Abstract
PURPOSE:
To determine whether tension in the spermatic cord of rats causes lesions in the testis, epididymis or vas deferens.
METHODS:
Forty Wistar rats were randomly allocated into four groups. A traction force of 1.6 Newton (N) in group I and 1 N in group II was applied to the right spermatic cord. Group III was the sham, and group IV served as the control.
RESULTS:
Testicular lesions occurred on the right side in 66.7% of the rats and on the left side in 46.1% of the rats. The testes showed a decreased number of Sertoli cells, necrosis and a decreased number of germ cells in the seminiferous tubules. Anatomopathological changes in the vas deferens were not identified. There was no decrease in the thickness of the muscle wall of the vas deferens. In the right epididymis, 71.8% of the animals showed a reduction and 5% showed an absence of intraluminal sperm. In the left epididymis, 37.5% of the rats showed a reduction. The volume and the final testicular weight of the right side in group IV was different from those in the other groups.
CONCLUSIONS:
Anatomopathological lesions were found in the testis and epididymis.
Spermatic Cord; Vas Deferens; Testis; Orchiopexy; Hernia, Inguinal; Rats
Introduction
Diseases in which the surgical treatment involves handling and intraoperative traction of the spermatic cord and of the testis include indirect inguinal hernias and cryptorchidism.
Hernias may be defined as the protrusion of an organ or part of an organ or tissue through an abnormal opening in the wall of the cavity containing them. In the case of indirect inguinal hernias, this protrusion is formed inside a hernia sac in the spermatic cord. For repair of indirect inguinal hernias, the hernia sac is pulled along with the spermatic cord in order to be dissected and ligated at the level of the deep inguinal ring.
Cryptorchidism is a condition in which the testis has not completely descended to its anatomical position within the scrotum; however, it has not deviated from the normal path for descent of the gonad. In orchidopexy, the spermatic cord is pulled and dissected to allow testicular mobilization to the scrotum. From a surgical point of view, the limiting factor to place the testis in the scrotum is the length of the spermatic vessels. If the testis is fixed under tension, these vessels may be damaged and may cause testicular atrophy11. Thorup J, Haugen S, Kollin C, Lindahl S, Läckgren G, Nordenskjold A, Taskinen S. Surgical treatment of undescended testes. Acta Paediatr. 2007 May;96(5):631-7. PMID:17381472..
The aim of this study was to determine whether tension in the spermatic cord of rats causes lesion in the testis, epididymis or vas deferens.
Methods
This study was approved by the Animal Research Ethics Committee of the Federal University of Minas Gerais (UFMG), with protocol number 86/2012. Animal handling followed the guidelines recommended by the Brazilian College of Animal Experimentation (COBEA). Forty adult male albino Wistar rats (128.8 ± 3.5 days), weighing on average 424.7 ± 37.9 g, were used and randomly allocated into four groups of 10 animals. The animals were fed a specific diet (Nuvital(r)) and water ad libitum.
The animals were anesthetized with intramuscular injections of 10% ketamine hydrochloride at a dose of 60 mg/kg of body weight and xylazine at a dose of 10 mg/kg of body weight.
Surgical access was made through a suprascrotal incision. The right side was chosen for the procedure and the left side for the control. The right spermatic cord, testis and epididymis were exposed through this incision. A window was created in the right spermatic cord between the vas deferens and the testicular vessels, near the epididymis. A Kenzaki Release 11(r) carbon steel fishing hook with a barbless shank and a barbless point was introduced into this window. A 1-m-long thread of 2.0 monofilament nylon was used to tie the hook to the dynamometer (Figure 1), first passing through a simple pulley (EQOO8.56, CIDEPE(r)).
The hook was introduced into the window created in the right spermatic cord and is tied to surgical thread in order to help with the traction process.
The dynamometer and the pulley were fixed on a 46.5 cm x 62 cm magnetic board (TBF-106, MINIPA(r)) and assembled on a 97 cm x 85 cm x 34 cm multiuse demonstration structure (TBF-100, MINIPA(r)) with a 93 cm x 32 cm shelf (TBF-101, MINIPA(r)). A traction force measured through the dynamometer was continuously applied to the right spermatic cord distally and in the horizontal direction for five minutes (Figure 2). The gubernaculum was not sectioned. Subsequently, the organs were repositioned in the scrotum, and the surgical wound was closed with 4.0 monofilament nylon thread. The left spermatic cord of the rats was not surgically manipulated in the 1st surgery and therefore, not subjected to traction force.
Preoperative traction of the right spermatic cord. The arrow indicates the direction of the stretching force.
In group I (n=10), a perioperative traction force of 1.6 Newton (N) was imposed on the right spermatic cord. In group II (n=10), a traction force of 1 N was applied to the right spermatic cord. The value of 1 N equals 0.10197 kilograms-force (kgf). Group III (n=10) served as the sham group and was subjected to the aforementioned procedures; however, no traction force was applied to the right spermatic cord. Group IV (n=10) was used as the control group and did not undergo the 1st surgical intervention; hence, this group's right spermatic cord was not tractioned.
The dimensions and the initial volume of the right and left testes were measured using ultrasonography during the preoperative period before starting the surgery by a veterinarian specialized in diagnostic imaging using a portable GE(r) Logic E ultrasound device.
Another measurement of the dimensions and the final volume of each testis using ultrasound was performed in all rats from group I 24 to 36 days after the 1stsurgery, when the traction force was applied to the right spermatic cord. Then, 28 to 36 days after the 1st operation, all animals from group I were subjected to a second surgery. The testes, epididymis and vas deferens were removed from their origin in the cauda epididymis in this surgery. The left and right testes were weighed after dissection and excision of the epididymis. Subsequently, the animals were euthanized with an overdose of anesthetic that was three times the conventional dose; sodium pentobarbital was administered at a dose of 150 mg/kg intraperitoneally.
The organs were fixed in 10% buffered formalin. Paraffin blocks were sectioned to yield 4 µm-thick slices, and the slides obtained were stained with hematoxylin and eosin (HE).
The study of histological parameters was performed using light microscopy and a Holtermann(r) microscope reticle with a scale of 0 to 10 mm with 0.05 mm divisions. The anatomopathological analysis and interpretation were performed by a physician specialized in anatomic pathology and cytopathology.
Each testis was examined and given a histological grade from zero to six, based on the seminiferous tubule assessment method described by Kolbe et al.22. Kolbe A, Sun CC, Hill JL. Unpredictability of capsulotomy in testicular torsion. J Pediatr Surg. 1987 Dec;22(12):1105-9. PMID:3440895..
The initial 3 cm of the vas deferens from its origin in the cauda epididymis was sectioned, and six to eight cut levels were observed in each section. The vas deferens of rats undergoes changes in its structure over its entire length; therefore, the histological characteristics of its proximal, junctional, distal and terminal regions33. Hamilton DW, Cooper TG. Gross and histological variations along the length of the rat vas deferens. Anat Rec. 1978 Apr;190(4):795-809. PMID:637322. were examined to measure the external diameter, the thickness of the muscle layer and the luminal diameter of this organ. Thus, cut levels of the vas deferens wall with similar regional histology characteristics were measured. Four and two levels of cuts were selected to measure the vas deferens of the distal and proximal regions, respectively. It is noteworthy that the means of P1, P2, P3 and P4 were evaluated for the distal regions and the means between P1 and P2 for the proximal regions. The luminal diameter was measured from the apex of the columnar cell from one side to the apex of columnar cell of the other side on the longer axis. The external diameter was measured on the longest axis of the sections. Muscle thickness was measured from the base of the epithelium to the outermost point of the muscle at two points of each level chosen, considering the larger and thinner points of the muscle wall.
The criteria for abundance, reduction or absence of sperm in the lumen, presence of microabscesses, necrosis and interstitial inflammation were analyzed in the epididymis.
A significance level of 5% was used. Comparisons of the characteristics evaluated and of the groups were performed using the F test (ANOVA) when the usual model assumptions were met and Kruskal-Wallis when they were not. When a significant difference between groups was found, one of several techniques for multiple comparisons between means was used to find the differences. Was calculated the testicular volume variation (final testicular volume - initial testicular volume) and variation in the testicular volume compared to the inicial measurement [(final testicular volume - initial testicular volume) / initial testicular volume].
Results
One animal from group IV was excluded due to granulomatous orchitis diagnosed in the right testis on histopathological examination. Therefore, 39 rats were considered in this study. Group IV had nine rats.
Testicular volume measured using ultrasonography
A significant difference was observed when comparing the testicular volume between groups only for the right side, p-value ≤ 0.05 for Tukey's test and 0.05/6 = 0.008 for the Bonferroni test (Table 1). Thus, at least two groups differed with respect to this characteristic. The ANOVA F statistic showed no significance for the initial testicular volume.
Group IV had a higher final testicular volume than groups I and III and smaller (disregarding the sign) changes in testicular volume variation and variation in the right testicular volume (RTV) compared to the inicial measurement than groups I and III. Moreover, the testicular volume variation in group I (-0.8 ± 0.5) was higher than in group II (-0.3 ± 0.2).
Testicular weight
A significant difference (p-value ≤ 0.05) was found between groups for the testicular weight on the right side (Table 2). Thus, at least two groups differed with respect to this characteristic. The final testicular weight of the left side did not differ between groups.
Tukey's test showed that the final testicular weight on the right side in group IV was significantly higher than in the other groups (I, II, III).
Evaluation of the testes according to Kolbe's histological grading
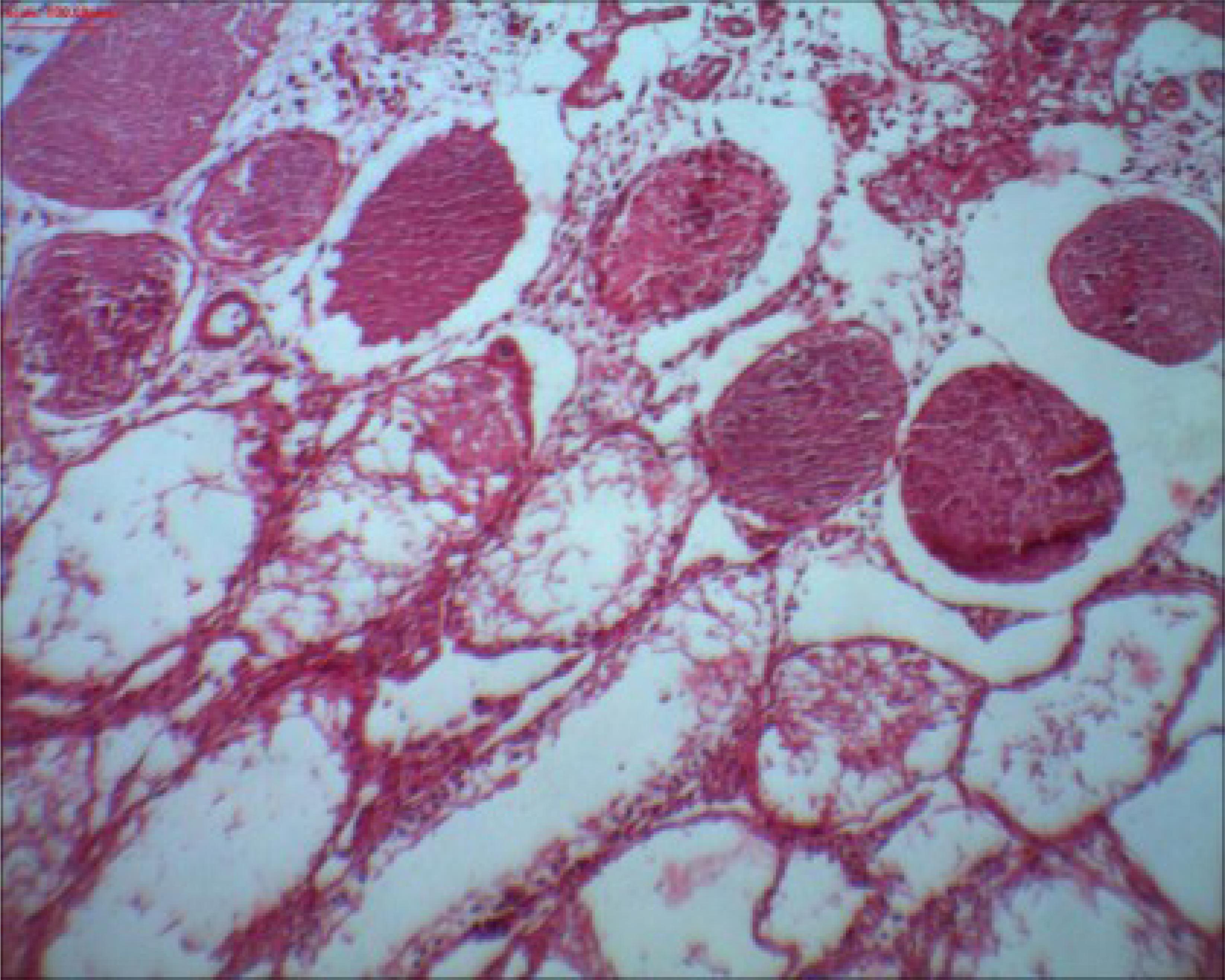
Thirteen (33.3%) rats showed normal histology in the right testis, and 26 (66.7%) had testicular histopathological lesions with progressive severity (Figure 3). Twenty-one (53.9%) rats had normal histology of the left testis, and 18 (46.1%) showed testicular histopathological lesions with progressive severity.
Right testicle of rat 9 from group II showing a reduction in the number of germ cells in the seminiferous tubules. Note the presence of Sertoli cells. Grade 1 according to Kolbe's histological classification. HE Staining. x40 magnification.
In the comparison between the groups and the classification of the testes between the two sides according to Kolbe's histological grading (Table 3), the ANOVA F statistic was statistically significant (p ≤ 0.05); thus, Tukey's test was used to determine the differences between each group two by two. When the right testis was classified according to Kolbe's histological grading, group I (2.6 ± 0.8) had a higher classification than the other groups (II, III and IV), and groups II (1.1 ± 0.7) and III (1.3 ± 1.1) had a higher classification than group IV (0.1 ± 0.3).
On the left side, group I (1.0 ± 0.0) had a higher classification than groups III (0.1 ± 0.3) and IV (0.0 ± 0.0). Group II (0.7 ± 0.5) also obtained a higher classification than groups III (0.1 ± 1.1) and IV (0.0 ± 0.0).
Histological testicular necrosis
The comparison of histological testicular necrosis on the right side between the groups is shown in Table 4. None of the 39 rats showed testicular necrosis on the left side; therefore, it was not possible to compare them. However when evaluating the right side, 50% of rats showed necrosis in group I (Figure 4), 40% of rats showed focal and sparse necrosis in group III, and groups II and IV showed no cases of necrosis.
Right testicle of rat 1 from group I showing multiple seminiferous tubules with grade 4 necrosis according to Kolbe's histological classification. HE Staining. x100 magnification.
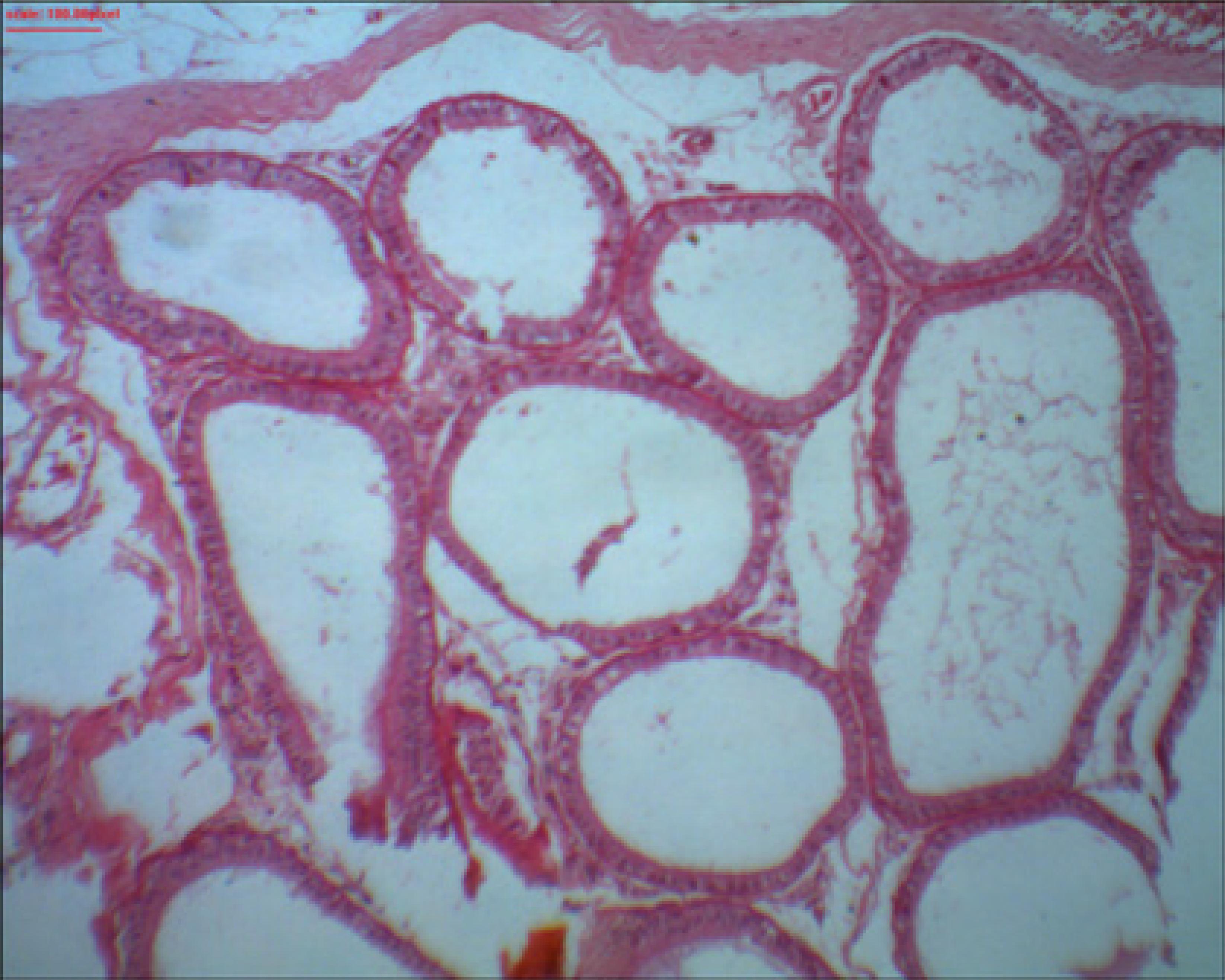
Presence of sperm in the lumen of the epididymis
The comparison of the presence of sperm in the lumen of the epididymis on the right and left sides between the groups is shown in Table 5. There was a significant difference (p-value ≤ 0.05) in the abundance and reduction of intraluminal sperm on both sides.
Groups I (80%) and II (60%) had the largest reduction in intraluminal sperm on the left side. On the right side, groups I (90%), II (100%) and III (90%) had the greatest reductions (Figure 5). Group IV remained constant, i.e., there was no sperm reduction. The abundance of intraluminal sperm is similarly interpreted.
Right epididymis of rat 1 from group I showing the absence of intraluminal sperm. HE staining. x100 magnification. Microabscess, necrosis and interstitial inflammation were not found in the epididymis.
An association of the absence of intraluminal sperm on the left side could not be quantified due to the high number of zeros, and the right side was not statistically significant at the 5% alpha level.
Dimensions of the vas deferens wall
No statistical significance was observed in the proximal region of the right side; however, the group IV showed greater luminal diameter of the distal region of the right side than groups I, II and III. Group I (453.1 ± 96.8) exhibited greater muscle thickness of the distal region of the right side than group IV (386.8 ± 63.7).
There was a difference in the luminal diameter of the proximal region of the left side in group II in relation to groups I and IV. Group II (400.0 ± 66.7) showed a lower luminal diameter than the rats in groups I (570.0 ± 107.2) and IV (555.6 ± 162.9). There was a difference in the muscle thickness of the distal region of the left side in group I in relation to groups II and IV, revealing that the muscle thickness in group I was higher than in these two other groups (Table 6).
Discussion
There is a high incidence of surgical diseases in the retroperitoneal, inguinal, pelvic and scrotal regions of human beings, and in some cases, their treatment may inadvertently cause damage to the constituents of the spermatic cord, to the testis and to the epididymis. Surgeons know that they should delicately handle these structures; however, they do not know the intensity of the traction force being applied during the intraoperative period.
The traction imposed on the right spermatic cord changed the final testicular volume. To verify this fact, testicular ultrasonography was used because this exam is recognized as the most accurate method for measuring testicular volume in situ 11. Thorup J, Haugen S, Kollin C, Lindahl S, Läckgren G, Nordenskjold A, Taskinen S. Surgical treatment of undescended testes. Acta Paediatr. 2007 May;96(5):631-7. PMID:17381472. , 44. Gouletsou PG, Galatos AD, Leontides LS. Comparison between ultrasonographic and caliper measurements of testicular volume in the dog. Anim Reprod Sci. 2008 Oct;108(1-2):1-12. PMID:17723281. , 55. Lin CC, Huang WJ, Chen KK. Measurement of testicular volume in smaller testes: how accurate is the conventional orchidometer? J Androl. 2009 Nov-Dec;30(6):685-9. doi: 10.2164/jandrol. 108.006460.
https://doi.org/10.2164/jandrol.108.0064...
. Testicular volume reflects spermatogenesi, because approximately 70 to 80% of testicular mass consists of seminiferous tubules, and such structures are correlated with sperm count, sperm motility, sperm morphology and the daily production of sperm44. Gouletsou PG, Galatos AD, Leontides LS. Comparison between ultrasonographic and caliper measurements of testicular volume in the dog. Anim Reprod Sci. 2008 Oct;108(1-2):1-12. PMID:17723281..
To measure the wall of the vas deferens in the rat, it is necessary to know their histological peculiarities. The vas deferens of the adult rat is 4.5 to 6 cm long, originates proximally from the end of the cauda epididymis in the scrotum and ends distally in the ejaculatory duct in the pelvis. It is a muscular tube that has three layers of smooth muscle arranged as follows: the middle layer is circular and the outer and inner layers are longitudinal33. Hamilton DW, Cooper TG. Gross and histological variations along the length of the rat vas deferens. Anat Rec. 1978 Apr;190(4):795-809. PMID:637322. , 66. Shandling B, Janik JS. The vulnerability of the vas deferens. J Pediatr Surg. 1981 Aug;16(4):461-4. PMID:7277139. , 77. Kennedy SW, Heidger PM Jr. Fine structural studies of the rat vas deferens. Anat Rec. 1979 May;194(1):159-79. PMID:355773.. The vas deferens contracts unidirectionally during ejaculation, releasing the seminal fluid in the ejaculatory duct. This contractile activity is biphasic in rodents and is mediated by the autonomic nervous system88. Pampal A, Ozen IO, Ekingen G, Demirogullari B, Helvacioglu F, Take G. The morphological evaluation of ipsilateral and contralateral vasa deferentia in a rat model of unilateral spermatic cord torsion. Pediatr Surg Int. 2010 Mar;26(3):287-92. doi: 10.1007/s00383-009-2533-5.
https://doi.org/10.1007/s00383-009-2533-...
. The vas deferens of rats is suspended by a mesentery that is continuous with the epididymis. In the vas deferens, the following regions can be differentiated: proximal, junctional, distal and terminal. These regions have different histological characteristics:
a) In the proximal region, the vas deferens has thin muscle, which allows the intraluminal visualization of its whitish content. As a result, the vas deferens in this location is narrower compared to the vas deferens of the distal region. The topology of the lumen in this region is a tapered cylinder; therefore, the lumen diameter is large, but gradually decreases from the proximal portion towards the distal portion of the vas deferens. The columnar epithelium is thin and the lamina propria is narrow, offering little vascularization. This remarkable luminal reduction is due to the progressive increase in epithelial cell height along the vas deferens and the variations in the diameters of the epithelium and lamina propria, which are increased in the distal segment and decreased in the proximal segment of the vas deferens.
b) In the junctional region, which is located between the proximal and distal regions of the vas deferens, the epithelium has a crenellated appearance, resembling small jagged parapets built on top of towers, forts or castles protecting the shooters. This signals the beginning of the formation of the mucosal folds, which are characteristic of the distal region of the organ.
c) In the distal region, the musculature of the vas deferens is thickened, which does not allow the visualization of its intraluminal content. The columnar epithelium is tall with mucosal folds and thickened lamina propria. In the living animal, the distal region is colored pink due to the increased vascularization when compared with the proximal region. Thus, a dramatic change occurs in the lumen geometry, which displays a stellate shape. It is not known if the lumen of the vas deferens increases during ejaculation.
d) In the terminal region of the vas deferens, before its confluence with the duct of the seminal vesicle to form the ejaculatory duct, the typical epithelium of the distal region is replaced with a simple epithelium containing nests of cells that phagocytose the sperm. An obvious ampullary region is not observed in the vas deferens33. Hamilton DW, Cooper TG. Gross and histological variations along the length of the rat vas deferens. Anat Rec. 1978 Apr;190(4):795-809. PMID:637322..
The cause of testicular and vas deferens lesions is most likely related to vascularization. Experiments have shown that mobilization of the vas deferens with dissection of the artery of the vas deferens in rats resulted in a significant reduction in fertility and fecundity and also caused histological changes in the testis and epididymis. This most likely happened due to sympathetic denervation of the vas deferens, with consequent loss of motility and functional obstruction of the organ or possibly due to changes in testicular function. The clinical applicability of this theory is related to the fact that, in orchidopexy in humans, an extensive mobilization of the vas deferens may be required, resulting in lesions in the artery of the vas deferens99. Smith EM, Dahms BB, Elder JS. Influence of vas deferens mobilization on rat fertility: implications regarding orchiopexy. J Urol. 1993 Aug;150(2 Pt 2):663-6. PMID:8100864..
A change in the number of sperm in the epididymis may be influenced by: changes in testicular function, changes in blood flow and temperature of the epididymis, loss of tubular sympathetic tone and obstruction of the transit of sperm1010. Billups KL, Tillman S, Chang TS. Ablation of the inferior mesenteric plexus in rat: alteration of sperm storage in the epididymis and vas deferens. J Urol. 1990 Mar;143(3):625-9. PMID:2304184..
Lesions in the contralateral testis caused by unilateral testicular damage are one of the most controversial aspects. The exact etiology of these lesions is still unclear. The neurovascular regulation of blood flow to the testicles and the possibility of reperfusion lesions after contralateral vascular compromise are hypotheses that attempt to explain this phenomenon. Another theory is that possible contralateral damage is immunological. However, it seems that autoimmunization in humans is an unlikely cause, at least in the long term, of contralateral testicular lesion1111. Ceylan H, Karakok M, Guldur E, Cengiz B, Bagci C, Mir E. Temporary stretch of the testicular pedicle may damage the vas deferens and the testis. J Pediatr Surg. 2003 Oct;38(10):1530-3. PMID:14577081.
12. Choi SE, Kook MC, Kim CJ, Lee SC, Park KW, Jung SE, Kim WK. Effects of compression/stretching of the spermatic cord and blunt dissection on testicular growth and fertility. J Pediatr Surg. 2009 Nov;44(11):2163-7. doi: 10.1016/j.jpedsurg.2009.03.025.
https://doi.org/10.1016/j.jpedsurg.2009....
13. Turner TT. On unilateral testicular and epididymal torsion: no effect on the contralateral testis. J Urol. 1987 Nov;138(5):1285-90. PMID:3499519.
14. Jesus LE. Acute scrotum. Rev Col Bras Cir. 2000 Jul-Aug;27(4):271-8. doi.org/10.1590/S0100-69912000000400008.
https://doi.org/10.1590/S0100-6991200000...
15. Nguyen L, Lievano G, Ghosh L, Radhakrishnan J, Fornell L, John E. Effect of unilateral testicular torsion on blood flow and histology of contralateral testes. J Pediatr Surg. 1999 May;34(5):680-3. PMID:10359163. - 1616. Kolettis PN, Stowe NT, Inman SR, Thomas AJ Jr. Acute spermatic cord torsion alters the microcirculation of the contralateral testis. J Urol. 1996 Jan;155(1):350-4. PMID:7490885..
Blunt dissection of the spermatic cord in rats causes greater changes in testicular volume than the compression and stretching of this structure1212. Choi SE, Kook MC, Kim CJ, Lee SC, Park KW, Jung SE, Kim WK. Effects of compression/stretching of the spermatic cord and blunt dissection on testicular growth and fertility. J Pediatr Surg. 2009 Nov;44(11):2163-7. doi: 10.1016/j.jpedsurg.2009.03.025.
https://doi.org/10.1016/j.jpedsurg.2009....
.
Ceylan et al.1111. Ceylan H, Karakok M, Guldur E, Cengiz B, Bagci C, Mir E. Temporary stretch of the testicular pedicle may damage the vas deferens and the testis. J Pediatr Surg. 2003 Oct;38(10):1530-3. PMID:14577081. published the earliest reference found on the effects of stretching of the spermatic cord with traction force on the testis and vas deferens measured by a dynamometer. These authors reported having found decreased muscle thickening in the vas deferens wall of rats in group I in their experiment. They argued that the slightly greater increase in luminal diameter of the vas deferens in the animals of this group may have resulted in a significant decrease in wall muscle thickness of the organ. They mentioned that the reduced thickness of the muscular wall of the vas deferens in rats from group I was found in measurements performed on sections obtained from the vas deferens 2 cm distal to the epididymis.
However, these changes may not have actually occurred, given the following:
a) The histological differences between the four distinct regions of the vas deferens wall were not mentioned in the article reporting that study; therefore, they were most likely not used as a methodological parameter;
b) Considering that the length of the vas deferens of rats ranges from 4.5 to 6 cm and structural changes occur over its length, sectioning of the vas deferens by the anatomic pathologist during the macroscopic examination does not necessarily specify whether the histological pattern of one or more regions of this structure can be found in the study;
c) The size of the lumen is not concatenated with a significant decrease in the thickness of the muscle wall, as suggested by these authors. The gradual narrowing of the lumen of the proximal region to the distal region of the vas deferens is due to a progressive increase in epithelial cell height along the vas deferens and to changes in the epithelial and lamina propria diameters, which together are increased in the distal segment and decreased in the proximal segment of this structure. The luminal diameter of the vas deferens wall is greatest in the proximal region and smallest in the distal region33. Hamilton DW, Cooper TG. Gross and histological variations along the length of the rat vas deferens. Anat Rec. 1978 Apr;190(4):795-809. PMID:637322.;
d) That author reports that the group I rats have a slightly larger luminal diameter and thinner muscles compared with the other groups. This suggests that the cut levels of the vas deferens in the examined histological slides may have histological characteristics of different regions of the wall of this organ;
e) Regarding the alleged reduction in muscle thickness, there is information in the literature that in the second proximal centimeter of the vas deferens, the muscle wall of this organ is already thickened77. Kennedy SW, Heidger PM Jr. Fine structural studies of the rat vas deferens. Anat Rec. 1979 May;194(1):159-79. PMID:355773.. Therefore, it is very likely that the cut levels of the vas deferens examined in the histological slides by these authors have histological characteristics of the proximal and junctional regions of the vas deferens. In this area, the vas deferens most likely has a slightly larger lumen with some crenellations and thick muscles, merged with the portion of the vas deferens that has greater luminal diameter and thin muscles. If there is no previously established histological criteria, measurement and comparison of the dimensions of the vas deferens wall without observing the histological characteristics of its four distinct regions, depending on the proposed objectives, constitute a methodological flaw. Thus, it is essential to consider all of the aforementioned evidence to avoid an erroneous result.
Variations in the diameter and in the structure of the vas deferens along its length have been described in an experiment on the effects on the vas deferens of post-pubertal rats after they had previously been damaged when the rats were prepubertal. However, the authors of this study explained that some of their measurements were not statistically significant due to the small size of the sample1717. Benge BN, Jordan GH. Prepubertal vasal injury: its effect on postpubertal vas deferens. J Urol. 1993 Apr;149(4):906-9. PMID:8455272..
Conclusions
The traction force applied to the right spermatic cord of rats caused injury in the testis and the epididymis. Anatomopathological changes were not identified in the vas deferens, and there was no decrease in the muscle wall thickness of this organ.
Acknowledgements
To Prof. Dr. Danilo Nagib Salomão Paulo, Professor of the Fundamentals of Surgery, College of Sciences of Santa Casa de Misericordia of Vitoria, Espirito Santo and the following medical students from College of Sciences of Santa Casa de Misericordia of Vitoria, Caroline Oliveira Brêtas, Guilherme Posses Bridi, Rafael Falk Zanello, Augusto Ribeiro de Jesus Oliveira, Luisa Carvalho Benedito, Murilo Silva Andrade and Gustavo Dias Santiago de Amorim for their participation in the experiment.
References
-
1Thorup J, Haugen S, Kollin C, Lindahl S, Läckgren G, Nordenskjold A, Taskinen S. Surgical treatment of undescended testes. Acta Paediatr. 2007 May;96(5):631-7. PMID:17381472.
-
2Kolbe A, Sun CC, Hill JL. Unpredictability of capsulotomy in testicular torsion. J Pediatr Surg. 1987 Dec;22(12):1105-9. PMID:3440895.
-
3Hamilton DW, Cooper TG. Gross and histological variations along the length of the rat vas deferens. Anat Rec. 1978 Apr;190(4):795-809. PMID:637322.
-
4Gouletsou PG, Galatos AD, Leontides LS. Comparison between ultrasonographic and caliper measurements of testicular volume in the dog. Anim Reprod Sci. 2008 Oct;108(1-2):1-12. PMID:17723281.
-
5Lin CC, Huang WJ, Chen KK. Measurement of testicular volume in smaller testes: how accurate is the conventional orchidometer? J Androl. 2009 Nov-Dec;30(6):685-9. doi: 10.2164/jandrol. 108.006460.
» https://doi.org/10.2164/jandrol.108.006460 -
6Shandling B, Janik JS. The vulnerability of the vas deferens. J Pediatr Surg. 1981 Aug;16(4):461-4. PMID:7277139.
-
7Kennedy SW, Heidger PM Jr. Fine structural studies of the rat vas deferens. Anat Rec. 1979 May;194(1):159-79. PMID:355773.
-
8Pampal A, Ozen IO, Ekingen G, Demirogullari B, Helvacioglu F, Take G. The morphological evaluation of ipsilateral and contralateral vasa deferentia in a rat model of unilateral spermatic cord torsion. Pediatr Surg Int. 2010 Mar;26(3):287-92. doi: 10.1007/s00383-009-2533-5.
» https://doi.org/10.1007/s00383-009-2533-5 -
9Smith EM, Dahms BB, Elder JS. Influence of vas deferens mobilization on rat fertility: implications regarding orchiopexy. J Urol. 1993 Aug;150(2 Pt 2):663-6. PMID:8100864.
-
10Billups KL, Tillman S, Chang TS. Ablation of the inferior mesenteric plexus in rat: alteration of sperm storage in the epididymis and vas deferens. J Urol. 1990 Mar;143(3):625-9. PMID:2304184.
-
11Ceylan H, Karakok M, Guldur E, Cengiz B, Bagci C, Mir E. Temporary stretch of the testicular pedicle may damage the vas deferens and the testis. J Pediatr Surg. 2003 Oct;38(10):1530-3. PMID:14577081.
-
12Choi SE, Kook MC, Kim CJ, Lee SC, Park KW, Jung SE, Kim WK. Effects of compression/stretching of the spermatic cord and blunt dissection on testicular growth and fertility. J Pediatr Surg. 2009 Nov;44(11):2163-7. doi: 10.1016/j.jpedsurg.2009.03.025.
» https://doi.org/10.1016/j.jpedsurg.2009.03.025 -
13Turner TT. On unilateral testicular and epididymal torsion: no effect on the contralateral testis. J Urol. 1987 Nov;138(5):1285-90. PMID:3499519.
-
14Jesus LE. Acute scrotum. Rev Col Bras Cir. 2000 Jul-Aug;27(4):271-8. doi.org/10.1590/S0100-69912000000400008.
» https://doi.org/10.1590/S0100-69912000000400008 -
15Nguyen L, Lievano G, Ghosh L, Radhakrishnan J, Fornell L, John E. Effect of unilateral testicular torsion on blood flow and histology of contralateral testes. J Pediatr Surg. 1999 May;34(5):680-3. PMID:10359163.
-
16Kolettis PN, Stowe NT, Inman SR, Thomas AJ Jr. Acute spermatic cord torsion alters the microcirculation of the contralateral testis. J Urol. 1996 Jan;155(1):350-4. PMID:7490885.
-
17Benge BN, Jordan GH. Prepubertal vasal injury: its effect on postpubertal vas deferens. J Urol. 1993 Apr;149(4):906-9. PMID:8455272.
-
Financial source: none
-
1
Research performed at Laboratory of Animal Experimentation, Research Center, College of Sciences of Santa Casa de Misericordia de Vitoria, Espirito Santo, Brazil. Part of Master degree thesis, Postgraduate Program in Sciences Applied to Surgery and Ophthalmology, School of Medicine, Federal University of Minas Gerais (UFMG), Brazil. Tutor: Edson Samesima Tatsuo.
Publication Dates
-
Publication in this collection
Aug 2014
History
-
Received
25 Mar 2014 -
Reviewed
22 May 2014 -
Accepted
24 June 2014