that the redistribution of body fat was associated with insulin resistance and various seric lipids abnormalities. 15 These abnormalities were lately described, generally, as lipodystrophy and/or HIV lipodystrophic syndrome (HIVLS).
The HIVLS was officially described by the Food and Drug Administration (FDA), North American organ that regulates the release and use of medications, in 1997. 16
The first morphologic signs of HIVLS were described around 2 years after the introduction of the protease inhibitors (PI). However, the introduction of the PIs coincides with the inclusion of a second nucleoside analog reverse-transcriptase inhibitors, stavudine. 17
Initially HIVLS was named "Crixbelly", because the first cases of body fat distribution were observed after the use of Crixivan® (Indinavir), a medication from the PI class. 18 The association between the use of indinavir and the body fat distribution was described in 1998, with the use of computerized tomography that showed the increase of visceral fat in these individuals. 15 With the development of new PIs it was concluded that the redistribution of body fat was not an exclusive effect of Indinavir, and this denomination was abandoned.
Miller and collaborators, after observing the clinical similarities between patients with HIVLS and Cushing Syndrome, started to call it Cushing "pseudosyndrome". 19 However, subsequent studies did not show abnormalities of the hypothalamus-hypophisisadrenal tract in sero-positive HIV patients, and this nomenclature was also abandoned.
Currently various synonyms are used for the HIVLS, like body fat redistribution syndrome, metabolic syndrome associated to antiretroviral therapy or, more recently HIV/ARVT dyslipemic associated lipodystrophy. 20
2.2 CLINICAL ASPECTS
The first body alterations to be noted were the accumulation of fat on the abdominal region and on the posterior aspect of the neck, called humps. 17
Other anatomic changes include the facial, lower and upper limb lipoatrophy and a prominence of the superficial veins, associated or not with an accumulation of fat on the abdomen, cervical region and breasts. The metabolic abnormalities include lipidic alterations and abnormalities of the glucose homeostasis. The metabolic abnormalities might or might not be associated with the anatomic ones. 16
The lipidic alterations found on the HIVLS are the increase of the seric level of triglycerides (TGC) and/or total cholesterol, due to the low-density lipoproteins (LDL), with a tendency to the decrease of the levels of high-density lipoproteins (HDL). 17
The hypertriglyceridemia is due to, mostly, the increased rates of de novo lipogenesis and the delayed depuration of the TGC after eating. 21 Studies have also shown that a significantly increased proportion of those in use of PI had increased fasting seric levels of apoliproteins B and E, possibly due to their enhanced synthesis, which could be related to the manifestation of hyperlipidemia. 21 In addition to that, the so called polymetabolic syndrome, where the abdominal obesity, component of the HIVLS, relates to abnormalities of the lipidic metabolism, was present in 18% of the patients in use of ARV, mostly in patients using PI. 22
The glucose abnormalities can manifest as glucose intolerance, peripheric resistance to insulin or diabetes mellitus (DM). 17
The mechanism of action by which the ARV, like the protease inhibitors, cause resistance to insulin would be the decrease of the insulin mediated glucose captivation on striated muscles and adipocytes, interfering with the transmembrane glucose transporters GLUT-4, besides the role on the transcription factor steroid regulatory element binding protein-1c (SREBP-1), altering the glucose metabolism by producing imperfect expressions of the gamma peroxisoma proliferator activated receptor (PPAR-gamma). 23
The lactic acidosis seen on the syndrome is mostly caused by the transcriptase reverse nucleoside analog inhibitors. It is secondary to the mitochondrial dysfunction caused by the inhibition of the deoxyribonucleic acid (DNA) mitochondrial polymerase by this class of drugs. The setting of the lactic acidosis is slow and the symptoms are not specific. 24
It is still not clear if the loss of mineral density is a component of this same syndrome. 22 The avascular necrosis has been considered as a complication of the HIVLS, since the hyperlipidemia and the HIV infection itself are known risk factors for osteonecrosis of the head of the femur. 21
The metabolic abnormalities are associated with increased risk of cardiovascular events. 17,24
The hyperinsulinemia associated with insulin resistance is a recognized risk factor in patients not infected by the HIV and can contribute to the increased risk of acute myocardium infarction in patients receiving ARV. 23
Therefore, HIV positive patients with a significantly higher tendency to increased levels of fasting glucose and triglycerides and low levels of HDL cholesterol have an increased risk of atherosclerosis, coronaropathy and diabetes mellitus. 23 The risk of developing diabetes is 6 to 10% on these patients and it is further increased on those patients who are obese, have co infection by the hepatitis C virus or a family history of DM. 24 There are reports of 16% increase on the incidence of myocardium infarction per year with antiretroviral treatment. 24
Amongst the anatomical alteration of fat redistribution three groups are identified: lipoatrophy, lipohypertrophy and mixed forms.
Lipoatrophy and lipohypertrophy can occur independently or together on the same patient. 24
In lipohypertrophy there is an accumulation of central or localized fat. The accumulation of fat can develop on the abdomen, on the cervical region, on the dorsum, on the breasts or in other places in a localized form. The abdomen develops a round shape and the fatty tissue is commonly deposited intraabdominally, in the viscera or in between them. The increment of the intra-abdominal pressure can predispose to abdominal hernias that, eventually, might need corrective surgery. 17
It has also been observed a lipid accumulation on the upper trunk extending to the axillas in male patients, as well as on the anterior cervical and suprapubic regions in both sexes. 22,24
The increment of the beast volume in female patients happens basically due to the fat component, without obligatory association with glandular hypertrophy. In males gynecomasty (glandular hypertrophy) or pseudo-gynecomasty (fat accumulation) can develop. 17
Lipohypertrophy is more associated with advanced age at the beginning of the treatment, higher body mass index and use of protease inhibitors. 24
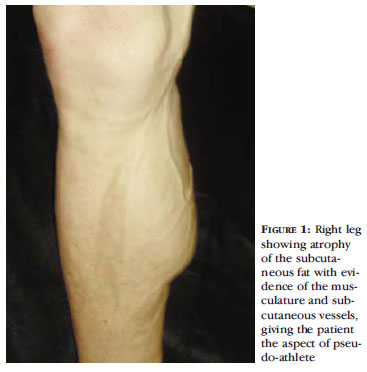
A peripheric loss of subcutaneous tissue is observed on the lipoatrophy. There is a narrowing of the upper and lower limbs, and the skin gets thinner, facilitating an almost anatomic visualization of the muscles groups and superficial blood vessels. These changes can give the patient pseudo-athletic appearance. (Figures 1 and 2). The evidence of the vascular pattern is also frequently mistaken by venous insufficiency (pseudo varicose veins). 17
For some authors the heterogeneity of the findings of the HIV associated lipodystrophy can reflect the existence of more than one syndrome. 23
2.3 DIAGNOSIS
There is not yet an universally accepted definition of HIVLS, which explains the difficulty in defining a case, as well as its prevalence, ethiology and treatment.
The most commonly used method used to define a case of lipodystrophy includes the subjective description of the body fat abnormalities. Two multicentric studies were performed aiming at defining a case of lipodystrophy. The Lipodystrophy Case Definition Study compared patients with and without clinical evidence of lipodystrophy, concordant between patients and doctors. Laboratory, anthropometric, and radiologic data, like dual X-ray absorptiometry (DEXA) and Computerized Tomography (CT) were compared between the two groups of patients. The generated definition of lipodystrophy had a sensibility and a specificity of 80%, but it happened to be more complex when used clinically. 25 The Fat Redistribution and Metabolic Changes Study in HIV Infection compared laboratory testings and anthropometric and radiologic data of body fat distribution amongst HIV infected patients and those not infected. This study showed that the only abnormality of body fat associated with HIV infection was the generalized lipoatrophy. This result does not explain the high prevalence of intra-abdominal obesity in HIV positive patients, but agrees with other studies that lipoatrophy is the hallmark body abnormality in HIV infected individuals. 26
Some diagnostic criteria were suggested during the First International Workshop on Lipoatrophy and Adverse Reactions to Drugs, which took place in San Diego, in June 1999. The clinical criteria described were the hollow face, depressed temples, hollow eyes, prominent zygomatic arch, emaciated aspect, prominent non-varicose veins on arms and legs, loss of cutaneous folds, loss of contour and of fat on the buttocks. The fat accumulation was categorized in 5 areas: increase of the abdominal circumference, pectoral enlargement, accumulation of dorsum-cervical fat, accumulation of facial fat (although possibly less common than facial lipoatrophy), and the presence of lipomas. The methods for evaluating and monitoring the fat include patients report, clinical evaluations, anthropometric measures and imaging exams. 21
Objective criteria for the diagnosis of lipodystrophy have not yet been established. The lack of standardized values in relation to fat on the general population and the heterogeneity of the clinical manifestations of lipodystrophy make this even harder. A gold-standard technique to measure body fat is not yet available. However, some methods have been utilized, like anthropometry, bioimpedance, DEXA, computerized tomography, magnetic resonance and ultrasound. 27
Anthropometry and impedanciometry can not measure regional fat. CT and MR are expensive methods, which restricts their use. The use of ultrasound is promising because it is simple, non-invasive, and available at low-cost, however it is more operatordependant that the other techniques. The fact that measures of absolute values of regional fat do not clarify the occurrence of abnormalities of the fat is also a limiting aspect. 27
High resolution magnetic resonance made possible the identification of a clear disorganization of the adipose tissue of HIV positive patients and the alterations on the tissue architecture seem to appear earlier than the alterations detected by DEXA or clinical examination. 28
The ultrasound showed a moderate concordance between its findings and the lipoatrophy reported by the patient or the doctor at clinical evaluation. According to some authors, the anatomy of the face, the age of the patient and the quality of the skin interfere with the way how the subcutaneous fat is perceived exteriorly. Even though, they still regard the ultrasound as a potential tool for the evaluation of the patients, keeping in mind its low cost, accessibility and absence of radiation. 29
In view of all these limitations, the reports of loss or accumulation of fat in specific areas and the determination of the intensity level clinically evaluated and concordant between doctor and patient remain the best way of defining the problem individually. 27
Most studies about lipodystrophic syndrome are based on the presence of subjective symptoms reported by patients, on the presence of clinical signs observed at physical evaluation by the doctor or on the combination of both. These observations may or may not be confirmed by diagnostic methods. 30
Objective measures of facial fat are even harder to obtain than body fat measures. A questionnaire of the FRAM study asked the patients to evaluate any change on facial fat on the cheeks area, close to the nose and mouth, and grade the changes from 1 to 6. A similar study of grading from 1 to 7 was used by health professionals to evaluate the fat on the region of the cheeks in each participant. A longitudinal ratio of the data obtained from the patients and the health professionals or, even, follow-up with serial photographs can be used, if the patient agrees. 26
The diagnosis of lipoatrophy is still frequently based on the perception of the patient and on the clinical evaluation, which has shown a good correlation. 31
2.4 EPIDEMIOLOGY
It is hard to evaluate the prevalence of HIVLS, as there is no clear definition of the disease, with well defined criteria for the characterization of a case, neither are there precise diagnostic methods for the detection of fat redistribution or the quantification of loss or gain of body fat.
As the condition is made up of various body morphology alterations, such as atrophy, hypotrophy or hypertrophy that might be present jointly or individually, it is difficult to place the patients in well defined groups.
The prevalence of lipodystrophy described on the literature varies greatly, with articles reporting rates of 7% to 84% amongst patients with HIV/AIDS, in use or not of antiretrovirals. Such variation is due to, possibly, the diagnostic criteria used, taking into consideration their lack of standardisation. 17,21,32,33
The prevalence, as a whole, of at least one body alteration is around 50%. 32,34,35
In a study by Cabrero and collaborators with 965 patients seen in 98 different health establishments showed that the perception by the patients of some body alteration was reported in 55,1% of the cases. As for the doctors perception of to patients' body alterations, it was reported in 55,2% of the cases. The most common alteration described was the lipoatrophy, reported by 46,8% of the patients and by 49,4% of the doctors, followed by lipohypertrophy. There was no difference on the perception of body alterations between men and women. The concordance in terms of the alterations between patients and doctors was 83%. 32
Hendrickson and collaborators also agreed that lipoatrophy is one of the manifestations most commonly associated with the use of ARV and reported a variation on its frequency between 13% to 63%. 36
Viskovic and collaborators consider lipoatrophy the most common and disfigurating of all the body alterations associated with the syndrome. From a total of 151 HIV-positive patients evaluated 39% reported lipoatrophy is some area, while 45% of the doctors noted the presence of fat loss at clinical examination. Amongst the patients, 11% reported facial lipoatrophy, while the doctors observed clinically detectable facial lipoatrophy in 15% of the patients. 37
2.5 PHYSIOPATHOGENESIS
The exact mechanism that leads to the development of the anatomic and metabolic alterations is not yet clear. Various hypothesis came up but none of them explains all the aspects of these alterations, which are probably multifactorial. Mitochondrial toxicity related to the use of NARTIs; deregulation of tumoral necrosis α (TNFα); inhibition of cytochrome p 450 related to the protease inhibitors; local effect of HIV on the production of cortisol and abnormalities of other steroid hormones have been cited, amongst others. 38
When HIVLS appeared it was initially thought to be associated with PI use, a frequent component of the ARVT. Studies suggested that PI mediated the lipoatrophy by changing the steroids regulator element, linking itself to protein 1, which is involved with the adipocyte differentiation.
Ledru and collaborators also showed that the PI have some effect on the cell protease, which contributes to accumulation of T cells, that produce TNFα. This seems to favour the lipodystrophy contributing to changes to the lipidic metabolism. Other authors also showed that the levels of TNFα and its receptors seem to be associated with the development of lipodystrophy in patients under ARVT. 39
More recently, the NARTIs, another frequent component of ARVT, have been implicated as causative of lipodystrophy.
Amongst the NARTIs, lipodystrophy is more associated with the use of stavudine and zidovudine. Lipoatrophy develops in 30% of the patients after 2 years of use of stavudine, but only in 6% of the patients using tenofovir. 24
The NARTIs deplete the mitochondrial deoxyribonucleic acid (DNA) inhibiting the DNA mitochondrial polymerase, which may result in the apoptosis of the adipocytes. It has been suggested that the timidine analoge NARTIs (stavudine, zidovudine) are more toxic to the mitochondrial DNA that the new non-timidine analoge NARTIs, like abacavir, although all the drugs of this class can cause depletion of the mitochondrial DNA. 24
Lipohypertrophy is more associated with the use of PI, although efavirenz, a NNARTIs, is involved with the development of pseudo-gynecomasty. Although the lipoatrophy is more related to NARTIs, efavirenz is also implicated with the progression of the lipoatrophy. 17
Pacenti and collaborators identified genes modulated by the PI and the NARTIs on early adipogenesis and propose that the regulation of transcription and modulation factors of the Wnt gene are the pathway by which the PI cause the inhibition of the adipocyte differentiation and the negative regulation of the expression of specific markers for adipocytes, like leptin, MRAP, Cd36/FAT and S100A8. The effect of the NARTIs on the differentiation of the adipocytes and on the profile of the genic expression was milder than that of the PI, although the NARTIs have shown some modulation on the expression of tissue inhibitors of metalloproteinase and transcription factors, like Aebp1, that can have a role on the determination of the adipocytes phenotype. The authors concluded that the abnormal expression of these genes can be the basis to the ARVT associated lipodystrophy. 40
The genetic predisposition is another important factor on the genesis of lipodystrophy. Ranade and collaborators identified a subgroup of patients that was especially vulnerable to the metabolic adverse effects caused by the ARVT. After genetic analysis they identified the resitine gene as being implicated on the susceptibility to HIV associated lipodystrophy. 41
The H halogroup of the mitochondrial DNA was also identified as having strong association with the presence of atrophy in patients treated with nucleoside analog reverse-transcriptase inhibitors. On the other hand the T halogroup showed marginal significance as a protective factor to the development of lipoatrophy on this same group of patients. 36
Some studies suggest that the fat redistribution and the metabolic abnormalities of the HIV infection are associated with alterations of the endocrine function of the adipose tissue. The adipose tissue, as well as acting on fat storage, is an active endocrine tissue and the biggest determinant of sensibility to insulin, modulating the glucose and lipidic metabolism through the secretion of adipocytokines.
Verkauskiene and collaborators showed that HIV-infected children with signs of body fat redistribution have low levels of adiponectine, associated with insulin resistance and dyslipidemia. On this study the concentration of leptin did not show significant effect on the body fat redistribution. 42
Lipoatrophy can occur in the absence of therapy with PIs or NARTIs, with studies suggesting that the antiretroviral drugs are not the only causative factor. On the HIV Outpatient Study, 1.077 patients were evaluated in relation to the alterations of body fat distribution. Lipoatrophy was associated with the use of indinavir, a PI, for more than 2 years and with any use of stavudine, a NARTI. However, independently, risk factors not associated with the use of the drugs were strongly associated with lipoatrophy, including advance age (> 40 years), white skin, CD4 count of < 100 cells/mm3, decrease of body mass index, and longest duration and severity of the HIV disease itself. The number of non-pharmacologic risk factors increased the probability of developing lipoatrophy. The results suggest that the cause of lipoatrophy is multifactorial and that it might be a result of long term HIV infection. The expression of tumoral necrosis factor α (TNFα) by subcutaneous adipocytes in vitro is higher amongst patients with lipoatrophy, and this suggests that the permanent activation of inflammatory cytokines in HIV infection can mediate the lipoatrophy. 35
Inteleukin 6 (IL-6) is a multifunctional cytokine that acts as inflammatory, immune and metabolic mediator. As such, its involvement in various events associated HIV infection is considered. Increased production of IL-6 in HIV infected patients and in use of antiretroviral therapy is known. 43 Saumoy and collaborators evaluated the influence of the 174G>C genotype of IL-6 on the risk of developing the fat redistribution syndrome in HIV infected patients in use of combined antiretroviral therapy. No significant difference was found on the distribution of IL-6 genotype amongst patients with and without the fat redistribution syndrome, and IL-6 genotype was not considered a modulating factor of the speed of the installation of this syndrome. The expression of IL-6 messenger RNA on the subcutaneous adipose tissue did not show significant difference amongst HIV-1 infected patients with or without the fat redistribution syndrome, although it was significantly higher on the control group of non-infected patients (p < 0,001). Amongst the infected patients the plasmatic level of IL-6 did not differ between patients with or without lipodystrophy (p = 1). There was also no significant difference on the plasmatic levels of IL-6 between patients that received or not timidine analogs (p = 0,8). 44
Other risk factors for HIV facial lipodystrophy already identified are the use of protease inhibitors, age, low CD4, high viral load, duration of ARV, white race and female sex. 45
Other not yet identified influences might also be associated with the development of HIVLS.
Whatever the HIVLS ethiology, be it drug therapy, genetic predisposition, immune reconstitution, cytokine activation, direct action from the HIV virus, hormonal influences, or other non indentified influences, the fact is that the fat loss is apparently irreversible. 46
3. FACIAL LIPOATROPHY
3.1 DEFINITION
Of all the areas involved by lipoatrophy, one of the most frequent components of syndrome, the face is the place where the fat loss is more evident and has a higher impact.
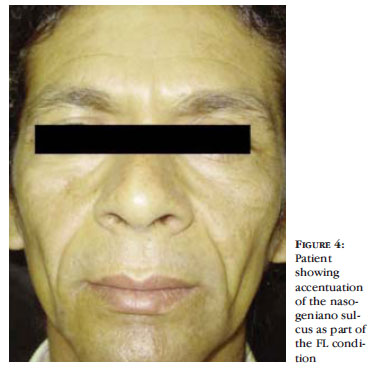
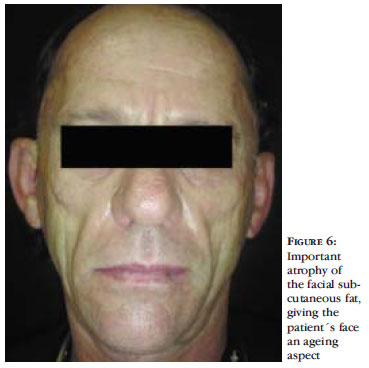
The facial lipoatrophy (FL) consists of progressive loss of the facial fat, mostly due to the decrease of the malar fat (Bichat fat) and of the temporal fat. The FL leads to the development of new cutaneous sulcus and the accentuation of expression sulcus, as well as areas of depression and evidenciation of the bone framework (Figures 3 to 6). All this leads to a wrinkling of the face and gives the patient an aspect of premature ageing and, in women, the loss of facial fat leads to a loss of the facial femininity. 47 Additionally, the thinned and gaunt face, with the aspect of a "diseased face", brings back the old stigma of the "AIDS face", together with the fear of involuntary revelation of the diagnosis. 27
3.2 CLASSIFICATION
The lack of criteria for the diagnosis and measuring of the fat loss on facial lipoatrophy is also a complicating factor in order to establish a classification of the severity of the disease.
The Facial Lipoatrophy Index (FLI) was developed by Brazilian doctors, based on the parameters used for the classification of the severity of psoriasis. This instrument aims at measuring the level of atrophy and the level of improvement with the treatment, in a objective way. 17
The FLI evaluates three facial regions (Figure 7). The malar region corresponds to the zygomatic and the buccal areas, being limited by the infraorbital border and the lower border of the mandible. Other anatomic structures considered are the zygomatic bone, the projection of the body of the mandible, the zygomatic major muscle, the canine fossa and the maxilla. 17
The temporal region corresponds to the anterior portion of the temporal fossa, limited by temporal line of the frontal bone and the zygomatic arch (zygomatic process of the temporal bone and the temporal process of the zygomatic bone). 17
The pre-auricular region corresponds to the masseter region, between the zygomatic arch and the angle and the lower border of the mandible. 17
The depth and the extension of the involved areas on the malar, temporal and pre-auricular regions are evaluated, separately. 17 The depth of the atrophic areas is graded from 0 to 4, 0 being the absence of atrophy, 1 mild, 2 moderate, 3 severe and 4 very severe. The extension of the involved area is graded from 0 to 5, 0 being the absence of involvement, 1 less than 20% of the evaluated area, 2 from 21% to 50%, 3 from 51% to 70%, 4 from 71% to 90% and 5 from 91% to 100%.
A partial number is calculated for each evaluated area, multiplying the grading related to the depth by the grading of the involved area, and yet by a correction factor.
The correction factor was specified for each facial region and corresponds to the level of importance of each one of them on the facial atrophy. The correction factors used are 0,7 for the malar region, 0,2 for the temporal and 0,1 for the pre-auricular.
Since the fat loss is not symmetrical, the most involved side is considered on the evaluation.
At the end, the partial grades of the three regions are added, coming to the final index.
The Ministry of Health uses a classification of facial lipoatrophy in grades I to IV, based on the FLI.17
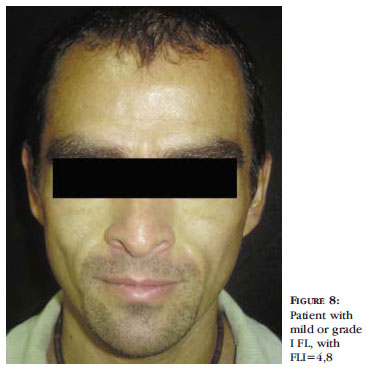
Grade I, or mild facial atrophy, corresponds the FLI from 0 to 5,9. In this case there is a mild depression but no evidence of the anatomic structures of the area neither loss of the facial contour. The skin is normal at digital pressure. (Figure 8).
Grade II, or moderate, corresponds to FLI 6,0 to 10. The depression is more visible, with beginning of visualization of the anatomic structures, specially the zygomatic arch, and increment of the nasal labial sulcus. There is no loss of facial contour or projection of the maxilla. At digital pressure the skin depresses normally but there is a delay in its return to rest status (Figure 9).
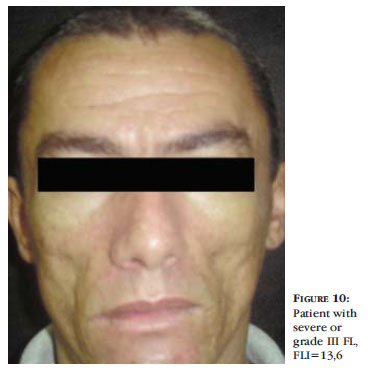
Grade III, or severe, corresponds to FLI 10,1 to 15. The structures of the malar region are well noted, like the zygomatic bone. There is also visualization of the canine fossa, partial visualization of the zygomatic major muscle and mild to moderate depression of the lower border of the mandible. Loss of facial contour and projection of the mandible might develop. At digital pressure, the skin depresses a little and takes longer to return to the rest status (Figure 10).
Grade IV, or very severe, corresponds to FLI of 15,1 to 20 (Figure 11). There is almost complete visualization of the anatomic contours, revealing the bone and muscular framework of the face. There is loss of the facial contour, with visualization of the upper and lower faces of the zygomatic arch on the temporal and pre-auricular regions. At digital pressure the skin almost does not depress.
The FLI can vary from 0 to 20, and the Ministry of Health preconises treatment for patients with FLI grading equal to or above 6.17
Other classifications are adopted on the international literature, all of them with some degree of subjectivity as they are evaluator-dependant
3.3 PSYCHOLOGIC IMPACT
Changes to body image can be extremely disturbing in terms of psychosocial wellbeing, enhancing the stigma of the disease. Despite also visible on the arms, legs and buttocks, lipodystrophy is more apparent on the face. 30
With the progression of the condition, many patients start to present with the typical face, characteristic of the lipodystrophic syndrome. This brought back the stigma of AIDS and a need for specialists that work with HIV/AIDS patients to identify these alterations and seek treatment options. 38, 48, 49
The patients have described the facial lipodystrophy as being a visible marker for the identification of HIV carriers, perceived as the "aids face" or, yet, the "Kaposi sarcoma of the 21st century". Additionally, this causes problems for family and social relationships which, in some cases, trigger disturbances on the social relations, leading to social isolation of the patients. One of most important consequences of the lipodystrophy is the abandonment of the treatment by the patients because of the psychosocial effects of the body fat redistribution. 16, 48
In view of the prevalence of the alterations of the fat redistribution, it is certain that the HIV related facial atrophy is becoming epidemic. It stigmatizes the affected individuals with great impact on the quality of life of these patients. Usually, these patients have a good control of the disease, but their facial appearance suggests the contrary. 45
The patients with facial lipoatrophy feel exposed and have no way of keeping their condition under control. This can result in discrimination at work and affect relationships, sexual function and even adherence to treatment. It influences the patients sense of well being, as well as their body image and self-esteem. In some cases the patients become socially isolated. 47
It is a fact that facial lipoatrophy has a great psychological impact and can reduce the patients complacency with the treatment. 50
3.4 TREATMENT
Due to the fact that the causes of HIV associated lipodystrophy are not well known, and that is still not evident how the syndrome develops, treatment trials are difficult to be outlined. Currently some treatments are available for facial lipodystrophy, and they can be conservative or interventionist, medicamental or surgical, with variable side effects.
3.4.1 CONSERVATIVE TREATMENTS
Amongst the conservative treatments of FL, the possibility of adjustment of the antiretroviral therapy, by using drugs which are less related to the development of HIVLS, has been discussed. The change of the antiretroviral therapy in response to lipoatrophy must be cautious due to the risk of a viral rebound or to adverse effects to the used drugs. 14
Some studies showed that the change of a timidine nucleoside analog reverse-transcriptase inhibitor to a non-timidine analog results in a discreet increase of the peripheric fat after 24 weeks, measured by computerized tomography and DEXA, although the effect was not clinically evident. 14 The change of stavudine for abacavir or tenofovir showed maintenance of the immunologic pattern, with the advantage of non progression of the anatomic alterations and, even, a discreet improvement. 17 The prolonged interruption of the treatment (more than 6 months), however, does not lead to a clinically evident improvement of the atrophy is some studies.
In any case, when changing the therapeutic regimens the doctor should consider the sensibility of the virus to the drugs and the severity of the disease, in addition to the potential risks of the drug therapy.14
Another possible modality of medicamental treatment is the use of antidiabetic agents.
The thiazolidinediones (rosiglitazone, pioglitazone) are antidiabetic agents that improve insulin resistance in type 2 diabetes. They can cause fat gain in some patients and can increase fat mass in some familial forms of lipoatrophy. Some studies show conflicting results in relation to gain of subcutaneous adipose tissue with the use of rosiglitazone. Large scale studies are necessary.14
Studies with metformine are also non-consensual and the majority of them have a short follow-up. Some data suggest decrease of subcutaneous fat with its use, including visceral and of the limbs, being more useful in patients with glucose disturbances. 51
Amongst the effects of the growing hormone (GH) the anabolic one stands out. The therapeutic use of GH started 49 years ago. Since 1985 the recombinant GH has been used, which allowed for hypophisary hormone replacement with less risks for the patient. The most common indications for the use of GH are deficitary growth, be it idiopathic or secondary, adults with deficiency or inefficiency of GH or loss of body weight due to AIDS. 52 The FDA approved a type of growing hormone to treat muscular loss in seropositives when they present with hormonal suppression. The use of this treatment in public health programs is limited by its high cost, around 36.000 dollars a year. The international literature reports that a short term treatment increases the total weight and the lean body mass, with consequent improvement of the physical capacity and quality of life. In HIV/AIDS the regimen to be employed and the duration of the treatment have not yet been defined. 53
Although they are usually used to treat loss of body mass, the anabolic steroids can, in some cases, decrease the subcutaneous fat and worsen the lipoatrophy in HIV. 14 However, Honda and collaborators observed a significant thickening of the soft tissues on the face on the 3rd and 6th months after receiving 5 units of recombinant GH daily for 6 months, in HIV-1 patients with moderate to severe facial atrophy. The authors concluded that GH is effective and relatively safe for the treatment of moderate to severe facial atrophy and that the cost-benefit ratio should be better discussed. 54
The fact that the benefits obtained with the use of recombinant GH do not last for more than 12 weeks after the interruption of the GH and the decrease of the sensibility to insulin, already compromised on the syndrome, also limit its use. 55
Some new medical treatments have been suggested for HIVLS, but they await more scientific studies in order to evaluate their true value for clinical use.
Leptin is an amino acid product from the human leptin gene. It regulates the energetic, neuroendocrine and functional homeostasis of the organism. The recombinant human leptin is an emerging therapeutical possibility for the lipoatrophy caused by its genetic deficiency, and it might have some indication for the HIV/AIDS associated lipoatrophy. 56
As one of the causes of lipoatrophy would be the mitochondrial toxicity caused by the ARV, anti-oxidants and mitochondrial co-factors could also be of some value on its prevention or treatment.
Nutritional orientation and physical exercise are adjuvant components of the treatment of the body and metabolic alterations of the HIVLS. Aerobic exercises decrease the level of TGC and cholesterol, specially the LDL and, by burning fat, they help revert some of the body alterations associated with central fat accumulation. Resistance exercises help with the muscular mass gain, improving the aspect of the thorax and upper and lower limbs, as well as being useful on the treatment of osteopenia. A diet rich in fibers and adequate in energy and proteins can prevent the development of deposits of body fat. 17 However, these measures have no impact on the lost facial subcutaneous fat.
3.4.2 SURGICAL TREATMENTS
It is currently believed that the changes of the body fat distribution associated with the antiretroviral therapy are irreversible and there are no therapeutic strategies, discussed in various studies, that can promote enough recovery of the adipose tissue to allow for a consistent clinical perception.
For the Formation and Information Foundation about HIV/AIDS treatments in Spain, the surgical treatment is the only one to revert the manifestations of lipodystrophy, be it atrophy, hypertrophy or a mixed case.57
A promising technique for the facial lipoatrophy consists of subcutaneous filling.
The use of cutaneous fillers was introduced in 1981, when bovine collagen started to be implanted onto the skin in order to minimize the facial wrinkles. Since then new materials have been developed to improve effectivity and safety parameters. 58
The ideal filler should be a non-toxic material, that does not induce hyper sensibility or foreign body reactions, that does not degenerate with the passing of time or induce calcification, that is inert and easily implantable. These substances must be biocompatible, must not induce allergic reactions, be easy to deal with and stable with time. Besides, the cost must be accessible to the patients.
3.4.2.1 CUTANEOUS FILLERS
The injectable fillers are, currently, important tools of the non-invasive arsenal of rejuvenation procedures, correction of acquired or congenital facial defects and, more recently, for the treatment of HIV/AIDS associated facial lipoatrophy.
According to its availability, chemical composition and degradation, the fillers can be classified as temporary or permanent, organic or inorganic, autologous or heterologous. 58
In relation to the duration, some studies use yet a third sub-group, that of the semi-permanent fillers. Some authors define the semi-permanent products as those with durability between 1 and 2 years. The permanent ones would then have duration of over 2 years and the temporary ones a duration of less than a year. 59
Some fillers, when implanted, increase the facial volume by filling and direct expansion of the receptive sites. It is the case of silicon, collagen and polyacrilamides. 59 Others also create volume directly, but stimulate a foreign body reaction on the tissue for a period of time, stimulating a progressive and durable collagen deposit. Examples of fillers in this category would be the PMMA, polylactic acid and calcium hydroxyapatite. 59
3.4.2.1.1 POLYMETHILMETHACRYLATE
Currently the PMMA stands out as a therapeutic option for FL because it is the product available at the Unified Health System, from the Ministry of Health, for the treatment of HIV/AIDS facial lipodystrophy.
When trying to find a more durable filler, a series of non-reabsorbable particles have been tested in rats, and the polymethilmethacrylate molecules came out as the best tolerated, with the lowest index of allergic reactions. 60
The acrylic acid and its derivates were well known since 1890, but it was only in 1901 that solid and transparent acrylic acid polymers were made available. 61
Polymethilmethacrylate was first synthetised in 1902. It was patented as Plexiglas in 1928 and its major medical application was as a bone cement. 62, 63 Initially available in the shapes of slats, in 1937 the material was also found as granules and mouldable powder. 61
The neurosurgeons started to use PMMA during the Second World War in cranioplasties, due to the strength and lightness of the material. PMMA is still being used on the reconstruction of cranial defects due to its excellent tissue compatibility, easiness of manipulation in surgeries, resistance, and radiolusccency of the material, as well its accessibility, low thermal and electrical conductance and lightness. 61 Already in 1946, PMMA represented approximately 95% of the prosthesis market.
Medical research progressed and PMMA also started to be used for the fixation of femoral orthopaedic prosthesis. The use of PMMA as bone cement was introduced by Charnley and Smith on the sixties. Since then it has been largely used in surgeries to fill the spaces between prosthesis and bones. 61
The inert chemistry and biocompatibility of polymethilmethacrylate were accepted since Jude introduced the first hip prosthesis made of polymethilmethacrylate in 1947. 64
The use in ophthalmology also brought new understanding of the PMMA. The first hard ophthalmic lenses from PMMA were made by Kevin Tuohey in 1948. Although PMMA has favourable optic properties, its low permeability to the oxygen limits its long term use. PMMA has been the standard material of the surgeries of intra-ocular lenses implantation since its introduction in 1949, by Harold Ridley. Although the material has being used for more than 40 years, the major problems are still a consequence of its relatively low surface energy, which can result in damage to the corneal endothelium like in post-operative adherence of inflammatory cells to the intra-ocular lenses. 61
Until now the PMMA continues to be used as bone cement in orthopaedics, as cranio-facial reparation material in neurosurgery, as intra-ocular lenses material in ophthalmology and as dental cement in odontology. 61,65
The PMMA molecule has been shown to be chemically inert and previous allergenic tests are not necessary, if it is not associated with other fillers. 66 Experiences in animals have shown that the keys to biocompatibility with the skin are the spheric shape of the particle, its smooth and regular surface and the size of the polymethilmethacrylate microspheres. 60,67 The size of the molecules is important because very small particles can be easily phagocytised and the bigger ones do not easily pass through size 26 needle. The repeated washing of the microspheres reduces the impurities and renders the product more tolerable by reducing the number of foreign body giant cells around the injected PMMA particles. 67
The injected microspheres provoke a tissue stimulus that induces a neoformation of collagen fibbers.
The tissue stimulation induced by the PMMA microspheres happens due to the discreet inflammatory process produced by monocytes, histiocytes and fibroblasts at the place of application and that can, later on, make collagen fibers. 68 Allen and collaborators, in a longitudinal study, checked the cellular reactions after the injection of inert implants. These reactions were followed by a series of events of variable magnitude. During the first 24 hours, neutrophils and small round cells predominate; in 48 hours there is the predominance of monocytes; in 7 days there is already the formation of foreign body type giant cells. In 2 weeks the cell response is already moderate; in 4 weeks the fibroblasts appear; in 6 weeks the collagen deposition is intensified; in 8 weeks the chronic inflammatory cells are disposed along a solid collagen deposition. From then on the cellular reaction to the foreign body stabilizes and in six months some giant cells and a mild degree of cellular response are present, and there is a conversion of the fibroblasts to fibro cells. From then on a higher permanency of the implant on the place has been observed and, in this context, the collagen compounds are mixed with PMMA microspheres, generating great expectations for the researchers and the medical community. 69
On the presentations internationally available the PMMA microspheres were initially suspended in gelatine. From the 578 patients that received the product initially, 15 developed granulomas in 6 to 18 months after application. They concluded therefore, that the impurities stimulated the macrophages and were the cause of the formation of the granulomas. Besides, some patients developed palpable nodulations that were attributed to the rapid absorption of the gelatine that carried the microspheres, which caused their agglutination. This vehicle was then substituted by a collagen solution that, being more viscous, proved to last longer in the tissue. After the application of the product in the deep dermis, the collagen is degraded by the body in 1 to 3 months and completely substituted by the patient's collagen in a similar period, warranting as a result an increase in volume. 70
The collagen used in foreign presentations is from bovine origin. The antigenicity of the bovine collagen is decreased by the action of a pepsin that removes the final most antigenic portion of the collagen molecule, without destroying the helicoidal nature of the collagen fibers. 71
The commercial presentations available internationally are a 20% suspension of purified PMMA microspheres, from 30 to 42 micrometers in diameter, in a solution of 3,5% bovine collagen. It also contains 0,3% of lidocaine that reduces the discomfort after the application. 67 This product was approved and made available in more than 50 countries since 1994, with an estimate of 400.000 patients treated since then, with a complication rate of 0,01%. It is commercialized under the name of Artecoll since 1996 on the European Union, since 1998 in Canada and since 1999 in Mexico. The product was approved by the FDA in October 2006, and it is commercialized under the name of Artefill, with a more uniform size of the spheres. 63
As the bovine collagen is a foreign protein, 3% of the patients can develop an immunologic reaction, possibly a type IV allergic reaction, although antibodies to bovine collagen can be demonstrated on the serum of the patients. This way, a previous allergic test is mandatory. A small amount of pure collagen solution, usually 0,05 to 0,1 ml, is injected intradermically on the forearm surface. The reading is done in 72 hours and, again, after 1 month. The presence of edema and/or erythema renders the test positive. Around 1,2% of the patients with negative test develop immunologic reaction in a subsequent application; therefore, a second test must be performed 30 days after the first. Some authors even suggest a new test for treatments after a period of 12 months. 70
The association of bovine collagen to PMMA increases the cost of the product substantially, which is a limiting factor in situations where great amounts of the filler are needed, as on the treatment of facial lipodystrophy, making it basically not viable for use in public health programs.
Most of the articles published on the international literature about PMMA implants refer to products with collagen association (Artecoll or Artfill).
The injectable product used in Brazil consists of microspheres of polymerized PMMA varying from 30 to 50 micromeres in size, involved in a magnesium carboxi-gluconate-hidrolactic gel. The microspheres /gel proportion is 3:10. It is available in 10 ml jars or in 1 to 3 ml ready to use syringes, kept under room temperature. 58,66 It was initially introduced in Brazil in 1996. As it does not contain any animal product in its structure, there is no need of a previous allergic test.
The PMMA implant has an immediate and long lasting result, being also a biocompatible and inert filler, which gives it the characteristics of a permanent implant.
As the PMMA microspheres are non biodegradable and too large to migrate or to be phagocytised by macrophages, a permanent tissue augmentation is expected, consisting of 80% of the volume of the autologue conjunctive tissue. However, animal studies using PMMA microspheres have shown conflicting results. Lemperle and collaborators suggest that the PMMA particles are resistant to phagocytosis and degradation and have no carcinogenic potential. This study attributes the resistance to phagocytosis to the smooth surface of the particles and reports that, after 4 months, a delicate fibrous capsule is formed around each particle, which prevents the moving of the implanted material. 60 However, McClelland and collaborators suggest that PMMA in heterologue collagen have the capacity to evoke immune response and that the microspheres are susceptible to phagocytosis and elimination. 72 A permanent and long lasting result is expected in any case.
The National Agency of Sanitary Surveillance (Agência Nacional de Vigilância Santitária - ANVISA) approved the use of PMMA for the treatment of the HIV facial lipodystrophy. 73 However, this product has also been used on the treatment of nasogeniano sulcus, correction of bone eminences atrophy, mainly malar and mentonian, on the Romberg's hemifacial atrophy, correction of nasal dorsum, correction of scars and of atrophic earlobe. 68
PMMA is used in many countries and the number of treated patients worldwide surpassed 250.000 patients in 2005. Amongst those, only 0,01% developed granuloma formation. 74,75
Brazil is one of the countries with the largest experience with the use of PMMA for cutaneous filling. Although very satisfactory results have been achieved on the treatment of FL with the PMMA implant, there are still few scientific papers on the issue. (Figure 12).
3.4.2.1.2 OTHER CUTANEOUS FILLERS
The literature describes the use of other fillers for the treatment of FL. In the United States and Canada some forms of injectable liquid silicone have been used with success to treat the HIV facial lipoatrophy. The term silicone was described for a family of polymers with one basic element: silicon. These polymers vary in their viscosity, from an oil to a jelly form. The pure silicone indicated for cutaneous filling is the syloxane, that is a class of chemical composts with alternate chains of silicone, oxygen and methane. The pure form, filtered and sterile, is the one recommended for use as filler. 76
The combination of puncture and deposit of silicone causes an inflammatory reaction with migration of the polymorphonucleocytes, followed by a moderate lymphocytic infiltrate. This infiltrate can be observed during six months. There is a mild phagocytic activity and a small number of giant cells can be evidenced that, in general, do not progress to the formation of granulomas. The small volumes of silicone injected soon accommodate on the deep dermis and subcutaneous tissue and are circundated by pseudo capsules of pre-existent collagen, that later brings on a fine capsule of neoformed collagen. 76
The immediate reactions are erythema, edema and eventually echimosis. Soon after the injection the development of small papules can happen on the site and they disappear after a few hours or in up to 3 weeks. There are reports of dyschromy but they are not frequent. Exaggerated elevation can happen due to excessive amount of volume applied and hypercorrection. The cases of granuloma formation are linked to the impurity of the material, injection of the product into an inadequate place or injection of large volumes. The migration of the silicone, which in many cases is the biggest fear of the professionals and patients, only happens when volumes over 1 ml are injected in the same place, which sometimes is necessary when treating FL. 76
In some studies, the liquid silicone seemed to be the treatment with the best cost-benefit ratio in the United States. 77 However, a longer follow-up of the patients is needed in order to ascertain the efficacy, durability, and long term safety of the injectable liquid silicone for the treatment of the HIV facial lipoatrophy. 77
A limiting factor in relation to this product is that its use is prohibited in many countries, including Brazil.
The polyacrylamide gel is a non-degradable, non-allergenic and non-toxic polymer composed by 96% non-pyrogenic water and 4% polyacrylamide. It is the only one of the fillers in which a thin capsular layer of collagen develops around the gel, isolating it from the host tissue. As a result of the encapsulation process the implant can be promptly identified and, if necessary, easily removed by expression of the capsule with the extrusion of the material from its interior. 78 Due to this fact the polyacrylamide is considered as an injectable prosthesis. 79
The polyacrylamide gel is non-degradable and maybe considered biologically inert. 79 The cosmetic effects of the filling with polyacrylamides are permanent, with no need of further treatments. 78
The high cost of the polyacrylamides renders the use of this filler basically non viable in public health. 78
The polylactic acid was the first filler to be approved by the FDA for the treatment of HIV facial lipoatrophy associated with the use of ARV. The decision of the FDA was based in 4 studies that documented the safety and efficacy of the product in 278 patients with facial lipoatrophy. 80
The polylactic acid is a synthetic polymer, biodegradable and immunologically inert. After being injected, the micro particles of polylactic acid can stimulate collagen production, which provides a gradual and progressive volume augmentation of the lipoatrophic area. 81 The polylactic acid is part of the alphahydroxiacids and it has been available for over 30 years for various uses in Medicine. 47
The polylactic acid is injected into the deep dermis with the objective of increasing the number and activity of fibroblasts, resulting in an enhanced collagen synthesis. It has two modes of action. Initially there is a temporary increase in volume of the treated area and it is essential that the patients are informed so as not to be disappointed when this initial volume is reduced. 47 The initial volume is created by the injection of an amount of sterile water used for the reconstitution of the polylactic acid, which is reabsorbed in 48 to 72 hours. 50 The second mode of action is the stimulus to the collagen formation.
Many sessions may be necessary before the desired effect on the contour of the face is noted. 47 The polylactic acid is completely degraded in nine months. 47
Carey and collaborators performed a randomised, multicentric study, with follow-up periods of 24 and 96 weeks, comparing adult patients with facial lipoatrophy induced by antiretrovirals that received injections of polylactic acid into the deep dermis with a control group. These authors showed that the treatment of facial lipoatrophy with polylactic acid in adult patients infected by the HIV promoted only a modest augmentation of the facial thickness but not of the facial volume. In contrast, the patients perception of the increment on well being, quality of life and cosmetic benefits was significant. The polylactic acid does not interfere with the fat loss in other areas of the body. The authors also emphasize that additional comparative studies are necessary in order to define the optimum treatment for HIV facial lipoatrophy. 81
However, because it is a biodegradable product, the effect of the polylactic acid is temporary and re-treatment is eventually necessary. Moreover, multiple sessions of applications are necessary to administer the polylactic acid. Besides, there are reports of subcutaneous nodules after the administration of the material. Adding to that is the high cost of this filler. So, other alternative options for the patient with facial lipoatrophy are important. 80
The hyaluronic acid is a polysaccharide, component of the soft tissues, and it is identical in all species and types of tissues. There are commercial presentations already approved by the FDA.
The injectable hyaluronic acid is obtained by ways of bacterial fermentation and there is a low incidence of adverse reaction. This incidence has dropped even more over the last years, from 1/1400 patients in 1999 to 1/1800 patients in 2000. This decrease is explained by the production of more purified forms of hyaluronic acid by the pharmaceutical industries. 82
The hyaluronic acid has been used successfully to treat HIV facial lipoatrophy. However, as in other temporary fillings, great volumes are frequently necessary in order to achieve a complete correction, which tends to decrease after 6 to 12 months. The high cost of injecting great volumes and the need for a repeated treatment are important limiting factors. 45
The gel of calcium hydroxyapatite is an injectable filler composed by 30% microspheres of calcium hydroxyapatite and 70% of an aqueous gel carrier. Despite being synthetic, its components are identical to the mineral portion of the bones and teeth. It is a biocompatible, non-toxic and non-antigenic material. 24
It was approved by the FDA in 2006 for the correction of the signs of facial fat loss in HIV patients. 24
This implant provides an immediate correction. The carrier gel is absorbed in few weeks leaving the microspheres that act as the matrix for the neocollagenesis and formation of new tissue. The great limiting factor for its use is also the high cost, adding to that the fact that it is a new filler and there are no long term studies.
As the HIV facial lipodystrophy is caused by the loss of the patients own subcutaneous fat, it would seem logical that the transference of autologous fat would be the most appropriate therapeutic option. A recent study reported 29 patients with HIV facial lipodystrophy that received transplant of autologous fat by the Coleman method. The technique was considered reliable and photographic records taken 6 months after the treatment showed durability of the fat implant. However, the authors noted that most patients with facial lipoatrophy associated with HIV do not have adequate fat donor areas, so they are not eli-gible for this procedure. 80 Jones performed the fat implant in 10 patients with facial lipoatrophy associated with HIV with the same methods and similar results. However, in almost all the cases the correction did not persist for more than 12 weeks. Another recent study also suggested that although autologous fat filling is effective in this condition, the patients with facial lipoatrophy associated with HIV have minimal fat donor areas and the treatment requires additional sessions of filling as time goes by. HIV patients generally lose the subcutaneous fat on the abdome and buttocks which are, usually, the fat donor sites. 80
Comparative studies of groups treated with different fillers available on the market must be done in order to better establish the cost-benefit of each pro duct; the high cost of most fillers limits their use for the FL treatment.
In an attempt to obtain new fillers to be used, above all, in treatments for facial rejuvenation, the tendency is that new products are developed and made available on the market. The cost of the recently released products, the durability of the materials and the existence of research that ensure their efficacy and safety are important factors that should support the use of these fillers in medical practice, particularly its use on the treatment of FL.
REFERENCES
- 1. Center for Disease Control and Prevention. Pneumocystis pneumonia - Los Angeles. Morbid Mortal Wkly Repor. 1981;30:250-2.
- 2. Center for Disease Control and Prevention. Kaposi's Sarcoma and Pneumocycstis Pneumonia Among Homosexual Men - New York City and California. Morbid Mortal Wkly Repor. 1981;30:305-8.
- 3. Sabino EC, Barreto CC, Sanabani S. AIDS: Etiologia e Subtipos do HIV. In: Veronesi R, Focaccia R, editores. Tratado de infectologia. São Paulo: Atheneu; 2005. p. 111-7.
- 4. Veronesi R, Focaccia R, editores. Tratado de infectologia. São Paulo: Atheneu; 2005. p.118-137.
- 5. Marques AR, Masur H. História Natural da Infecção pelo HIV. In: Veronesi R, Focaccia R, editores científicos. Tratado de infectologia. São Paulo: Atheneu; 2005. p.143-6.
- 6. Rizzo LV. Imunopatogênese. In: Veronesi R, Focaccia R, editores científicos. Tratado de infectologia. São Paulo: Atheneu; 2005. p.138-142.
- 7. Lomar AV, Diament D. Terapia Anti-retroviral. In: Veronesi R, Focaccia R, editores científicos. Tratado de infectologia. São Paulo: Atheneu; 2005. p. 235-241.
-
8World Health Organization (WHO). [Internet]. Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS epidemic update. Geneva, 2009. [cited 2010 Sep 7]. Available from: http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf
-
9World Health Organization (WHO). [Internet]. Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS epidemic update. Geneva, 2007. [cited 2008 Jul 21]. Available from: http://www.aids.gov.br/data/documents/storedDocuments/
- 10. Sadala MLA, Marques AS. Vinte anos de assistência a pessoas vivendo com HIV/aids no Brasil: a perspectiva de profissionais de saúde. Cad Saúde Publ. 2006;22:2369-78.
-
11Ministério da Saúde. [Internet]. Boletim Epidemiológico DST/AIDS. Ano IV nº 01.Brasília, 2007. [acesso 15 Jul. 2007]. Disponível em http://www.aids.gov.br/main.asp?ViewID
- 12. Brasil. Ministério da Saúde. Fundação Nacional de Saúde. Guia de Vigilância Epidemiológica. 5. ed. Brasília: Funasa; 2002. 842 p. (Aids/Hepatites Virais; vol. 1).
- 13. Brasil. Ministério da Saúde. Secretaria de Assistência a Saúde. Programa Nacional de DST/Aids. Consenso sobre a terapia anti-retroviral em adultos. Brasília: Ministério da Saúde; 1996.
- 14. Martinez E, Mocroft A, García-Viejo MA, Pérez-Cuevas JB, Blanco JL, Mallolas J, et al. Risk of Lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 2001;357:592-8.
- 15. Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51-8.
- 16. Collins E, Wagner C, Walmsley S. Psychosocial impact of the lipodystrophy syndrome in HIV infection. AIDS Read. 2000;10:546-51.
- 17. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, AIDS e Hepatites Virais. Manual de tratamento da lipoatrofia facial: recomen dações para o preenchimento facial com polimetilmetacrilato em portadores de HIV/AIDS. Série A. Normas e Manuais Técnicos. Série Manuais 81. Brasília: Ministério da Saúde; 2009. 44 p.
- 18. Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871-5.
- 19. Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Pseudo-Cushing's syndrome in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:68-72.
- 20. Valente AM, Reis AF, Machado DM, Succi RC, Chacra AR. HIV lipodystrophy syndrome. Arq Bras Endocrinol Metab. 2005;49:871-81.
- 21. Behrens GMN, Stoll M, Schimidt RE. Lipodystrophy Syndrome in HIV Infection: What is it, What Causes it and How Can it Be Managed? Drug Saf. 2000;23:57-76.
- 22. Gkrania-Klotsas E, Klotsas AE. HIV and HIV treatment: effects on fats, glucose and lipids. Br Med Bull. 2007;84:49-68.
- 23. Castelo Filho A, Abrão P. Alterações metabólicas do paciente infectado por HIV. Arq Bras Endocrinol Metab. 2007;51:93-6.
- 24. Finucane KA, Archer CB. Dermatological aspects of medicine: highly active antiretroviral therapy and the treatment of human immunodeficiency virus. Clin Exp Dermatol. 2010;35:107-9.
- 25. Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG, et al. An objective case definition of lipodystrophy in HIV-infected adults. Lancet. 2003;361:726-35.
- 26. Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860-9.
- 27. Milinkovic A, Martinez E. Current perspectives on HIV-associated lipodystrophry syndrome. J Antimicrob Chemother. 2005;56:6-9.
- 28. Josse G, Gensanne D, Aquilina C, Bernard J, Saint-Martory C, Lagarde JM, et al. Human immundeficiency vírus atrophy induces modification of subcutaneous adipose tissue architecture: in vivo visualization by high-resolution magnetic resonance imaging. Br J Dermatol. 2009;160:741-6.
- 29. Viskovic K, Richman I, Klasnic K, Hernandez A, Krolo I, Rutherford GW, et al. Assessment of Ultrasound for Use in Detecting Lipoatrophy in HIV-Infected Patients Taking Combination Antiretroviral Therapy. AIDS Patient Care STDS. 2009;23:79-84.
- 30. Baril JG, Junod P, Leblanc R, Dion H, Therrien R, Laplante F, et al. HIV-associated lipodystrophy syndrome: A review of clinical aspects. Can J Infct Dis Med Microbiol. 2005;16:233-43.
- 31. Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, et al. Fat distribution in men with HIV infection. J Acquire Immune Defic Synd. 2005;40:121-31.
- 32. Cabrero E, Griffa L, Burgos A; HIV Body Physical Changes Study Group. Prevalence and Impact of Body Physical Changes in HIV Patients Treated with Highly Active Antiretroviral Therapy: Results from a Study on Patient and Physician Perceptions. AIDS Patient Care STDS. 2010;24:5-13.
- 33. Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845-56.
- 34. Carter VM, Hoy JF, Bailey M, Colman PG, Nyulasi I, Mijch AM. The prevalence of lipodystrophy in an ambulant HIV-infected population: It all depends on the definition. HIV Med. 2001;2:174-80.
- 35. Carr A. HIV lipodystrophy: Risk factors, pathogenesis, diagnosis and management. AIDS. 2003;17(Suppl 1):S141-8.
- 36. Hendrickson SL, Kingsley LA, Ruiz-Pesini E, Poole JC, Jacobson LP, Palella FJ, et al. Mitochondrial DNA Halogroups Influence Lipoatrophy After Highly Active Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2009;51:111-6.
- 37. Bugge H, Negaard A, Skeie L, Bergersen B. Hyaluronic acid treatment of facial fat atrophy in HIV-positive patients. HIV Med. 2007;8:475-82.
- 38. Li HY, Silva ACCM, Santos S. Síndrome Lipodistrófica e HIV/AIDS. J Bras AIDS. 2002;3:23-35.
- 39. Ledru E, Christeff N, Patey O, de Truchis P, Melchior JC, Gougeon ML. Alteration of tumor necrosis factor- T-cell homeostasis following potent antiretroviral therapy: contribution to the development of human immunodeficiency virus-associated lipodystrophy syndrome. Blood. 2000;95:3191-8.
- 40. Pacenti M, Barzon L, Favaretto F, Fincati K, Romano S, Milan G, et al. Microarray analasys during adipogenesis identifies new genes altered by antiretroviral drugs. AIDS. 2006;20:1691-1705.
- 41. Ranade K, Geese WJ, Noor M, Flint O, Tebas P, Mulligan K, et al. Genetic analysis implicates resistin in HIV lipodystrophy. AIDS. 2008;22:1561-8.
- 42. Verkauskiene R, Dollfus C, Levine M, Faye A, Deghmoun S, Houang M et al. Serum Adiponectin and Leptin Concentrations in HIV-Infected Children with Fat Redistribution Syndrome. Pediatric Research. 2006;60:225-30.
- 43. Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480-4.
- 44. Saumoy M, López-Dupla M, Veloso S, Alonso-Villaverde C, Domingo P, Broch M, et al. The IL-6 system in HIV-1 infection and in HAART-related fat redistribution szndromes. AIDS. 2008;22:893-903.
- 45. Baril JG, Junod P, Leblanc R, Dion H, Therrien R, Laplante F, et al. HIV-associated lipodystrophy syndrome: A review of clinical aspects. Infect Dis Med Microbiol. 2005;16:233-43.
- 46. Jones D. HIV Facial Lipoatrophy: Causes and Treatment Options. Dermatol Surg. 2005;31:1519-29.
- 47. Kavouni A, Catalan J, Brown S, Mandalia S, Barton SE. The face of HIV and AIDS: can we erase the stigma? AIDS Care. 2008;20:485-7.
- 48. Paton NI, Earnest A, Ng YM, Karim F, Aboulhab J. Lipodystrophy in a cohort of human immunodeficiency virus-infected asian patients: prevalence, associated factors, and psychological impact. Clinical Infectious Disease. 2002;35:1244-9.
- 49. Pujol RM, Domingo P, Xavier-Matias-Guiu, Francia E, Sanbeat MA, Alomar A, et al. HIV-1 protease inhibitor associated partial lipodystrophy: clinicopathologic review of 14 cases. J Am Acad Dermatol. 2000;42:193-8.
- 50. Narins RS. Minimizing adverse events associated with poly-L-latic acid injection. Dermatol Surg. 2008;34(Supp1):S100-4.
- 51. Wohl DA, Brown TT. Management of Morphologic Changes Associated with Antiretroviral Use in HIV-Infected Patients. J Acquir Immune Defic Syndr. 2008;49(Supply 2):S93-100.
- 52. Wannmacher L. Hormônio de crescimento: uma panacéia? Organização Pan-Americana da Saúde/Organização Mundial da Saúde - Brasil. 2006;3:1-6.
- 53. Yin MT, Glesby MJ. Recombinant human growth hormone therapy in HIV-associated wasting and visceral adiposity. Expert Rev Anti Infect Ther. 2005;3:727-38.
- 54. Honda M, Yogi A, Ishizuka N, Genka I, Gatanaga H, Teruya K, et al. Effectiveness of Subcutaneous Growth Hormona in HIV-1 Patients with Moderate to Severe Facial Lipoatrophy. Intern Med. 2007;46:359-62.
- 55. Spinola-Castro AM, Siviero-Miachon AA, Silva MTN, Guerra-Junior GO papel do hormônio de crescimento no tratamento dos distúrbios endócrino-metabólicos do paciente com a síndrome da imunodeficiência adquirida (Aids). Arq Bras Endocrinol Metab. 2008;52:818-32.
- 56. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative Review: The Role of Leptin in Human Physiology: Emerging Clinical Applications. Ann Intern Med. 2010;152:93-101.
- 57. Fundación para la Formación e Información sobre Tratamiento en el VIH/sida(FIT). Tratamiento quirurgico de la lipodistrofia asociada a la infección por VIH. Conclusiones de uma Reunión Multidisciplinar. Enferm Infecc Microbiol Clin. 2007;25:26-38.
- 58. Odo MEY, Chichierchio AL. Práticas em Cosmiatria e Medicina Estética - Evolução dos Implantes e Toxina Botulínica.1. ed. São Paulo: Tecnopress; 2000.
- 59. Sturm LP, Cooter RD, Mutimer KL, Graham JC, Maddern GJ. A Systematic Review of Permanent and Semipermanent Dermal Fillers for HIV-Associated Facial Lipoatrophy. AIDS Patient Care STDS. 2009;23:699-714.
- 60. Lemperle G, Ott H, Charrier U, Hecker J, Lemperle M. PMMA microspheres for intradermal implantation. I. Animal Research. Ann Plast Surg. 1991;26:57-63.
- 61. Frazer RQ, Byron RT, Osborne PB, West KP. PMMA: an Essential Material in Medicine and Dentistry. J Long Term Eff Med Implants. 2005;15:629-39.
- 62. Alster TS, West TB. Human-derived and new synthetic injectable materials for soft-tissue augmentation: Current status and role in cosmetic surgery. Plast Reconstr Surg. 2000;105:2515-25.
- 63. Lemperle G, Gauthier-Hazan N, Lemperle M. PMMA microspheres (Artecoll) for long-lasting correction of wrinkles: Refinements and statistical results. Aesthetic Plast Surg. 1998;22:356-65.
- 64. Cohen SRMD, Holmer REMD. A long-lasting injectable wrinkle filler material: report of a controlled, randomized, multicenter clinical trial of 251 subjects. Plast Reconstr Surg. 2004;114:964-76.
- 65. Reichnberger MA, Stoff AF, Ritcher D. Polymethilmethacrylate for managing frontal bone deformities. Aesth Plast Surg. 2007;31:397-400.
- 66. Carvalho Costa IM, Salaro CP, Costa MC. Polymethylmethacrylate facial implant: a successful personal experience in Brazil for more than 9 years. Dermatol Surg. 2009;35:1221-7.
- 67. Lemperle F, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354-66.
- 68. Wolfram D, Tzankov A, Pisa-Katzer H. Surgery for Foreign Body Reactions due to Injectable Fillers. Dermatology. 2006;213:300-4.
- 69. Allen O. Response to subdermal implantation of textured microimplants in humans. Aesthetic Plast Surg. 1992;16:227-30.
- 70. Haneke E. Polymethyl methacrylate microspheres in collagen. Semin Cutan Med Surg. 2004;23:227-232.
- 71. Lemperle G, Hazan-Gauthier N, Lemperle M. PMMA microspheres (Arte cool) for skin and soft tissue augmentation. II. Clinical investigations. Plast Reconstr Surg. 1995;96:627-34.
- 72. McClelland M, Egbert B, Hanko V, Berg RA, DeLustro F. Evaluation of artcoll ploymethylmethacrylate implant for soft-tissue augmentation: biocompatibility and chemical characterization. Plast Reconstr Surg. 1997;100:1466-74.
- 73. Ministério da Saúde. Programa Nacional de DST/AIDS. Secretaria de Vigilância em Saúde. Tratamento da lipoatrofia facial em pacientes de HIV/AIDS com polimetil metacrilato (PMMA). Brasília: Ministério da Saúde; 2006.
- 74. Gelfer A, Carruthers A, Carruthers J, Jang F, Berstein S. The natural History of Polymethylmethacrylate Microspheres Granulomas. Dermatol Drug. 2007;33:614-20.
- 75. Carruthers A, Carruthers J. Polymethylmethacrylate Microspheres / Collagen as an tissue augmentation agent: Personal experience over 5 years. Dermatol Surg. 2005;31:1561-4.
- 76. Pinheiro AMC, Oliveira Filho J, Costa IMC. Preenchimentos Cutâneos: Principais Preenchedores Cutâneos: Indicações e Técnicas. In: Gadelha AR, Costa IMC. Cirurgia Dermatológica em Consultório. São Paulo: Atheneu; 2009. p. 527-48.
- 77. Orentreich D, Leone AS. A case of HIV-associated facial lipoatrophy treated with 1000-cs liquid injectable silicone. Dermatol Surg. 2004;30:548-51.
- 78. Loutfy MR, Raboud JM, Antoniou T, Kovacs C, Shen S, Halpenny R, et al. Immediate versus delayed polyalkylimide gel injections to correct facial lipoatrophy in HIVpositive patients. AIDS. 2007;21:1147-55.
- 79. Jones DH, Carruthers A, Fitzgerald R, Sarantopoulos GP, Binder S. Late-Appearing Abscesses after Injections of Nonabsorbable Hidrogel for HIV-Associated Facial Lipoatrophy. Dermatol Surg. 2007;33(Suppl 2):S193-8.
- 80. Carruthers J, Carruthers A. Facial and Tissue Augmentation. Dermatol Surg. 2005;31:1604-12.
- 81. Carey DL, Baker D, Rogers GD, Petoumenos K, Chuah J, Easey N, et al. A randomized, open-label study of poly-L-lactic acid for HIV-1 facial lipoatrophy. J Acquir Immune Defic Syndr. 2007;46:581-9.
- 82. Denton AB, Tsaparas Y. Injectable hyaluronic acid for the correction of HIV-associated facial lipoatrophy. Otolaryngol Head Neck Surg. 2007;136:563-7.
Publication Dates
-
Publication in this collection
01 Dec 2011 -
Date of issue
Oct 2011
History
-
Received
12 Nov 2010 -
Accepted
28 Nov 2010