ABSTRACT
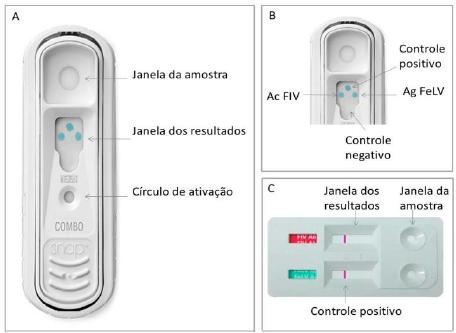
FIV and FeLV are Retrovirus associated mainly with feline neoplasms. Two point-of-care tests are commercially available in Brazil for diagnosis of these infections: a bidirectional flow immunochromatography kit (IDEXX SNAP ® Combo) and a lateral unidirectional flow immunochromatography kit (ALERE/BIONOTE Anigen Rapid). The aim of this study was to compare SNAP ® and ALERE tests. Blood samples obtained from 178 cats were evaluated using both tests. Quantitative real-time polymerase chain reaction (qPCR) was used as confirmatory test for all samples. The sensitivity and specificity of SNAP ® test was 100% for FIV, and for ALERE test was 96.15% and 98.68%, respectively. The sensitivity and specificity for FeLV was 93.02% and 96.30% for SNAP ® test and 90.70% and 97.78% for ALERE test. Three samples with a qPCR positive result for FeLV obtained a false negative result in both SNAP ® and ALERE tests. There was no statistically significant difference between the two methods. Considering qPCR as gold standard method, the SNAP® test showed higher sensitivity and specificity for FIV, and the ALERE test presented higher specificity for FeLV. The results showed good agreement among the tests.
Keywords:
feline retrovirus; FIV; FeLV; serological diagnosis